Introduction
Tail biting in pigs is a serious welfare problem, especially in conventional husbandry systems, with multifactorial causes (van Putten, Reference van Putten1969; Bracke et al., Reference Bracke, Hulsegge, Keeling and Blokhuis2004a, Reference Bracke, Hulsegge, Keeling and Blokhuis2004b; Blokhuis et al., Reference Blokhuis, Nunes Pina, Bracke, Sanaa, Edwards, Gunn, Martineau, Mendl and Prunier2007). Several risk factors are described in the literature, ranging from high stocking densities to deficiencies in feed quality or accessibility (Moinard et al., Reference Moinard, Mendl, Nicol and Green2003), problems with the housing climate (Hunter et al., Reference Hunter, Jones, Guise, Penny and Hoste2001), lack of appropriate enrichment material (Zonderland et al., Reference Zonderland, Wolthuis-Fillerup, van Reenen, Bracke, Kemp, den Hartog and Spoolder2008), group size and group composition (Zonderland et al., Reference Zonderland, Bracke, den Hartog, Kemp and Spoolder2010) as well as poor health status (Day et al., Reference Day, Spoolder, Burfoot, Chamberlain and Edwards2002a) or genetic factors (Breuer et al., Reference Breuer, Sutcliffe, Mercer, Rance, Beattie, Sneddon and Edwards2003). Due to the multifactorial causes of tail biting, any of the above-mentioned factors or any other factors can result in a tail biting outbreak, whereby Schrøder-Petersen and Simonsen (Reference Schrøder-Petersen and Simonsen2001) stated that stress plays an important role for the occurrence of tail biting. This abnormal behaviour leads to tail injuries with different degrees of severity, from scratches and small bite marks to a complete loss of the tail with large wounds. Thus, tail biting has enormously negative effects on pig health and welfare and, thus, also results in substantial economic losses in pig production.
Besides the risk factors named above, the human–animal relationship also influences animal welfare and productivity and can therefore probably influence the occurrence of tail biting in pigs. Pleasant or gentle interactions (stroking or talking in a gentle voice) may reduce fear of humans and induce a positive association. Ursinus et al. (Reference Ursinus, Van Reenen, Reimert and Bolhuis2014) illustrated that the occurrence of tail biting is correlated with animals’ fearfulness: animals that actively performed tail biting showed a higher fear response compared with non-tail biters. Day et al. (Reference Day, Burfoot, Docking, Whittaker, Spoolder and Edwards2002b) stated a positive effect of gentle interactions on feed intake in growing pigs and that early handling of animals might reduce the stress associated with changes in the animals’ environment (e.g. rehousing, mixing, change in food). Furthermore, interacting with the animals could be seen as some kind of positive enrichment: other studies have confirmed that provision of raw material or new enrichment materials may be able to reduce the occurrence of tail biting. Thus, since interactions between humans and animals have an effect on the animals’ behaviour and the performance, the aim of the present study was to evaluate whether intensified human–animal interaction, i.e. regular gentle and unforced interactions, is able to reduce the occurrence of tail biting in weaned piglets.
Materials and methods
Animals and housing
The animals were kept at a conventional farrow-to-fattening farm with a closed system in Schleswig-Holstein, Germany. Data collection took place from November 2016 to February 2017. In total, data from 662 crossbred piglets (Duroc × (Yorkshire × Landrace)) in four batches were included in the analysis. All animals were given an iron injection, vaccinated against mycoplasma and circovirus and marked with ear tags in the farrowing pens. Tails were not docked and males were not castrated. The piglets were weaned on average at 21 days with an average weaning weight of 5.4 ± 0.11 kg. After the rearing period of 7 weeks, the piglets reached an average weight of 25 ± 2.3 kg before they were re-housed in the fattening unit.
From the second week of age until weaning, the piglets received a pre-starter diet (14.6 MJ ME, 175 g/kg protein, 14.5 g/kg lysine). After weaning, they were fed ad libitum with piglet-growing foods (PGF) using a sensor-controlled liquid feeding system in three phases: PGF I (day 22–36; 14.4 MJ ME, 176 g/kg protein, 14.0 g/kg lysine), PGF II (day 37–50; 14.0 MJ ME, 170 g/kg protein, 14.0 g/kg lysine) and PGF III (day 51–70; 13.6 MJ ME, 170 g/kg protein, 13.0 g/kg lysine). The trough was refilled when the filling level dropped below a certain threshold, i.e. there was always feed in the trough for the animals. The animal feeding space ratio was 2:1. New feed was blended with the previous one for 3 days.
The rearing unit consisted of four smaller and four larger compartments which contained two pens each (Fig. 1). Directly after weaning, a maximum number of 50 piglets were housed in each of the 12 m2 pens of the smaller compartments (0.24 m2/animal). These pens contained a heated resting area with a rubber floor mat with a cover to maintain an adequate temperature for the piglets. The rest of the pen consisted of a plastic slatted floor. Chains and plastic bite sticks, as well as a shovel of chopped straw spread twice a day over the rubber floor mat, were provided as enrichment material. Water was accessible through two bowl drinkers and one nipple drinker. Due to the stable construction, as well as legal requirements for animal space ratio within the stable, piglets were re-housed in the 17.5 m2 pens of the larger compartments (0.35 m2/animal) after 3 weeks. These pens had also rubber floor mats in the resting area and a plastic slatted floor in the rest of the pen. Inorganic material such as plastic balls mounted on chains was provided as enrichment material. Additionally, the animals had access to chopped straw provided in conventional straw racks, which were filled twice a day. Water was accessible via one bowl drinker and two nipple drinkers. Pen walls of both the small and large compartments were concrete, so the animals could hear but not see each other. The environmental temperature was regulated automatically by forced ventilation. It was set to 28.0 °C on day 1 of rearing and decreased stepwise to 24.0 °C on day 40. The animals had full artificial lighting between 06.00 and 19.00 h.
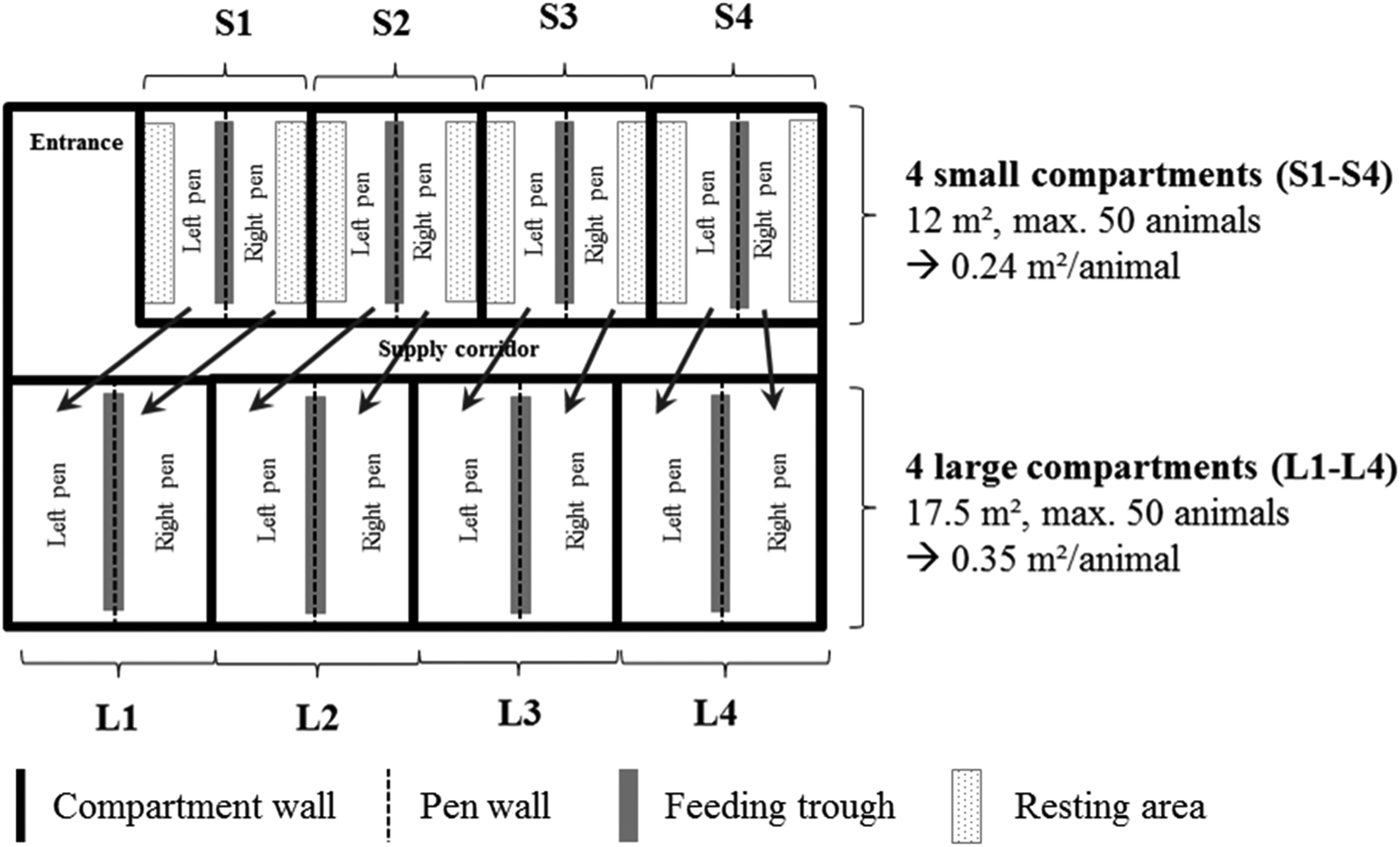
Fig. 1. Illustration of the rearing unit consisting of four small compartments (S1–S4) and four large compartments (L1–L4), each containing two pens (arrows show the rehousing procedure from the small to the large compartments after 3 weeks).
Experimental setup
The study included four batches with one control group and one trial group each. Data recording covered the whole rearing phase. The piglets of one batch were weaned simultaneously and re-housed in the rearing unit, sorted by weight, i.e. the animals of the control and the trial group had nearly the same weight. The number of animals in the different batches ranged from 130 to 190 piglets. In each batch, only two of the four compartments were used; one for the control group and the other for the trial group and each compartment contained one pen for male and one pen for female piglets.
In total, 16 piglet groups were involved in the present study, separated into eight piglet groups per treatment group. The control group had on average 41 animals per pen and the trial group had on average 42 piglets per pen. However, the number of animals varied between the batches (control group: batch 1: 47, batch 2: 31, batch 3: 41, batch 4: 46; trial group: batch 1: 46, batch 2: 35, batch 3: 38, batch 4: 48). During the whole experimental procedure, the animals included in the current study were affected by an E. coli infection, confirmed by a pathological analysis, which led to high animal losses of 10/100 animals in the control group and 16/100 animals in the trial group.
In the control group, only routine daily animal control was performed, i.e. a visual inspection of the animals, the feeding and drinking system, and further pen equipment was carried out by the farmer without any interaction with the animals. In the trial group, the human–animal interaction was intensified compared with the control group: in addition to the daily animal control, one operator interacted with the animals for 15 min per pen on 3 days of the week. The operator crouched or knelt down, depending on the curiosity or obtrusiveness of the animals, spoke calmly with the animals and tried to attract their attention with chopped straw. The piglets were petted if they allowed it without showing signs of stress or discomfort. The intensified human–animal interaction in the trial groups started with the first day in the rearing unit and was carried out until the end of the rearing period, always at the same time of day (14:00 h).
Data recording
All data recording was carried out by one observer, who was trained intensively by one experienced veterinarian. Data recording started with the human approach test, which was carried out according to Thodberg et al. (Reference Thodberg, Jensen and Herskin1999) at pen level once a week during the rearing period (week of age 4 to week of age 11). This resulted in eight scorings for each batch. The observer entered the pen and stood directly in front of the pen walls without speaking or further movements. From this moment on, the time until the first piglet physically touched the observer was recorded.
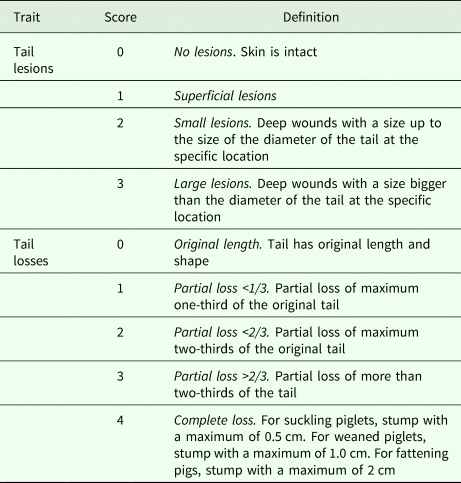
After the human approach test, the piglets’ tails were scored once a week during the rearing period (week of age 4 to week of age 11), which resulted in eight scorings for each batch. Two different traits were scored (tail lesions and tail losses) at pen level using the classification scheme presented in Table 1, following the German national scoring scheme for pigs (‘Deutscher Schweine Boniturschlüssel’) (Anonymous, 2016). Different intensities of open and fresh wounds on the tail were scored: starting with superficial lesions including scabs and small bite marks, followed by small and large lesions scored as deep wounds of increasing size. The occurrence of fresh blood was not recorded. Tail losses, on the other hand, only recorded the length of the pigs’ tails regardless of whether or not this tail loss had happened recently, i.e. if there was a fresh wound or not.
Table 1. Classification scheme for tail lesions and tail losses following the German national scoring scheme for pigs (‘Deutscher Schweineboniturschlüssel’) (Anonymous, 2016)
Statistical analysis
The statistical software package SAS 9.4 (SAS® Institute Inc., 2013) was used for statistical analysis. Mixed linear models were carried out for normally distributed data using the MIXED procedure. Generalized mixed linear models were applied for the analysis of binomial and multinomial data using the GLIMMIX procedure. The Akaike's information criteria corrected (AICC) (Hurvich and Tsai, Reference Hurvich and Tsai1989) and the Bayesian information criteria (BIC) (Schwarz, Reference Schwarz1978) were used to compare the different models. The model with the smallest AICC and BIC values was chosen. In general, the fixed-effects treatment group, batch, week of age (except for tail losses) and sex were added stepwise to the models. Interactions between the fixed effects were also tested. Here, only significant interactions were included in the final models.
Human approach test
The time until the first animal physically touched the operator was scored pen-wise in the human approach test. The data were transformed logarithmically in order to obtain a normal distribution. These transformed data were analysed using the MIXED procedure. The model for time taken to approach the human in the approach test included the fixed-effects treatment group (control group, trial group), batch (1, 2, 3, 4), week of age (4, 5, 6, 7, 8, 9, 10, 11), sex (male, female) and the interaction between treatment group and week of age. The significance of differences in the least square means between the treatment groups in a specific week of age were adjusted by the Bonferroni–Holm correction (Holm, Reference Holm1979). For illustration purposes, the least square means were back-transformed.
Tail lesions
Tail lesions were scored pen-wise on a four-point scale (categories 0–3). Due to low frequencies in categories 2 (small lesions) and 3 (large lesions), these two categories were combined. The multinomial data of tail lesions were analysed using the GLIMMIX procedure with a multinomial distribution using the cumlogit link function. The model for tail lesions included the fixed-effects treatment group (control group, trial group), batch (1, 2, 3, 4), week of age (4, 5, 6, 7, 8, 9, 10, 11), sex (male, female) as well as the interaction between treatment group and batch.
Tail losses
Tail losses were scored pen-wise on a five-point scale (categories 0–4). Due to low frequencies in categories 3 (partial loss >2/3) and 4 (complete loss), these two categories were combined. Additionally, only the last observation at the end of rearing (11th week of age) was used in the model. The multinomial data of the tail losses were analysed using the GLIMMIX procedure with a multinomial distribution with the cumlogit link function. The model for the tail losses included the fixed-effects treatment group (control group, trial group), batch (1, 2, 3, 4) and sex (male, female) as well as the interaction between treatment group and batch.
Results
All values illustrated in the Results section are least square means (LSMeans) derived from the statistical models described in the Materials and method section and described as proportion.
Human approach test
The fixed-effects treatment group, sex and week of age as well as the interaction between treatment group and week of age showed a significant influence on the time to approach recorded in the human approach test (P < 0.05). The fixed-effect batch showed no significant influence.
Interaction between treatment group and week of age
Figure 2 illustrates the output of the mixed linear model for the interaction between treatment group and week of age. Except for the 4th and the 7th week of age, piglets in the trial group were quicker to approach humans than those in the control group. Furthermore, the time taken to approach showed a clear reduction in the trial group, whereas more fluctuation could be observed in the control group. However, significant differences between the two treatment groups could only be detected in the 8th, 10th and 11th weeks of age.
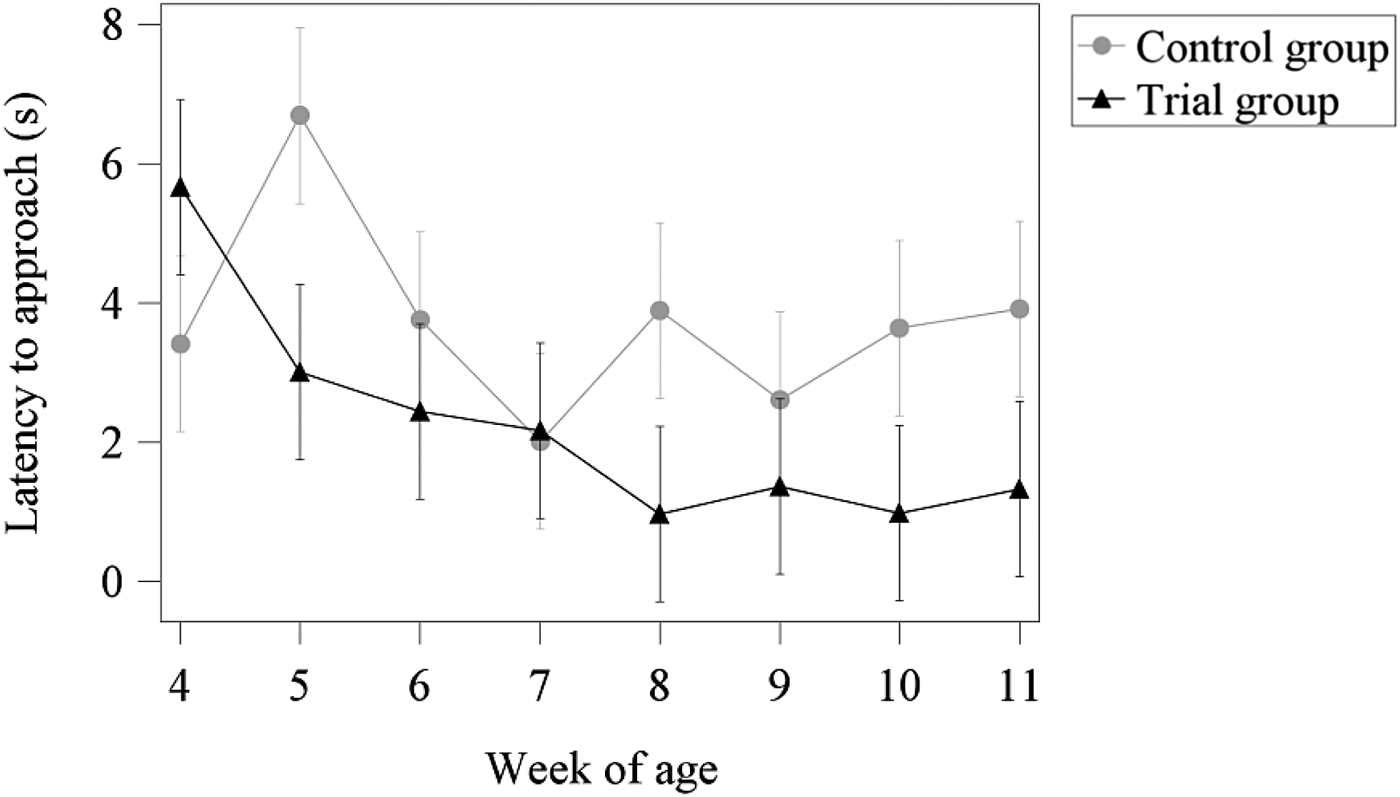
Fig. 2. Estimated (LSMeans) latency to approach in seconds for the interaction between treatment group and week of age.
Sex
Significant differences in the time taken to approach were also recorded between male and female piglets: female piglets, at 2 ± 1.1 s, showed a significantly lower time to approach compared with male piglets at 3 ± 1.1 s (P < 0.05). The P values were calculated based on the logarithmically transformed latency to approach.
Tail lesions
All fixed effects (treatment group, batch, week of age and sex), as well as the interaction between treatment group and batch, included in the model showed a significant influence on tail lesions (P < 0.05).
Interaction between treatment group and batch
Figure 3a illustrates the results for tail lesions in relation to treatment group and batch. It shows clearly that fewer tail lesions in each batch of the trial group were seen compared with the control group. The differences were more prominent between the two treatment groups in batches 1 and 3 compared with batches 2 and 4.

Fig. 3. Estimated frequencies (LSMeans) for the tail lesions depending on the interaction between treatment group and batch (a), the week of age (b) as well as the sex (c).
Week of age
Figure 3b shows the development of tail lesions in relation to week of age. The number of tail injuries increased directly after weaning, until the 7th week of age when 43/100 animals had no tail injuries, 29/100 animals showed superficial lesions and 28 animals/100 animals had small-to-large lesions. From week 7 to 10, the level of tail lesions in all three categories remained relatively stable. Only in the 11th week of age could a decline in the amount of tail lesions be observed.
Sex
Figure 3c shows the results for tail lesions in male v. female piglets. Small but significant differences were obtained for sex, with male piglets showing more tail lesions compared with females (P < 0.05).
Tail losses
All fixed effects included in the model (treatment group, batch and sex) as well as the interaction between treatment group and batch showed a significant influence on tail losses (P < 0.05).
Interaction between treatment group and batch
Figure 4a illustrates the estimated frequencies of tail losses for the interaction treatment group and batch in the last week of rearing (11th week of age). In accordance with the results of the tail lesions, the trial group showed higher numbers of animals with intact tails over all four batches compared with the control group. Furthermore, batches 3 and 4 in particular had fewer animals with partial losses of more than one-third of the tail in the trial group compared with the control group.

Fig. 4. Estimated frequencies (LSMeans) for the tail losses depending on the interaction between treatment group and batch (a) and sex (b) for the last week of rearing (11th week of age).
Sex
Figure 4b illustrates the estimated frequencies of the tail losses separated by sex in the last week of rearing (11th week of age). In accordance with the tail lesion results, more female piglets had intact tails compared with males. In particular, the numbers of tail losses of more than one-third was lower for females compared with males.
Discussion
The aim of the present study was to evaluate the effect of intensified human–animal interaction on the prevention of tail biting in pigs. Therefore, in the rearing phase, three times a week one person entered the pen and interacted calmly and in a friendly manner with the piglets in the trial group. In both treatment groups, piglets’ tails were scored once a week regarding tail lesions and tail losses and compared with each other. Additionally, a human approach test was performed to evaluate the human–animal relationship between the two treatment groups.
Human approach test
Interaction between treatment group and batch
The results of the human approach test show that, except for the 4th and 7th weeks of age, piglets from the trial group were quicker to approach the observer compared with the control group. However, these differences were significant only for the 8th, 10th and 11th weeks of age. For the trial group, a clear decrease in the time taken to approach with increasing age could be obtained, whereas the control group showed more fluctuations over the single weeks with no clear reduction compared with the value of the 4th week of age. Overall, the results showed that there was a clear effect of treatment group depending on the week of age. In the trial group, animals showed a habituation effect to the person interacting with the animals, which shortened the time taken to first physical contact between human and animals. In the literature, previous studies have stated a habituation effect due to the increasing number of tests (Hemsworth et al., Reference Hemsworth, Barnett and Hansen1986; Hemsworth and Barnett, Reference Hemsworth and Barnett1992; Scheffler et al., Reference Scheffler, Stamer, Traulsen and Krieter2014a) or due to the increasing age of the animals (Janczak et al., Reference Janczak, Pedersen and Bakken2003). Also, Grandin and Shivley (Reference Grandin and Shivley2015) stated that younger pigs had a stronger reaction towards a sudden novel stimulus (stamping of a boot) compared with older pigs, because their responses were less likely to be affected by learning or habituation. However, according to this explanation, the control group should also show a decrease in the time taken to approach, which was not observed in the present findings. Therefore, one explanation might be the intensified human–animal interaction, which was the only difference between the two treatment groups. Here, the animals of the trial group learned that no negative interactions were performed by the observer; in fact, they received a reward (chopped straw) when they interacted with the human, which resulted in reduced fearfulness of the animals. Previous studies have shown that animals receiving regular contact with humans (Hemsworth and Barnett, Reference Hemsworth and Barnett1991; Jones and Waddington, Reference Jones and Waddington1993), or which have learned to associate humans with a food reward (Pajor et al., Reference Pajor, Rushen and de Passillé2000), had a shorter time to approach. Also, Hemsworth et al. (Reference Hemsworth, Barnett and Hansen1986) and Andersen et al. (Reference Andersen, Berg, Bøe and Edwards2006) stated that the approach to humans depends on the quality of handling as well as on the animals’ fear of humans. Thus, in the present study, a positive effect of the intensified human–animal interaction could be observed, which was confirmed by the lower time to approach of the animals in the trial group.
Sex
The results show a significant difference in the time to approach between male and female piglets, with female piglets showing significantly lower values compared with the males. The influence of sex on the outcome of the human approach test is discussed in the literature, with various findings. The present findings agree with those of Scheffler et al. (Reference Scheffler, Traulsen and Krieter2014b), who attributed the differences to negative experiences in the handling of male piglets due to castration (van Erp-van der Kooij et al., Reference van Erp-van der Kooij, Kuijpers, Schrama, Ekkel and Tielen2000; Chaloupková et al., Reference Chaloupková, Illmann, Neuhauserová, Tománek and Vališ2007; Siegford et al., Reference Siegford, Rucker and Zanella2008; Rault et al., Reference Rault, Lay and Marchant-Forde2011). However, in the present study, the male piglets were not castrated, and male and female piglets were handled identically. In the study of Reimert et al. (Reference Reimert, Rodenburg, Ursinus, Duijvesteijn, Camerlink, Kemp and Bolhuis2013), female piglets were also faster to approach the observer. They argued that female animals could have a higher motivation to explore novel stimuli or be less fearful (Brown et al., Reference Brown, Dewey, Delange, Mandell, Purslow, Robinson, Squires and Widowski2009). Moreover, the study of Lay et al. (Reference Lay, Matteri, Carroll, Fangman and Safranski2002) stated that male piglets had a higher basal cortisol concentration compared with female piglets, which may cause female piglets to be less susceptible to stress than male piglets. In the study of de Oliveira et al. (Reference de Oliveira, Paranhos da Costa, Zupan, Rehn and Keeling2015), female piglets tended to allow more stroke attempts compared with male piglets. In contrast, the study of Muns et al. (Reference Muns, Rault and Hemsworth2015) detected no sex effect. The higher motivation to explore and lower susceptibility to stress in female piglets can be seen as a possible explanation for their faster time to approach in the human approach test.
Tail lesions and tail losses
Interaction between treatment group and batch
For tail lesions and tail losses, a significant interaction between treatment group and batch was obtained. However, the trial group showed lower numbers of tail lesions and tail losses in all four batches.
One possible explanation for these results could be the intensified human–animal relationship, less fear and thus less stress for the animals because they became accustomed to interaction with the humans. These results are in accordance with the results of the human approach test. Here, the animals of the trial group showed a significantly lower time to approach compared with the control group, which as discussed above shows reduced fearfulness of the animals due to the intensified human–animal interaction. Previous studies confirmed these results. de Oliveira et al. (Reference de Oliveira, Paranhos da Costa, Zupan, Rehn and Keeling2015) and Zupan and Zanella (Reference Zupan and Zanella2017) stated that if animals were not habituated to the handler, they showed an increased fear response. Furthermore, Zupan and Zanella (Reference Zupan and Zanella2017) stated that fearful animals may have a higher tendency to become more stressed by environmental changes. This increased stress may then be a trigger for the occurrence of tail biting behaviour. Previous studies have illustrated that tail biting is caused by multifactorial triggers (Moinard et al., Reference Moinard, Mendl, Nicol and Green2003; von Borell and Schäffer, Reference von Borell, Schäffer, Aland and Madec2009; Taylor et al., Reference Taylor, Parker, Mendl, Edwards and Main2012; Holling et al., Reference Holling, Tölle, Otto and Blaha2016). One of these triggers is stress, which plays an important role in the occurrence of tail biting (Schrøder-Petersen and Simonsen, Reference Schrøder-Petersen and Simonsen2001). Also, Ursinus et al. (Reference Ursinus, Van Reenen, Reimert and Bolhuis2014) stated that tail biting occurrence is correlated with the fearfulness of the animals. In the current study, the performers of tail biting showed increased avoidance of a novel object and were more alert and stressed compared with non-tail biters, which suggests greater fear. Day et al. (Reference Day, Spoolder, Burfoot, Chamberlain and Edwards2002a) described a positive effect of gentle interactions on feed intake and that early handling may be able to reduce the disruption associated with changes in the animals’ environment. The significant differences between the two treatment groups in the present study confirm these findings.
Another possible explanation for reduced tail lesions and tail losses in the trial group could be the improved occupation of animals through greater variety in their daily routine. Additionally, they also received new enrichment material (chopped straw) during the interaction with the observer, which could also reduce the development of tail biting. These findings were also confirmed in previous studies, which were able to illustrate a significant reduction in tail biting due to the provision of small amounts of raw material as enrichment material (Fraser et al., Reference Fraser, Phillips, Thompson and Tennessen1991; Day et al., Reference Day, Burfoot, Docking, Whittaker, Spoolder and Edwards2002b; Zonderland et al., Reference Zonderland, Wolthuis-Fillerup, van Reenen, Bracke, Kemp, den Hartog and Spoolder2008; Veit et al., Reference Veit, Traulsen, Hasler, Tölle, Burfeind and grosse Beilage2016).
Week of age
An increase in tail lesion injuries was seen from the 4th week of age but, looking at the development of tail lesions, increases could only be seen until the 7th week of age. From the 7th until the 10th week of age, the amount of tail lesions remained relatively stable and decreased again in the 11th week of age. Other studies confirm this temporal shift in the occurrence of tail biting (Abriel and Jais, Reference Abriel and Jais2013; Abriel et al., Reference Abriel, Jais and Bernhardt2014; Veit et al., Reference Veit, Traulsen, Hasler, Tölle, Burfeind and grosse Beilage2016, Reference Veit, Büttner, Traulsen, Gertz, Hasler, Burfeind, grosse Beilage and Krieter2017). Similar to the present findings, tail biting in other studies showed a clear increase 2–3 weeks after weaning. Furthermore, Abriel and Jais (Reference Abriel and Jais2013) and Abriel et al. (Reference Abriel, Jais and Bernhardt2014) found that this shift was even more delayed in enriched pens (lower stocking density, additional trough, more enrichment material, e.g. straw rack), with a less severe course of tail biting. Veit et al. (Reference Veit, Traulsen, Hasler, Tölle, Burfeind and grosse Beilage2016) stated that this shift could be explained by the number of changes the piglets faced during and after the weaning process. In the early rearing phase, piglets are confronted with a large number of changes (separation from the sow, new pen mates, new environment, changed feeding) to which they need to adapt. According to Lallès et al. (Reference Lallès, Bosi, Smidt and Stokes2007), under natural conditions, weaning is a gradual process that is not completed until 10th to 12th weeks of age. According to Wechsler (Reference Wechsler1995), in conventional housing systems, the animals often fail to control stressful situations using evolved coping strategies. It is argued that abnormal behaviour such as tail biting can result based on this failure, i.e. unsuccessful coping. Furthermore, according to Hötzel et al. (Reference Hötzel, de Souza, Costa and Machado Filho2011), the re-housing and mixing of piglets after weaning leads to fights for rank order, which represents an additional stressor for piglets and which might also trigger the occurrence of tail biting. Since only tail lesions and tail losses were analysed in the current study, i.e. the outcome of a tail biting occurrence, and not the active behaviour, the point at which the animals started to bite each other cannot be determined definitely. According to the study of Zonderland et al. (Reference Zonderland, Kemp, Bracke, den Hartog and Spoolder2011), 82/100 animals were observed biting pen mates’ tails and 96/100 animals received tail bites before any tail damage was actually observed in the pen, which may be an explanation for the 2–3-week shift seen in the present data.
Sex
In the present study, female piglets showed fewer tail lesions and tail losses. Although these results were statistically significant, the differences between female and male piglets were only small. The influence of sex on occurrence of tail biting is discussed in the literature with various conclusions. No clear pattern could be detected regarding which sex is most likely to be a tail biter or to have their tail bitten (Breuer et al., Reference Breuer, Sutcliffe, Mercer, Rance, Beattie, Sneddon and Edwards2003; Moinard et al., Reference Moinard, Mendl, Nicol and Green2003; Schrøder-Petersen et al., Reference Schrøder-Petersen, Simonsen and Lawson2003; Taylor et al., Reference Taylor, Main, Mendl and Edwards2010). Sinisalo et al. (Reference Sinisalo, Niemi, Heinonen and Valros2012) and Scollo et al. (Reference Scollo, Di Martino, Bonfanti, Stefani, Schiavon, Marangon and Gottardo2013) stated that in single-sex groups, male animals were not bitten significantly more than female or castrated animals. Also, Blackshaw (Reference Blackshaw1981) stated that independent of whether the animals were kept in single-sex or mixed-sex groups, no differences between female and castrated male animals regarding tail damage could be observed. In contrast, Keeling et al. (Reference Keeling, Wallenbeck, Larsen and Holmgren2012) analysed the tails of pigs at the abattoir and found that males were more likely to have damaged tails than females. Brunberg et al. (Reference Brunberg, Wallenbeck and Keeling2011) analysed tail biting of mixed-sex groups regarding both performers and receivers of the behavioural disorder and found no differences between the sexes concerning the number of received tail bites. However, female pigs showed a tendency to perform a higher proportion of severe tail bites. Also, other studies have confirmed that males receive more tail bites than females (Wallgren and Lindahl, Reference Wallgren and Lindahl1996; Kritas and Morrison, Reference Kritas and Morrison2004; Zonderland et al., Reference Zonderland, Bracke, den Hartog, Kemp and Spoolder2010). Zonderland et al. (Reference Zonderland, Bracke, den Hartog, Kemp and Spoolder2010) also stated that pigs in all-female groups showed more tail damage compared with all-male groups. However, with regard to mixed-sex groups, Zonderland et al. (Reference Zonderland, Bracke, den Hartog, Kemp and Spoolder2010) found that males had more tail damage than female animals. Thus, the present findings showed deviating results; here, male animals in single-sex groups had more tail lesions and tail losses compared with all-female groups. However, as already explained above, differences between the two sexes were only small.
Experimental setup
The trial group differed from the control group only in the intensified human–animal interaction, which was carried out three times a week by one person for the duration of 15 min in each pen. There were no other confounding factors which could bias the results. The compartments had the same construction, feeding system and ventilation system. Also, the enrichment material was the same for control and trial groups. Furthermore, the animals were all subjected to the same re-housing procedure, from the smaller compartments to the larger ones. Since the animals were sorted by weight after weaning, this confounding factor could also be excluded. This was a first attempt to see if any change in the behaviour of the animals could be observed by this relatively small variation between control and trial groups. However, this slight change in the performance of the human–animal interaction led to significant differences between the two treatment groups, as already discussed. In further studies, variations in the amount of intensified human–animal interactions should be evaluated in order to gain more insights into the effects of this treatment.
Furthermore, due to the E. coli infection of the animals included in the current study, animal health and welfare was affected negatively. Other studies have noted that poor health status is a risk factor in the development of abnormal behaviour such as tail biting (Schrøder-Petersen and Simonsen, Reference Schrøder-Petersen and Simonsen2001; Moinard et al., Reference Moinard, Mendl, Nicol and Green2003; Edwards, Reference Edwards2011). However, although the current trial group had slightly higher amounts of animal losses, it showed significantly fewer tail lesions and tail losses compared with the control group. In order to confirm that these results were caused by the different treatment groups and not by the impaired health condition of the animals, the study should be repeated including different intensities of human–animal interactions, i.e. varying length of time spent with the animals or varying number of days, to evaluate the effect on tail biting.
Conclusion
The results of the present study show that intensified human–animal interaction was able to influence the animals’ behaviour towards the human as well as towards their pen mates regarding abnormal behaviours such as tail biting. The trial group showed better results compared with the control group, i.e. fewer tail lesions and fewer tail losses. Furthermore, the results of the human approach test showed a lower time to approach for the animals of the trial group, indicating reduced fearfulness of the animals, which may be the result of a better human–animal relationship. If it is possible to integrate an intensified human–animal interaction into the daily practice of pig farms, the human–animal relationship could be enhanced as well as possibly reducing tail biting occurrence.
Financial support
None.
Conflict of interest
None.
Ethical standards
The authors declare that the experiments were carried out strictly following international animal welfare guidelines. The animals were kept following the ‘German Animal Welfare Act’ (German designation: TierSchG), the ‘German Order for the Protection of Animals used for Experimental Purposes and other Scientific Purposes’ (German designation: TierSchVersV) and the ‘German Order for the Protection of Production Animals used for Farming Purposes and other Animals kept for the Production of Animal Products’ (German designation: TierSchNutztV). No pain, suffering or injury was inflicted on the animals during the experiments.







