Article contents
Tick-borne viruses
Published online by Cambridge University Press: 19 April 2005
Abstract
At least 38 viral species are transmitted by ticks. Virus–tick–vertebrate host relationships are highly specific and less than 10% of all tick species (Argasidae and Ixodidae) are known to play a role as vectors of arboviruses. However, a few tick species transmit several (e.g. Ixodes ricinus, Amblyomma variegatum) or many (I. uriae) tick-borne viruses. Tick-borne viruses are found in six different virus families (Asfarviridae, Reoviridae, Rhabdoviridae, Orthomyxoviridae, Bunyaviridae, Flaviviridae) and at least 9 genera. Some as yet unassigned tick-borne viruses may belong to a seventh family, the Arenaviridae. With only one exception (African swine fever virus, family Asfarviridae) all tick-borne viruses (as well as all other arboviruses) are RNA viruses. Tick-borne viruses are found in all the RNA virus families in which insect-borne members are found, with the exception of the family Togaviridae. Some tick-borne viruses pose a significant threat to the health of humans (Tick-borne encephalitis virus, Crimean-Congo haemorrhagic fever virus) or livestock (African swine fever virus, Nairobi sheep disease virus). Key challenges are to determine the molecular adaptations that allow tick-borne viruses to infect and replicate in both tick and vertebrate cells, and to identify the principal ecological determinants of tick-borne virus survival.
- Type
- Research Article
- Information
- Copyright
- © 2004 Cambridge University Press
TICKS AS VECTORS OF ARBOVIRUSES
Ticks are not insects. The significance of this statement is considered in a review of the marked contrasts between the biology of ticks and that of insects, and the consequences for their vector potential (Randolph, 1998). Many unique features of ticks contribute to their remarkable success as virus vectors. This chapter will first consider the characteristics of ticks important in virus transmission and then present an overview of the tick-borne members of different virus families.
Tick life cycle and longevity
One of the most outstanding features of ticks is their remarkable longevity. The complete life cycle of ticks is usually measured in years, and individual stages can survive long periods without a bloodmeal (Sonenshine, 1991). Experimental data indicate that virus infections persist in ticks for the duration of the ticks' lifespan (Rehacek, 1965; Davies, Jones & Nuttall, 1986). Ecological and epidemiological data also support the observation that tick-borne virus survival is greatly dependent on persistent infections in tick populations (Blaskovic & Nosek, 1972).
Tick life stages (eggs, larvae, nymphs or adults) readily survive from one year to the next and ixodid tick species show marked differences in the number of generations completed within a year. The survival strategies of different tick species inhabiting temperate climates loosely reflect the prevailing conditions in which the ticks are found. For example, Rhipicephalus sanguineus, which prefers the warmer environment of southern Europe, can have up to two generations in one year and will remain active over winter. In contrast, Ixodes ricinus, which has a more northerly distribution and is active in colder climates, generally has two peaks of activity, in the spring and autumn. However, generally in each year it only feeds once and only passes through one developmental stage, thus taking at least three years to complete its life cycle. Ticks that do not find a host in the autumnal activity period will overwinter to become active again the following spring, hence the life cycle can take up to six years to complete. This may be regarded as a survival strategy to meet the demands of a harsh climate. Some tick species, such as Dermacentor spp., appear to show an intermediate strategy, with the adults overwintering and one generation being completed in each year.
The survival strategy of ticks is important for the survival of the viruses they transmit. Because of the exceptional longevity of ticks, they can carry tick-borne viruses over prolonged periods of time. As a result, ticks are not only vectors but also excellent reservoir hosts for the viruses they carry.
If the long-term survival of viruses depends on their tick vector, selection must favour infections that have no detrimental effect on the tick. This appears generally to be the case, although few studies have been published concerning arboviral effects on tick vectors. Differences were not detected in the reproductive output, moulting success and survival of uninfected R. appendiculatus, compared with ticks of the same population that were infected with Thogoto virus (THOV) at the larval stage (L. D. Jones, personal communication). However, the salivary glands of partially-fed adult female R. appendiculatus ticks infected with THOV secreted fluid in vitro at about 75% the rate of controls (Kaufman, Bowman & Nuttall, 2002). The significance of this observation is unknown. Detrimental effects of infection with African swine fever virus (ASFV) on adult Ornithodoros moubata ticks have been reported. A significant increase in mortality rates was observed amongst the adult ticks that fed on an infective compared with a normal (uninfective) blood-meal (Rennie, Wilkinson & Mellor, 2000). Such reports of adverse effects are exceptional whereas there are several reports that insect-borne viruses can adversely affect their vectors (Turell, 1988).
A specific mode of arbovirus persistence in the vector population is via vertical transmission in which virus from the infected female is transmitted via the egg to the succeeding generation. Although evidence from experimental studies of vertical transmission has been recorded for numerous tick-borne viruses, the levels of vertical transmission and filial infection in nature generally appear low. Certainly, the high levels of vertical transmission of certain insect-borne viruses associated with stabilized infections of their vectors (Turell, 1988) have not been recorded for any tick-borne viruses. If, as discussed above, tick-borne viruses rely on their vectors for persistence, then any deleterious effects of vertical transmission on ticks may outweigh the advantages to the virus. The balancing of costs and benefits of vertical transmission, together with the gains from co-feeding and non-viraemic transmission (not depending on the host viraemia) may explain why vertical transmission is common among tick-borne viruses but occurs at a low level.
Non-viraemic transmission has another important implication for vertical transmission. As mentioned above, vertical transmission is common among tick-borne viruses but occurs at an apparently low level. This has led to claims that vertical transmission is not a significant factor in the ecology and epidemiology of tick-borne viruses (Rehacek, 1965). A laboratory study of low level Tick-borne encephalitis virus (TBEV) infection in a population of larval ticks (detectable only by polymerase chain reaction) demonstrated that the infection was amplified by non-viraemic co-feeding to yield a significant number of infected nymphal ticks (Labuda et al. 1993). Opportunities for such amplification of vertically transmitted infections occur in the field where a low prevalence of TBEV infection in I. ricinus larvae has been documented (Danielova et al. 2002). Similar results have been reported for Colorado tick fever virus (CTFV; Calisher, 2001) and Crimean-Congo haemorrhagic fever virus (CCHFV; Gordon, Linthicum & Moulton, 1993), whereas a higher filial infection prevalence was reported for ASFV (Rennie, Wilkinson & Mellor, 2001). Because larvae that hatch from the same egg mass often quest in clusters, several of them may attach to the same individual host. This behaviour provides many opportunities for amplification of vertically-acquired tick-borne virus infections, in the vector population, through non-viraemic transmission between co-feeding larvae (Labuda et al. 1993; Danielova et al. 2002). As a result of such amplification, vertical transmission might be the difference between survival and extinction of certain tick-borne viruses in nature.
Host finding and host preferences in the virus transmission cycle
The most important requirement of ticks to accomplish their life cycle is to find a suitable vertebrate host. Some tick species prefer to feed on a particular vertebrate host species, whereas others feed on a range of hosts. As a rule, a successful tick vector species has a wide host range but there are notable exceptions, e.g. populations of the seabird tick, I. uriae, often feed year after year on the same seabird species. The number of hosts on which a tick feeds during its lifetime varies depending on the species of tick and its preferences and also on whether it is a one-, two-, or three-host tick. In Europe, most tick species are three-host (e.g. Ixodes spp., Rhipicephalus spp., Dermacentor spp. and Amblyomma spp.), of which the larva, nymph and adult each feed on a separate host and are free-living between feeding periods. Some species (e.g. Hyalomma spp.) are described as two-host ticks, where the larva and nymph feed on the same host, but the adult feeds on a different one. Thus, there is not only the possibility for transmission of viruses between hosts of the same species, but because of the range of potential hosts, there is also the important possibility of disease transfer between vertebrate species including humans.
Owing to their feeding preferences, ticks restrict the potential range of hosts for a virus. For example, I. ricinus has a very wide host range, including many species of mammals, birds and even lizards. In spite of such a variety of hosts, the majority of I. ricinus ticks feed on only a few mammalian species and tick infestation is frequently limited to only a part of the host population. Typically, the overdispersed distribution of ticks on their hosts results in a significant proportion of the hosts carrying large numbers of ticks that feed together.
Overdispersion arises from the non-random distribution of questing ticks and host genetic, behavioural and immunological heterogeneities. These factors determine the differential probabilities of an individual host picking up ticks. In Central Europe, the most abundant rodent hosts of immature I. ricinus are frequently yellow-necked mice (Apodemus flavicollis) and bank voles (Clethrionomys glareolus). Coincident aggregated distributions of I. ricinus larvae and nymphs on these species, in western Slovakia, resulted in 20% of the animals feeding about three quarters of both the larval and nymphal populations (Randolph et al. 1999). As a result, the number of larvae exposed to infection by feeding alongside potentially infected nymphs was doubled compared with the null hypothesis of independent distribution of these two tick stages. The observed pattern of co-feeding is typical for I. ricinus larvae and nymphs and not for other tick species occurring in western Slovakia. Overall, only 3% of I. ricinus nymphs were recorded on hosts that were not carrying at least one larva. In contrast, as many as 28% of D. reticulatus nymphs were found on hosts (from the same area and at the same time) that were not feeding with larvae of the same species. As both species are ‘competent’ vectors of TBEV in the laboratory, the virus can potentially be exchanged between ticks of each species where they co-exist. However, these tick species make differential use of voles and mice as hosts. More D. reticulatus have been recorded on C. glareolus than on A. flavicollis, while I. ricinus showed the reverse host association (Randolph et al. 1999). These particular patterns of tick infestation on transmission-competent rodent hosts help provide a quantitative explanation for the focality of TBEV as described by Randolph in this Supplement. The concept was first expounded by Pavlovsky as the ‘nidality’ of TBEV in Euroasia (Zilber & Soloviev, 1946). Focality and nidality reflect the fact that TBEV survival in nature results from the critically balanced relationships within the virus–vector–host triangle in the given environment.
Primary and secondary vector species
Certain tick species are crucial for the maintenance of virus transmission cycles and are considered primary vectors. All the ecological and physiological characteristics of such tick species appear to be well suited for maintaining certain tick-borne viruses in nature. Other species may be involved as secondary vectors. For example, TBEV is maintained in Europe primarily by I. ricinus ticks and in Asia by I. persulcatus. However, it seems that all competent tick species, occurring in sufficiently high numbers and having a sympatric distribution with the primary tick vector species, may become infected and subsequently transmit TBEV. Although experimental studies have shown that numerous tick species are competent vectors, their ecological roles vary. For example, the vector competence of I. hexagonus for TBEV has been demonstrated in the laboratory, including transmission of TBEV to hedgehogs, the principal host of this tick species, and TBEV has been isolated from field-collected I. hexagonus. Ixodes arboricola, a bird tick, was shown to be a competent vector in the laboratory. Similarly, Haemaphysalis concinna, H. inermis and H. punctata are competent vectors, and TBEV has been isolated from field collected specimens (Gresikova & Calisher, 1988). Yet, in the natural situation only the two primary vectors are able to perpetuate efficiently TBEV transmission cycles as documented many times over the huge territory in which TBEV is endemic. In contrast to the many tick species capable of transmitting TBEV, the epizootology of Louping ill virus (LIV) implicates I. ricinus as the exclusive vector even though Dermacentor reticulatus and Haemaphysalis punctata are present in the UK where the virus is endemic.
In the endemic area of Kyasanur forest disease, 36 species of ticks have been recorded. Of the 15 species of Haemaphysalis present in the area, Kyasanur forest disease virus (KFDV) has been isolated from H. spinigera and 8 other species which paints a picture even more complicated than that for TBEV. However, the record for the highest number of infected tick species goes to Crimean-Congo haemorrhagic fever virus (CCHFV), isolated from at least 31 different species and sub-species.
Taking a blood-meal
Ticks are pool feeders, yet another important feature distinguishing them from many blood-feeding insects. To create a specific feeding pool in the dermis, ticks attach themselves to the host skin using their chelicerae and toothed hypostome. Ixodid ticks may feed for a few days or up to two weeks, cementing their mouthparts into the skin. Only during the last day of attachment is the majority of the blood-meal taken up (Sonenshine, 1991). Such a profound physical and chemical assault on the host provokes the host's haemostatic, inflammatory and immune responses. Despite the massive armoury of rejection mechanisms, the tick manages to remain attached and achieve engorgement. The success of the tick is based upon a pharmacy of chemicals located in its complex salivary glands and secreted, in tick saliva, into the feeding pool (Nuttall, 1999; see also the chapter by Valenzuela (2004) in this Supplement). The main route of virus transmission by infected ticks is via saliva secreted during feeding (Kaufman & Nuttall, 1996). Virus transmitted by this route enters a skin site that is profoundly altered by the effects of tick saliva (Titus & Ribeiro, 1990). Tick saliva possesses pharmacologically active substances that have anti-haemostatic, vasodilatory, anti-inflammatory and immunosuppressive activities. Components of the host immune system that are modified by tick saliva include activation of complement and natural killer cells, antibody production, and T-lymphocyte proliferation and function (Wikel & Bergman, 1997; see also the chapter of Brossard & Wikel, 2004 in this Supplement). Modulation of host immunity at the feeding site not only allows the attached ticks to feed but also increases the rate at which other co-feeding ticks acquire infection from the same host. Thus tick blood feeding inadvertently provides an advantage to tick-borne viruses in that modulation of cells at the site of infection promotes virus transmission and survival.
Tick competence, digestion and moulting
In virus–vector systems other than those mentioned above, we do not observe a broad range of vector competence among tick species. For example, experiments comparing different methods of infecting ticks with Dhori virus and Dugbe virus have demonstrated the presence of a ‘gut barrier’ to virus infection. Dhori virus and Dugbe virus replicated in R. appendiculatus after inoculation into the tick hemocoel, a route of infection by-passing the gut. However, neither virus established an infection when the ticks were fed on an infective bloodmeal by the peroral route of infection (Steele & Nuttall, 1989). The presence of a gut barrier in ticks indicates that there is a specific interaction between virus (imbibed in the bloodmeal) and midgut cells. Although the nature of the gut barrier has not been determined, it appears to vary for different virus–tick systems.
The susceptibility of arthropod midgut cells to virus infection is one of the most important determinants of vector competence. Understanding the determinants of vector competence is important in explaining why certain tick-borne viruses have many tick vectors (e.g. CCHFV) whereas others have few (e.g. Nairobi sheep disease virus).
The initial stages of virus infection are likely to differ markedly for ticks and insects, and may be the principal reason why tick-borne viruses are rarely, if ever, transmitted by insects. Thus, viruses entering the tick midgut are exposed to different environmental conditions compared with those existing in, for example, the mosquito midgut. This is because ticks are heterophagous, i.e. blood-meal digestion is primarily an intracellular process occurring within midgut cells (Sonenshine, 1991). In contrast, the blood-meal of insect vectors is digested extracellularly (within the midgut lumen). Studies with mosquitoes and La Crosse virus (Bunyaviridae, Bunyavirus) indicate that cleavage of a protein exposed on the surface of virus particles (virions) is necessary to initiate vector infection (Ludwig et al. 1991). The necessary proteolytic conditions apparently occur in the midgut of mosquitoes, but such conditions may not be present in the midgut of heterophagic ticks.
If the method of blood-meal digestion in ticks exerts a strong selective pressure on arboviruses, the structure of the outer surface of tick-borne viruses is likely to differ significantly from that of related insect-borne viruses (given that virion–cell surface interactions are the first phase of infection). Presently, this hypothesis cannot be tested as there are insufficient data, for arboviruses, on the three-dimensional structure of virions and the nature of virus receptors. However, comparative sequence data have revealed significant differences in the virion surface proteins of midge-transmitted orbiviruses (e.g. Bluetongue virus) and the tick-transmitted orbivirus, Broadhaven virus (Iwata, Yamagawa & Roy, 1992); and in the surface envelope protein of tick-borne flaviviruses (e.g. TBEV) which contains a unique region of six continuous amino acids not found in the envelope protein of mosquito-borne flaviviruses, e.g. Yellow fever virus (Shiu et al. 1991). Recently, much attention has been given to the interaction of viral surface proteins with glycosaminoglycans, which are largely distributed on cell surfaces but vary with respect to their composition and quantity. For TBEV, it has been proposed that the affinity of the viral surface for glycosaminoglycan molecules such as heparin sulfate may be an important determinant of tissue tropism (Mandl et al. 2001). Attention has been also given to vector molecules. A lectin, named Dorin M, has been identified in the haemocytes and plasma of Ornithodoros moubata ticks. Since these lectin types were reported to function as non-self recognizing molecules, Dorin M may play a role in innate immunity and pathogen transmission (Kovar, Kopacek & Grubhoffer, 2000; and chapter by Grubhoffer, Kovar & Rudenko (2004) in this supplement). It remains to be determined whether and to what degree such molecules govern the specific adaptations of arboviruses to either tick or insect vectors.
As a result of the feeding behaviour of ticks, viruses must persist from one instar to the next in order to be transmitted to a vertebrate host. This means that the ‘extrinsic incubation period’, which is so important in determining the transmission dynamics of insect-borne viruses (Turell, 1988), is not significant for virus transmission by ixodid ticks because it is unlikely to exceed the comparatively long moulting period. However, the extrinsic incubation period is important in terms of virus survival, and in the rare cases of interrupted feeding by ticks (see next section).
In relation to virus survival during the extrinsic incubation period, the histolytic enzymes and tissue replacement associated with moulting provide a potentially hostile environment (Balashov, 1998). Several authors have suggested that the dynamics of viral replication within the tick reflect these events: a fall in virus titre, followed by an increase in the titre as the virus infects and replicates in replacement tick tissues (Rehacek, 1965; Burgdorfer & Varma, 1967). However, the replication of some viruses (e.g. THOV in R. appendiculatus, Langat virus in Ixodes ricinus) is not obviously correlated with any particular stage of the moulting period (Varma & Smith, 1972; Davies et al. 1986). These conflicting observations can be explained by the variety of infection strategies adopted by tick-borne viruses. The apparent targeting of specific cell types, tissues or organs may reflect mechanisms by which different tick-borne viruses have adapted to survive the moulting period, viz. by establishing an infection in at least one cell type that does not undergo histolysis.
An additional factor bearing on the extrinsic incubation period is the resorption and regeneration of salivary glands during moulting (see chapter by Bowman & Sauer (2004) in this Supplement). Hence virus infection of the salivary glands is likely to be a relatively late event in the infection cycle within ticks. A few reports describe virus in the salivary glands but the timing of infection varies. TBEV and Powassan virus infect the salivary glands prior to feeding; presumably they can be transmitted to the vertebrate host as soon as feeding is initiated (Rehacek, 1965; Chernesky & McLean, 1969). In contrast, THOV and Dugbe virus accumulate in the salivary glands after feeding commences (Booth et al. 1989, 1991), although in ticks infected in the preceding stadium, THOV is present in the salivary glands prior to blood feeding (Kaufman & Nuttall, 2004).
Interrupted feeding
The duration of the extrinsic incubation period (see previous section) is also important when ticks are interrupted in their feeding on a host. For example, a host may be killed or it may die from a virulent tick-borne virus infection. Partially fed infected ticks can detach from their deceased host and may successfully reattach and feed on a new host.
The consequences of interrupted feeding were investigated experimentally with THOV which kills hamsters before its nymphal or adult vector (R. appendiculatus) has completed engorgement (Wang & Nuttall, 2001). Ticks that had partially fed on infected hamsters were able to transmit the infection to new uninfected hosts on which they completed engorgement. The periods between feeds varied from 7 to 28 days, presumably within the extrinsic incubation period.
Although interrupted feeding of infected ticks is comparatively rare in nature, it may contribute to outbreaks of rapid and fatal tick-borne viral diseases such as Kyasanur forest disease in monkeys (Sreenivasan, Bhat & Rajagopalan, 1979). Additionally interrupted feeding provides an increased risk of transmission to humans and domestic animals during slaughter and game hunting.
TICK-BORNE ARBOVIRUSES
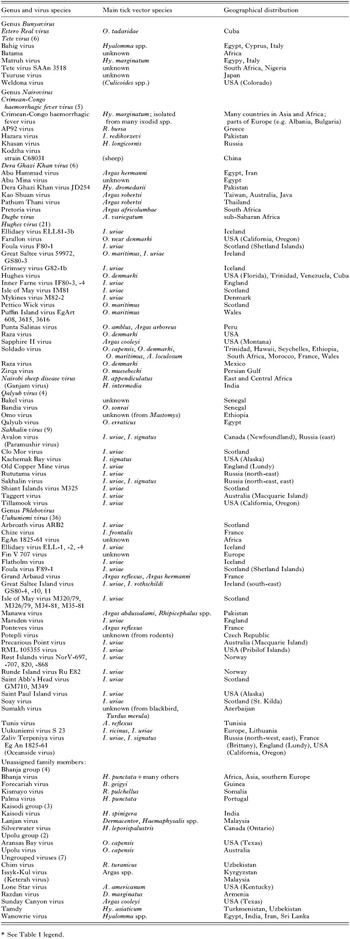
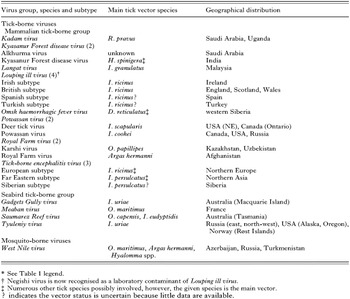
Arboviruses (arthropod-borne viruses) are taxonomically a heterogenous group of vertebrate viruses unified by a unique ecological feature, namely transmission by haematophagous arthropods in which they replicate. Intriguingly, all arboviruses (with one exception) are RNA viruses. The only DNA arbovirus (African swine fever virus, see next section) is transmitted by argasid ticks (Ornithodoros spp.). At least 500 arboviruses are registered (Karabatsos, 1985). About half of them are mosquito-borne and approximately one third are transmitted by ticks. Tick-borne viruses belong to 6 virus families (Tables 1–5). Each family is characterized by a unique genome organization and replication strategy. Thus tick-borne virus transmission has evolved independently at least six times during the phylogenetic period that can be traced today. Among virus families containing arboviruses, only the Togaviridae (genus Alphavirus) does not contain any tick-borne members, although some mosquito-borne alphaviruses (e.g. Sindbis virus) have occasionally been isolated from ticks (Gresíková et al. 1978).



Given that arboviruses represent the largest biological group of vertebrate viruses, it is reasonable to assume that their life style is a successful one. This is despite the fact that arboviruses, during their evolution, have solved a very specific problem: how to replicate successfully in two phylogenetically distinct systems, swapping between an arthropod cell and a vertebrate cell. Arboviruses have achieved this irrespective of whether they have a RNA genome that is double-stranded or single-stranded, segmented or non-segmented, or of positive or negative polarity.
Arbovirus groups having insect-borne members frequently also include tick-borne viruses. Insect-borne arboviruses outnumber tick-borne viruses in the Bunyaviridae and Flaviviridae families, but tick-borne viruses are exclusive to the nairoviruses, Uukuniemi virus group of phleboviruses, and coltiviruses. Interestingly, of approximately 200 named tick-borne viruses, 80% are members of the Orbivirus, Nairovirus, Phlebovirus and Flavivirus genera.
In general, the association between the arthropod and the transmitted virus is very intimate and highly specific. Comparatively few arthropod species act as vectors. In fact, less than 10% of the known tick species are incriminated as virus vectors and they are mostly found in large tick genera. For argasid ticks these are Ornithodoros and Argas and, among ixodid ticks, virus vectors are found mostly in the genera Ixodes, Haemaphysalis, Hyalomma, Amblyomma, Dermacentor, Rhipicephalus and Boophilus.
Some tick vector species transmit one or two virus species and a few transmit several species; Ixodes ricinus is a good example of the latter. It is widespread across most of the European continent reaching northern parts of Africa. In many forested areas it is the most abundant tick species with a very broad vertebrate host range. All these features make it a highly efficient vector of several arboviruses and also other pathogens (like Lyme disease borreliae). Indeed, I. ricinus is the main vector of viruses from three different virus families, e.g. TBEV and LIV of the Flaviviridae, Tribeč virus and Eyach virus of the Reoviridae, and Uukuniemi virus of the Bunyaviridae. Another example of a wide virus range transmitted by a single tick species is that of Ixodes uriae. It is a specific tick of seabird colonies with a circumpolar distribution along the sea shore. At least 6 virus species of the genus Orbivirus, Nairovirus, Phlebovirus and Flavivirus have been isolated from this tick species. Interestingly, viruses of the same genus or even group are vectored by both I. ricinus and I. uriae. These viruses are orbiviruses of Great Island virus (some 43 serotypes) transmitted by I. uriae and Kemerovo virus (all four serotypes) transmitted by I. ricinus; flaviviruses Tyuleniy virus and Gadgets Gully virus vectored by I. uriae and TBEV and LIV by I. ricinus; the phleboviruses Zaliv Terpeniya virus (a serotype of Uukuniemi virus) transmitted by I. uriae and Uukuniemi virus by both I. ricinus and I. uriae. The implications for the spread, vector specificity and evolution of these viruses are unknown. We can only speculate that tick genetics, physiology, ecology and life cycle are probably the key factors allowing both vector species to be involved in the maintenance of so many virus species in nature.
Returning to the basic question: how do arboviruses switch between replicating in vertebrate and invertebrate cells? Very little is known about the molecular mechanisms governing the relationship of arboviruses with ticks versus insects. The following characteristics of tick-borne members of different virus families and genera provide some clues and demonstrate the remarkable variety of viruses that have adapted to a life style using ticks as vectors.
Family Asfarviridae
Only a single genus, Asfivirus, is currently recognized within the Asfarviridae family and there is a single member, type species African swine fever virus (ASFV) (Table 1). The name of the family is derived from African swine fever and related viruses; ASFV represents the only known DNA arbovirus and is transmitted by tick bite or by a direct oral route (Vinuela, 1985; Dixon et al. 2000).
Virions of ASFV have a nucleoprotein core structure, 70–100 nm in diameter, within an icosahedral capsid, 170–190 nm in diameter, which is surrounded by internal lipid layers. Extracellular virions with an external lipid-containing envelope have a diameter of 175–215 nm. The viral genome comprises a single molecule of linear, covalently closed, double-stranded DNA, 170–190 kbp in size. The end sequences are present as two flip-flop forms that are inverted and complementary with respect to each other; adjacent to both termini are identical tandem repeat arrays about 2·1 kbp long. Whether these reflect adaptation to infect tick or vertebrate host cells, or both, is not known. The complete nucleotide sequence of the tissue culture adapted isolate has been published (Yanez et al. 1995) as well as partial sequences of two other virulent isolates. The genome comprises about 150 open reading frames, which are closely spaced (intergenic distances are generally less than 200 bp) and are read from both DNA strands. A few intergenic regions contain short tandem repeat arrays of unknown significance for virus virulence (Dixon et al. 2000).
Virions contain more than 50 proteins including a number of enzymes involved in nucleotide metabolism, DNA replication and repair or transcription, post-translational protein modification, an enzyme involved in synthesis of isoprenoid compounds, and factors needed for early mRNA transcription and processing. Enzymes packaged into virions further include RNA polymerase, poly(A) polymerase, guanyltransferase, and protein kinase. There are at least 8 characterized major structural proteins, and a further two DNA-binding proteins and seven proteins with putative transmembrane regions found in virions. In addition, 26 proteins with predicted transmembrane domains are encoded with some known to modify host cell function. Proteins that may modulate the host response to virus infection are also present. They include proteins similar to the T-cell surface protein CD2, IkB, the apoptosis inhibitors Bc12 and IAP, a protein similar to a Herpes simplex virus encoded neurovirulence factor ICP34.5, a myeloid differentiation antigen and the gadd34 protein. Large length variations are observed between genomes of different isolates, resulting from the gain or loss of members of five multigene families found in the genomic regions close to the termini, but such variations do not appear to result in any significant change in virus properties (Yozawa et al. 1994).
ASFV infects warthogs and bushpigs without any apparent ill effects. By contrast it causes severe disease, characterized by haemorrhage, in domestic and wild swine. The virus replicates in cells of the mononuclear phagocytic system and reticuloedothelial cells in lymphoid tissues and organs. Virus enters cells by receptor-mediated endocytosis and early mRNA synthesis begins in the cytoplasm immediately following entry. Virus DNA replication and assembly takes place in perinuclear areas (‘virus factories’) with peak DNA replication about 8 hours post infection. DNA replication may proceed by a self-priming mechanism. The cell nucleus is required for productive infection. Genes are expressed in an ordered cascade. Early genes are expressed prior to DNA replication; expression of late genes is dependent on the onset of DNA replication. Expression of some early genes continues throughout infection. Intermediate genes are expressed late but their expression does not depend on the onset of DNA replication. Virus morphogenesis takes place in the virus factories. Two layers of membrane, derived from the endoplasmic reticulum, are incorporated as internal lipid membranes. Formation of the icosahedral capsid is thought to occur on these membranes. The virus genome and enzymes are packaged into a nucleoprotein core. Extracellular virus has a loose-fitting external lipid envelope possibly derived by budding through the plasma membrane.
Soft ticks of the genus Ornithodoros are the main vectors of ASFV. O. moubata acts as the vector in parts of Africa south of the Sahara and is replaced by O. erraticus in southern Europe. ASFV replication in the tick midgut epithelium is required for infection of ticks (Kleiboeker et al. 1999). The virus can be transmitted in ticks trans-stadially, trans-ovarially, and sexually. Trans-ovarial transmission of ASFV in experimentally infected O. moubata ranged in filial infection prevalence from 1·2 to 35·5%. Immunohistochemistry showed that virus replicated in the developing larval cells and not in the yolk sac cells or within the outer layer of the eggs (Rennie et al. 2001). It appears that there is an increased mortality among adult Ornithodoros ticks infected by ASFV (Rennie et al. 2000).
Ticks transmit ASFV to warthogs, bushpigs and swine. Transmission between domestic swine can also occur by direct contact, ingestion of infected meat and fomites, or mechanically by biting flies. Disease is endemic in domestic swine in many African countries and in Sardinia. The virus was first introduced into Europe via Portugal in 1957 and became endemic in parts of the Iberian Peninsula from 1960 until 1995. Sporadic outbreaks that were successfully controlled have occurred in Belgium, Brazil, Cuba, the Dominican Republic, France, Haiti, Holland and Malta. Using restriction endonuclease analysis of genomic DNA, ASFV isolates can be distinguished into five groups (Gibbs, 2001). European and American isolates fall into one group and African isolates into the remainder, reflecting the likely African origin of the virus. American isolates appear to have originated in Europe.
Virus isolates differ in virulence and may produce a variety of signs ranging from acute to chronic, or they may produce inapparent infections. Virulent isolates can cause 100% mortality in 7–10 days. Less virulent isolates may produce a mild disease from which a number of infected swine recover and become carriers (Vinuela, 1985; Salas, 1994). A striking feature of ASFV infections is the absence of neutralizing antibody production. This has severely hampered attempts to produce an effective vaccine although the use of gene deleted virus strains has shown promise in protecting against virulent strains (Gibbs, 2001).
Family Orthomyxoviridae
The family contains three genera of influenza viruses (Influenzavirus A, B and C). In addition to these well known viruses causing respiratory diseases of humans, the fourth genus in the family (Thogotovirus) comprises tick-borne viruses (Table 1).
Genus Thogotovirus
The morphology and morphogenesis of these viruses show similarities with the influenza viruses. Virions are spherical or pleomorphic, 80–120 nm in diameter, and filamentous forms occur. The virion envelope is derived from cell membrane lipids and bear surface glycoprotein projections, 10–14 nm in length and 4–6 nm in diameter. Virions contain six or seven segments of linear, negative sense single-stranded RNA. Total genomic size is about 10 kb. Like the influenza viruses, each viral RNA segment possesses conserved regions of semicomplementary nucleotides at the 3′ and 5′ termini and mRNA synthesis is primed by host-derived cap structures. Both the 3′ and 5′ sequences of virion RNA are required for viral RNA promoter activity and the cap-snatching mechanism appears unique (Leahy, Dessens & Nuttall, 1997).
The type species, Thogoto virus (THOV), contains six single-stranded RNA segments. Four of them encode gene products that correspond to the viral polymerase (PB1, PB2 and PA) and nucleocapsid protein (NP) of influenza viruses (Weber et al. 1998). However, the fourth largest segment encodes a surface glycoprotein that is unrelated to any influenza viral protein but instead shows striking sequence homology to a baculovirus surface glycoprotein (Morse, Marriott & Nuttall, 1992). The same is true for Dhori virus (DHOV) (Freedman-Faulstich & Fuller, 1990). This unique glycoprotein is probably the key to the ability of members of the Thogotovirus genus to infect ticks (Nuttall et al. 1995). Influenza viruses use sialic acid residues on the surface of vertebrate cell membranes as receptors for infecting cells. Ticks are devoid of sialic acid. Clearly Thogotovirus members have got round this problem by evolving a different mechanism of cell infection to that of influenza viruses. A recent comparison of the glycoprotein sequences of eight THOV isolates, two DHOV isolates and one Batken virus isolate with the glycoprotein sequence of 10 nucleopolyhedrosis viruses (insect baculoviruses) suggests that the sequence similarity may represent convergent evolution rather than a common ancestry for the encoding gene (S. Turner, personal communication).
THOV has been isolated from Boophilus and Rhipicephalus spp. ticks in Africa (Kenya) and Europe (Sicily) as well as from Amblyomma variegatum ticks in Nigeria and Hyalomma spp. ticks in Nigeria and Egypt. THOV has also been isolated in the Central African Republic, Cameroon, Uganda and Ethiopia, and in several countries of southern Europe. Infections in sheep have been associated with high levels of abortion. THOV also infects cattle, goats and mongoose, and there are two reported cases of infections in humans associated with clinical conditions (Woodall, 2001). DHOV has a somewhat different but overlapping geographical distribution to that of THOV, occurring in India, eastern Russia, as well as Egypt and southern Portugal. DHOV virus has been isolated from Hyalomma spp. ticks. There is no detectable serological reactivity between THOV and DHOV and the structural differences (THOV has six RNA segments and DHOV has seven) and sequence diversity of 37% and 31% in the nucleoprotein and the envelope protein, respectively, support their separate species status. Batken virus, isolated from mosquitoes and ticks from Russia, cross reacts serologically with DHOV and shares 98% identity in a portion of the nucleoprotein and 90% identity in a portion of the envelope protein. These and other data suggest that Batken virus is closely related to DHOV (Frese et al. 1997). None of the viral proteins of members of the Thogotovirus genus are related antigenically to those of influenza viruses.
THOV has been used extensively in experimental studies of saliva-activated transmission in which the virus has been shown to exploit the pharmacological properties of its vector's saliva as described by Nuttall & Labuda in this Supplement. It has also been used to investigate reassortment using temperature-sensitive mutants to follow the exchange of genomic segments between viruses. The ability of THOV to reassort has been demonstrated in both ticks and a vertebrate host (Davies et al. 1987; Jones et al. 1987). However, the significance of such genetic exchange in the epidemiology of this virus is unknown.
Family Rhabdoviridae
The family Rhabdoviridae contains 6 genera, at least 6 other serogroups not assigned to any genus, and a number of individual unassigned viral species. The most important and best known representative is Rabies virus (genus Lyssavirus), one of the most lethal of all human pathogens. Typical rhabdoviruses infecting vertebrates have virions characteristically bullet-shaped, 100–430 nm long and 45–100 nm in diameter. The outer surface of virions is covered with projections (peplomers) comprising trimers of the viral glycoprotein. The viral genome is a single molecule of linear, negative sense single-stranded RNA and contains at least five open reading frames. Viruses generally have five structural polypeptides.
Many viruses of the genus Vesiculovirus are typical arboviruses replicating in both arthropods and vertebrates, such as Vesicular stomatitis virus (VSV). Phlebotomine sandflies are incriminated as vectors although recent data indicate that black flies (Simulium vittatum) transmit VSV by co-feeding on non-viraemic hosts (Mead et al. 2000). Isfahan virus has been isolated from sandflies and also from Hyalomma asiaticum ticks in Turkmenia (Table 1; Karabatsos, 1985). Antibody prevalence in humans is comparatively high in endemic regions but there are no reports of associated disease. The only other rhabdoviruses isolated from ticks are currently unassigned. These are Barur virus and Sawgrass virus of the Kern Canyon and Sawgrass virus groups, respectively (Table 1). Further studies are needed to confirm that ticks transmit these three rhabdoviruses biologically, and to determine whether other members of the Kern Canyon and Sawgrass virus groups are tick-borne.
Family Reoviridae
Reoviridae is a large family containing viruses with very diverse biological properties. The virions have icosahedral symmetry but may appear spherical in shape. Each virion has a capsid, which is made up of concentric protein layers organized as one, two, or three distinct capsid shells, having an overall diameter of 60–80 nm. Virions contain 10, 11, or 12 segments of linear double-stranded RNA, depending on the genus. The nine genera of the family can be divided into two groups. One group contains those viruses in which intact virus particles or cores have relatively large ‘spikes’ or ‘turrets’ situated at the 12 vertices of the icosahedron (members of the genera Orthoreovirus, Cypovirus, Aquareovirus, Fijivirus and Oryzavirus, as well as most of the unclassified or unassigned reoviruses from invertebrates). The second group of viruses have relatively smooth surfaces without prominent surface projections at their fivefold axes (members of the Orbivirus, Rotavirus, Coltivirus and Phytoreovirus genera). Orbiviruses and coltiviruses are arboviruses and include tick-borne viruses (Table 2).
Genus Orbivirus
Virions are spherical in appearance but have icosahedral symmetry. Although no lipid envelope is present on mature virions, they can leave the host cell by budding through the cell plasma membrane. During this process they transiently acquire an unstable membrane envelope. Unpurified virus is often associated with cellular membranes. The outer capsid has an ordered structure with icosahedral symmetry. The surface layer of the core particle is composed of capsomeres arranged as hexameric rings. These rings give rise to the name of the genus. The core particle also contains a complete inner capsid shell surrounding the 10 segments of double-stranded RNA that comprise the viral genome. Minor core proteins are attached to the inner surface of the subcore at the five-fold symmetry axes. Replication is characterized by production of viral ‘tubules’ and viral inclusion bodies and may be accompanied by formation of flat hexagonal crystals of the major outer core protein in the cytoplasm of infected cells.
Orbiviruses are transmitted between vertebrate hosts by a variety of haematophagous arthropods. They do not appear to cause disease in their natural hosts. Most orbiviruses associated with birds have been isolated from arthropod vectors that feed on birds, but evidence of infection of birds has been obtained for only a few orbiviruses (Nuttall, 1993).
The genus Orbivirus currently comprises 20 serological groups (Roy, 2001). The type species, Bluetongue virus (BTV), causes an economically important disease of sheep and is transmitted by midges of the genus Culicoides. It is one of the best characterized arboviruses. Approximately 60 tick-borne orbiviruses have been identified (Table 2). Most of these are variants (serotypes or topotypes) of Great Island virus (formerly in the Kemerovo serogroup) transmitted by ixodid ticks that parasitize seabirds (guillemots or murres, puffins, penguins, gannets, gulls etc.); at least 40 viruses have been isolated from the common seabird tick, Ixodes uriae. The distribution of Great Island virus reflects the bipolar distribution of I. uriae (Main et al. 1973; Nuttall, 1984; Chastel, 1988). Although these viruses are confined to seabird colonies, the presence of antibodies in various mammals including humans living in close proximity to the colonies (Lvov et al. 1972), and the recovery of I. uriae from ‘land’ birds (Arthur, 1963), indicate the possibility of extension of their normal range.
The four viruses of the species Kemerovo virus are maintained in the Palearctic region among small mammals and birds by two related species of ixodid ticks: Kemerovo virus by Ixodes persulcatus in western Siberia (Libikova et al. 1964), Tribeč virus and Lipovnik virus by I. ricinus in central Europe (Gresíková et al. 1965) and Kharagysh virus by I. ricinus in Moldova (Skofertsa et al. 1972). This group of viruses is listed under Great Island virus by the International Committee on Taxonomy of Viruses (van Regenmortel et al. 2000). We have identified Kemerovo virus as a distinct species because it showed limited genome segment reassortment with three representatives of Great Island virus, and none with Chenuda virus, Essaouira virus or Mono Lake virus (Nuttall & Moss, 1989). Speciation of Kemerovo virus and Great Island virus may be at a transitional stage in which ancestral links can be detected under highly selective experimental conditions. We have assumed genetic exchange between these two species cannot occur in nature, particularly given their different ecologies.
The species Chenuda virus includes 7 different serotypes from soft ticks of the genera Argas and Ornithodoros parasitizing birds (Hoogstraal, 1973). Chenuda virus was originally isolated from Argas reflexus hermanni collected from a pigeon house in Egypt (Taylor et al. 1966). Related viruses have been isolated from O. coniceps from pigeon nests in the Chatkal mountains and O. maritimus ticks infesting gulls (Larus argentatus) on an island in the Caspian Sea (Baku virus), gull colonies in Morocco (Essaouira virus and Kala Iris virus), and gull and shag (Phalacrocorax aristotelis) colonies in Brittany (Moellez virus). Two isolates of Great Saltee Island virus (GS80-5, GS80-6) were from eggs and adult male O. maritimus feeding in a breeding colony of shags on a small island off the south-east coast of Ireland. This virus is classed as a possible member of the species based on its geographical location, and vector and host associations.
Huacho virus, Mono Lake virus and Sixgun City virus are classified by the International Committee on Taxonomy of Viruses as belonging to the species Chenuda virus (van Regenmortel et al. 2000). Members of an orbivirus species are distinguished by their ability to exchange genomic segments. Based on this definition, we have recognised a distinct species, Mono Lake virus. The basis for this speciation is the inability of Mono Lake virus to demonstrate genome segment reassortment when tested experimentally with representatives of Chenuda virus, Great Island virus, and Kemerovo virus (Nuttall & Moss, 1989). Mono Lake virus was originally isolated from a pool of 10 adult Argas cooleyi ticks collected in 1966 from the nest of the California gull (L. californicus) on an island in Mono Lake, California. Other species members are recognized by their antigenic cross-reactivity with Mono Lake virus. These are Huacho virus, isolated from O. amblus nymphs collected from rocks of a guano seabird colony at Punta Salinas, and Sixgun City virus from Argas cooleyi collected in the nests of cliff swallows (Petrochelidon pyrrhonota) in Texas.
Chobar Gorge virus has two serotypes, Chobar Gorge virus and Fomede virus. They are associated with bats. Wad Medani virus has been isolated from sheep and from ixodid ticks of the genera Boophilus, Hyalomma, Ambylomma and Rhipicephalus in the Sudan, West Pakistan, India, Russia, and Jamaica. The closely related Seletar virus was isolated from B. microplus in Singapore and Malaysia.
The Great Island orbiviruses associated with seabird colonies provide a fascinating model in the study of virus evolution. Main et al. (1973) found two distinct but closely related serotypes, Great Island virus and Bauline virus, among puffins and petrels on Great Island, with little or no indication of other serotypes. They suggested that the antigenic identity of serotypes may be maintained by the isolation of the primary hosts of the vector and that each serotype developed in separate demes on the island. Yunker (1975) suggested that each virus within defined complexes has been influenced by common patterns of geography, ecology and behaviour. These included a discontinuous distribution, the nidiculous activity of the ticks that maintained them and the homing-colonial instinct of the bird hosts. Isolation produces a large number of strong insular variants, which in some cases develop into separate serotypes. Experimental studies have shown that Great Island serogroup viruses from ticks collected in seabird colonies in Scotland, Ireland, Iceland, Newfoundland, and Macquarie Island in the sub-Antarctic, can swap RNA segments. Thus, despite their extensive geographical distribution, this group of viruses constitutes a single gene pool clearly representing one virus species (Nuttall & Moss, 1989).
Structural and genetic studies have revealed significant differences between the tick-borne orbiviruses and (BTV, the type species of the genus) that may provide clues to their different ecologies. The most notable is in the structure and function of the two outer capsid proteins. In BTV and other insect-borne orbiviruses, VP2 and VP5 are the protein components of the outer capsid. VP2, the major determinant of the virus serotype, carries neutralising epitopes and the binding site for the vertebrate cell receptor. In contrast, the major determinant of serotype in the tick-borne orbiviruses is VP5 while VP4 is the second component of the outer capsid. VP4 probably bears the binding site for the vertebrate cell receptor as it determines neurovirulence. Interaction of VP4 and VP5 of Great Island virus (GIV) modulates viral pathogenicity. Thus, by reassortment, new viruses can be produced experimentally that are more or less virulent than either of their parental viruses (Nuttall et al. 1992). The significance of these studies for the natural ecology of GIV is unknown, particularly as the virus does not cause overt disease in its seabird hosts. However, a 4 year epidemiological study of guillemots (Uria aalge), the main amplifying hosts for GIV on the Isle of May, Scotland, revealed that GIV infections show a positive association with breeding success and might even influence colony structure (M. Nunn, personal communication). More needs to be done to determine the impact of GIV infections on seabirds and the role genetic reassortment plays in virus survival.
Comparison of three-dimensional models of BTV and Broadhaven virus (Great Island virus) indicate remarkable similarity except for differences in accessibility of the outer shell proteins (Schoehn et al. 1997). This may reflect the need to access and cleave VP2 of BTV within the midgut of its Culicoides vector. Cleavage of VP2 exposes the core protein, VP7, that bears the Arg–Gly–Asp (RGD) motif involved in insect cell infection. In contrast to insects, bloodmeal digestion in ticks occurs intracellularly. Furthermore, none of the viral proteins of tick-borne orbiviruses have been reported to carry a RGD motif. Hence tick-borne viruses most likely have evolved a mechanism of tick cell infection that does not rely on proteolysis in the midgut, unlike thier insect-borne relatives.
Genus Coltivirus
The most important feature distinguishing coltiviruses from the other members of the Reoviridae family is a genome comprising 12 segments of double-stranded RNA. During replication, viruses are found in the cell cytoplasm but immunofluorescent staining also reveals nucleolar fluorescence. Coltiviruses are transmitted to vertebrate hosts by ticks as well as mosquitoes (Brown et al. 1993). Tick-borne members of the genus are included in the Coltivirus subgroup A and mosquito-borne coltiviruses in subgroup B although it has been proposed that the latter group constitute a separate genus.
Coltivirus particles are 60–80 nm in diameter having two concentric capsid shells with a core that is about 50 nm in diameter. Electron microscope studies using negative staining reveal particles that have a relatively smooth surface capsomeric structure and icosahedral symmetry. Particles are found intimately associated with filamentous structures and granular matrices in the cytoplasm. The majority of the viral particles are non-enveloped, but a few acquire an envelope structure during passage through the endoplasmic reticulum.
Coltiviruses have been isolated from several mammalian species (including humans) and from ticks and also mosquitoes. Colorado tick fever virus (CTFV), the type species of the genus, is transmitted by ticks and causes an acute febrile illness in humans. There appears to be a single serotype involved in human infections. Because of the ease of isolation of the virus from patients and ticks, the geographical distribution has been determined as essentially that of the major vector, Dermacentor andersoni, in mountainous northwestern USA and Canada. Other tick species from which CTFV has been isolated include Dermacentor occidentalis, D. albipictus, D. parumapertus, Haemaphysalis leporispalustris and a species of Otobius. Vertebrate reservoirs include rodents, ground squirrels, pine squirrels, chipmunks, meadow voles and porcupines (Burgdorfer, 1977).
CTFV persists within erythrocytes in human patients and in experimentally infected animals (Emmons et al. 1972; Hughes, Casper & Clifford, 1974). Ticks become infected with CTFV on ingestion of a blood meal from an infected vertebrate host. Both adult and nymphal ticks become persistently infected and provide an overwintering mechanism for the virus. CTFV is transmitted trans-stadially and also trans-ovarially as demonstrated in Dermacentor andersoni ticks experimentally, and by the finding of related viruses from field collected Ixodes larvae in France and Germany (Calisher, 2001). Some rodent species have prolonged viraemias (more than 5 months), which may also facilitate overwintering, and virus persistence. Humans usually become infected when bitten by adult D. andersoni ticks but probably do not act as a source of reinfection for other ticks. Transmission from person to person has been recorded as the result of blood transfusion. The prolonged viraemia observed in humans and rodents is thought to be due to the intra-erythrocytic location of virions, protecting them from immune clearance (Emmons et al. 1972).
In 1972, a second serotype of CTFV was isolated from Ixodes ricinus ticks collected near Tubingen, West Germany (Rehse-Kupper et al. 1976). This virus is now recognised as a distinct species, Eyach virus. The virus has been associated with meningo-encephalitis in humans based on detection of specific IgM and IgG in patients with the disease. California hare coltivirus, isolated from Lepus californicus, is antigenically related to Eyach virus and may represent a distinct species. Several unassigned Chinese coltivirus isolates require further characterisation.
Family Bunyaviridae
The family Bunyaviridae (named after Bunyamwera virus, the prototype virus) consists of almost 400 named viruses making it the largest animal RNA virus family. Biologically, these viruses encompass a wide range of characteristics. Five genera are presently recognised within the family (Bunyavirus, Hantavirus, Nairovirus, Phlebovirus and Tospovirus). No arthropod vector has been demonstrated for rodent-associated hantaviruses transmitted among hosts by direct contact and aerosol-borne infections. Tospoviruses are transmitted by thrips between plants and are able to replicate in both thrips and plants. Viruses in the other three genera are typical arboviruses capable of replicating alternately in vertebrates and arthropods. Different viruses are transmitted by different species of a large variety of haematophagous arthropods. Some viruses of the family display a very narrow host range, especially for arthropod vectors. Tick-borne viruses are found in each of the three (Bunyavirus, Nairovirus and Phlebovirus) arbovirus genera (Table 3).
Viruses of the family Bunyaviridae have similar morphological features. The virions range between 80 and 120 nm in size, and display short (5–10 nm) surface glycoprotein projections embedded in a lipid bilayer envelope. The viral particles appear spherical or pleomorphic, depending on the method used for fixation for electron microscopic examination. An envelope, derived usually from cellular Golgi membranes, surrounds a core consisting of the genome and its associated proteins. All bunyaviruses have two glycoproteins, by convention designated G1 and G2.
Members of the family Bunyaviridae contain three unique molecules of single-stranded RNA of negative or ambisense polarity designated large (L), medium (M), and small (S) with a total size of 11–19 kb. The terminal nucleotides of each genomic RNA segment are base-paired forming non-covalently closed, circular RNAs and ribonucleocapsids. The terminal nucleotide sequences of genome segments are conserved among viruses in each genus but are different from those viruses in other genera. Genomic segments from different but closely related viruses can reassort when cells are coinfected with two viruses, but reassortment is limited to closely related viruses (a complex or a serogroup). All members of the family appear to utilize the same coding strategy, with the L RNA segment coding for a large (L) protein, the polymerase, the M RNA segment coding for the viral glycoproteins (G1 and G2), and the S RNA segment coding for a nucleocapsid protein (N). A number of non-structural proteins have also been described and assigned to specific segments. Each viral particle contains three internal nucleocapsids comprising genomic RNA associated with many copies of the N protein and a few copies of the L protein. Each nucleocapsid is arranged in a non-covalently closed circle which may be visualized in electron micrographs (Schmaljohn & Hooper, 2001).
Unlike most other enveloped viruses, viruses of the Bunyaviridae (with some exceptions) do not bud from the plasma membrane of infected cells. Rather, they mature by budding into intracytoplasmic vesicles associated with the Golgi apparatus. Virus release may occur through cell death and rupture or by transport of vesicles containing assembled virions to the cell surface. The glycoproteins are associated with the Golgi apparatus even when expressed independently of the other viral proteins indicating that the glycoproteins probably have specific processing and transport signals (Elliott, 1996).
Genus Bunyavirus
Classification of viruses belonging to the family Bunyaviridae was originally based on their serological relationships. These data have now been supplemented and largely supported by biochemical and genetic analyses. Viruses of the genus Bunyavirus are serologically unrelated to members of other genera.
At the genome level, the most important distinguishing feature is the consensus terminal nucleotide sequence of the L, M, and S genome segments. The N and NSs proteins are encoded in overlapping reading frames by the S RNA and are translated from the same complementary mRNA by alternative AUG initiation codon usage.
The genus Bunyavirus comprises over 150 viruses representing nearly 50 viral species. Most bunyaviruses are mosquito-borne and some have been isolated from Culicoides midges; only a few tick-borne viruses have been recorded (Table 3). Isolates of Bahig virus and Matruh virus (representatives of Tete virus) originate from Africa (Egypt, South Africa, Nigeria), Europe (Italy), Cyprus and Japan. Most isolates have been made from migratory birds whereas a few isolates are from ticks, yet it is believed ticks are the main vectors. Estero Real virus is the only other bunyavirus believed to be tick-borne.
Genus Nairovirus
The genus Nairovirus is named after Nairobi sheep disease virus (NSDV) and contains some of the most important tick-borne pathogens. It was divided into seven serogroups: Crimean-Congo haemorrhagic fever, Dera Ghazi Khan, Hughes, Nairobi sheep disease (NSD), Qalyub, Sakhalin and Thiafora group (Clerx, Casals & Bishop, 1981; Zeller et al. 1989a). These have now been replaced by 7 viral species, the NSD serogroup comprising two species: Dugbe virus and NSDV. All but one species (Thiafora virus) are tick-borne viruses (Table 3).
Virions are morphologically similar to other members of the family with very small surface units, which appear as a peripheral fringe. The L RNA segment is considerably larger than the L segments of other members of the family (Marriott & Nuttall, 1996), and the S segment does not encode a non-structural protein. The M segment encodes a single gene product, which is processed in a complex and poorly defined manner to yield the structural glycoproteins and at least three non-structural proteins. Processing of Gn (G2) is dependent on subtilase SKI-1, a cellular protease, whereas Gc (G1) processing is not. Determination of glycoprotein processing in ticks might provide important insights into nairovirus replication in the vector. Recent progress in applying reverse genetic technology to CCHFV offers the real prospect of understanding the biology of this important virus genus.
The most distinctive biological feature of nairoviruses is that they are all tick-borne, with relatively few isolates known also from mosquitoes (Dugbe virus and Ganjam virus) or Culicoides midges, although insect-borne transmission is unlikely. CCHFV is the most medically important member of the genus and the best-studied representative. This virus was originally isolated independently in two distant parts of the world (Democratic Republic of Congo and the Crimea) and subsequent laboratory comparison demonstrated the two viruses to be identical (Hoogstraal, 1979). It has one of the widest geographical distributions of the medically important arboviruses (Nuttall, 2001). Infections in humans result in haemorrhagic fever with severe typhoid-like symptoms and mortality rates up to 50% (Watts et al. 1989). Livestock movements and migratory birds play an important role in the transport of infected ticks. Although CCHFV has been isolated from at least 31 different tick species and subspecies (including two argasid species), the principal vectors are ixodid ticks of the genus Hyalomma (such as H. marginatum, H. rufipes, H. anatolicum and H. asiaticum). In contrast to the observation in ixodid ticks, CCHFV failed to replicate in argasid ticks indicating that CCHFV isolated from argasid ticks in nature merely represents virus survival in the recently ingested blood-meal (Shepherd et al. 1989). The distribution of CCHFV in scattered enzootic foci in sub-Saharan Africa, the Middle East and southern Eurasia falls within the limits of the distribution of the genus Hyalomma and human infections have been associated principally with bites by ticks of this genus (Swanepoel et al. 1987). Direct transmission from human to human is a frequent cause of nosocomial infections. Hares (Lepus spp.), hedgehogs (Erinaceus and Hemiechinus spp.) and cattle are probably important amplifying hosts in nature, although virus screening of wild and domestic animals has failed to identify viraemic host species. Transmission from infected to uninfected ticks co-feeding on non-viraemic hosts (e.g. sheep and ground-feeding birds) may be an effective transmission route (Nuttall, 2001).
The International Committee on Taxonomy of Viruses lists Kodzha virus as a variant of CCHFV and cites under this virus two strains, C68031 and AP92. Strain AP92 is from Greece where evidence of the disease in humans has not been recorded. The Greek strain is phylogenetically distinct from all other CCHFV strains examined to date and warrants a distinct name, as indicated in Table 3. Other viruses related to CCHFV, Hazara virus and Khasan virus, likewise have not been associated with disease.
Dera Ghazi Khan virus (DGKV) was isolated originally from Hyalomma dromedarii collected from a camel in western Pakistan (reference strain JD 254). At least 5 other related viruses are known. Unlike DGKV from ixodid species, the relatives have been isolated from argasid ticks, except for Abu Mina virus for which the tick vector is unknown.
Until recently, Dugbe virus was classified in the NSD serogroup but is now recognized as a distinct species. It is antigenically and genetically related to CCHFV but of comparatively low pathogenicity, which makes it a good model for experimental studies. Dugbe virus replicates and persists trans-stadially in orally infected Amblyomma variegatum ticks and can subsequently be transmitted by tick feeding to a vertebrate host (Steele & Nuttall, 1989). The virus has been isolated from numerous other tick species (e.g. Hyalomma truncatum, Boophilus decoloratus and Rhipicephalus appendiculatus) but has a much more restricted geographical distribution compared with CCHFV, possibly because it is not associated with birds.
Nairobi sheep disease virus (NSDV) is highly pathogenic for sheep and goats, and causes disease in humans. Some of the earliest work on tick-borne virus transmission is recorded for NSDV (Daubney & Hudson, 1931). More recent studies have been limited by the high degree of containment needed to handle this virus. Genetic and serological data indicate that Ganjam virus is an Asian variant of NSDV.
The largest group of nairoviruses representing a distinct species is that of Hughes virus. Hughes group viruses are transmitted by Ornithodoros ticks of the ‘capensis’ complex associated with seabirds, such as O. maritimus and O. denmarki, and the ixodid seabird tick, I. uriae. Hughes virus was first isolated from O. denmarki ticks collected in 1962 on Bush Key, Dry Tortugas, Florida and subsequently from the same tick species from Soldado Rock, off the south-west corner of Trinidad. The virus was also isolated from the blood of nestling terns (Sterna fuscata) captured on Soldado Rock. The number and geographical range of Hughes group viruses is comparable to that of orbiviruses of the Great Island group, which also circulate in seabird colonies (Table 2). The abundance of these two virus groups may simply reflect the level of activity in collecting ticks and isolating viruses from the attractive habitats of seabirds. Alternatively, evolutionary pressures resulting from unique features of their ecology may have given rise to a large number of distinguishable virus variants.
Qalyub virus was originally isolated from Ornithodoros erraticus in Egypt and many subsequent isolates were obtained from the same tick species (Darwish & Hoogstraal, 1981). The related Bandia virus was isolated from O. sonrai, a member of the O. erraticus group, and from rodents in Senegal. The capacity of viruses in this group to serve as human pathogens is unknown.
Genus Phlebovirus
The surface morphology of phleboviruses is distinct in having small round subunits with a central hole. The S RNA exhibits an ambisense coding strategy. It is transcribed by the virion RNA polymerase to a subgenomic virus-complementary sense mRNA that encodes the N protein and, from full-length antigenome S RNA, to a subgenomic virus sense mRNA that encodes a non-structural protein, NSs. The M segment gene order and coding strategy varies. Tick-borne phleboviruses (e.g. Uukuniemi virus) encode only G1 and G2 glycoproteins whereas the mosquito- and sandfly-vectored phleboviruses also have coding information for a non-structural protein, NSm (Schmaljohn & Hooper, 2001).
The Phlebovirus genus comprises some 30 viruses transmitted by phlebotomine sand-flies, mosquitoes, or ceratopogonids of the genus Culicoides. The type species is Rift Valley fever virus transmitted by mosquitoes. At least 10 viruses transmitted by ticks were formerly assigned to an independent Uukuvirus genus but are now included within the Phlebovirus genus based on similarities in their coding strategies (Simons, Hellman & Pettersson, 1990). Uukuniemi virus was originally isolated in southeast Finland from I. ricinus ticks (reference strain S 23). Subsequently, it was isolated in many other European countries predominantly from I. ricinus ticks. This virus appears to straddle terrestrial and marine biotopes, having been isolated from I. uriae collected in seabird colonies on the island of Runde in Norway (strain Ru E82). The other representatives of the species have been isolated repeatedly from passerines and seabirds or from their ticks, particularly in Scandinavia and in other parts of Palaearctic and Nearctic regions. They include Grand Arbaud virus (from Argas reflexus ticks in the Rhone delta of France), Manawa virus (from Argas abdussalami in West Pakistan), Oceanside virus (from Ixodes uriae collected at Three Arch Rocks and Yaquina Head, Oregon and Flat Iron Rock, California), Ponteves virus (from Argas reflexus, France), Precarious Point virus (from Ixodes uriae collected in penguin rookeries on Macquarie Island in the sub-Antarctic) and the closely related Murre virus and RML 105355 virus, St. Abbs Head virus (from I. uriae, east Scotland), and Zaliv Terpeniya virus (from I. uriae collected in Sakhalin and Kamchatka regions, Far-eastern Russia and the Murmansk region in the European part of Russia) (Table 3).
In addition to the bunya-, nairo-, and phlebovirus tick-borne members of the Bunyaviridae, there are at least 15 other tick-borne viruses that have yet to be assigned to a particular genus. The best known of these is Bhanja virus isolated from ticks of the genera Haemaphysalis, Boophilus, Amblyomma and Hyalomma in India, Africa (Nigeria, Cameroon, Senegal) and Europe (Italy, Balkan states). Palma virus is an isolate from Portugal closely related to Bhanja virus. Others include Silverwater virus from Haemaphysalis leporispalustris collected from snowshoe hare in Ontario and Upolu virus from Ornithodoros ticks collected in a tern colony (Sterna sp.) on a coral atoll in the Great Barrier Reef. Issyk-Kul virus appears to be transmissible by both mosquitoes and ticks, and has been associated with febrile illness in humans in Tajikistan.
Family Flaviviridae
Viruses within the family Flaviviridae are classified into three different genera (Flavivirus, Pestivirus, Hepacivirus) and exhibit very different biological characteristics. Many of the viruses in the genus Flavivirus are arboviruses; viruses in the other two genera only infect mammals. Indeed, on ecological grounds the composition of the Flaviviridae is questionable. It remains to be seen whether phylogenetic analyses support the taxonomic structure of the family.
Genus Flavivirus
Most flaviviruses (at least 50 species) are transmitted to vertebrate hosts by mosquitoes. They include such medically important pathogens as Yellow fever virus, Japanese encephalitis virus, West Nile virus (WNV), the four serotypes of Dengue virus and several others. Tick-borne viruses currently comprise 12 species and there are at least 14 species that are zoonotic viruses transmitted between rodents or bats with no known arthropod vector.
Flavivirus virions are enveloped, roughly spherical, with a diameter of 40–60 nm. The virion contains a nucleocapsid core of 20–30 nm composed of a single capsid protein. The envelope contains two virus-encoded proteins (envelope and membrane proteins). Immature, intracellular virions contain a precursor membrane protein, which is proteolytically cleaved during virus maturation. The viral genome is a single molecule of single-stranded RNA of approximately 11 kb which is positive-sense and infectious. The virion RNA appears to be identical to the mRNA. Three structural proteins and seven non-structural proteins are encoded from one long open reading frame flanked by terminal noncoding regions that form specific secondary structures required for genome replication, translation or packaging. Viral proteins are synthesized as part of a polyprotein of more than 3000 amino acids, which is co- and post-translationally cleaved by viral and cellular proteases (Lindenbach & Rice, 2001).
The envelope glycoprotein (E protein) is the major structural protein and plays an important role in membrane binding and inducing a protective immune response following virus infection. It carries epitopes detected by neutralization and haemagglutination-inhibition tests that have been used to identify different flaviviral subgroups and species. Within the E gene three genetic markers have been identified. These are a type specific tripeptide hypervariable region which provides a unique genetic signature for individual flaviviruses (Shiu, Ayres & Gould, 1991); a subgroup-specific pentapeptide motif (Gao et al. 1993); and a hexapeptide insertion (EHLPTA) typical of TBEV complex viruses (Marin et al. 1995).
Determination of the crystallographic structure of a soluble form of the E protein of a TBEV revealed that, unlike the spikes seen on many viruses, the flavivirus envelope protein is situated parallel to the virion surface in the form of head-to-tail homodimeric rods. Residues that influence binding of monoclonal antibodies occur on the outward facing surface of the protein (Rey et al. 1995). Interestingly, the putative receptor binding site of some mosquito-borne flaviviruses contains a putative integrin-binding motif Arg–Gly–Asp which is not found in the E protein of TBEV. There is an obvious analogy with the orbiviruses (see above).
All flaviviruses are serologically related, as demonstrated in binding assays such as ELISA and by haemagglutination-inhibition using polyclonal and monoclonal antibodies. Neutralization assays are more discriminating and have been used to define several serocomplexes within the genus Flavivirus. Comparative analyses of the nucleotide and amino acid sequence of the E gene have been used to investigate the phylogeny of flaviviruses (Marin et al. 1995). Cell fusing agent virus (CFAV) (tentatively placed in the genus Flavivirus) E gene was used as the outgroup based on the distant relationship of CFAV to tick- and mosquito-borne flaviviruses. The analyses showed that tick- and mosquito-borne flaviviruses diverged as two distinct and major genetic lineages. Interestingly, analyses of the E and non-structural NS5 gene sequences to compare mutational regimes indicated that mosquito-borne flaviviruses are evolving almost twice as fast as tick-borne flaviviruses (Marin et al. 1995; Gould, Zanotto & Holmes, 1997).
The tick-borne flaviviruses are currently classified into two groups: the mammalian tick-borne virus group and the seabird tick-borne virus group (Table 4). Three seabird tick-borne flaviviruses are recognized. These are Meaban virus (from France), Saumarez Reef virus (from Australia) and Tyuleniy virus (from Russian Far East). We have also placed Gadgets Gully virus (GGYV) in the seabird tick-borne virus group because of its known ecology. However, the International Committee on Taxonomy of Viruses lists it together with the mammalian group based on phylogenetic studies. Analysis of the E gene of GGYV places this virus at the edge of the mammalian tick-borne flavivirus cluster, possibly indicating that this virus is at the cusp between the two groups (Gaunt et al. 2001).
Medically, by far the most important tick-borne flaviviruses are those classically grouped into the TBEV serogroup, the most important being TBEV. TBE was recognised clinically in the Far East of the former USSR (now Russia) in the early 1930s. Severe cases of encephalitis were observed in humans residing in formerly unhabited areas. A special expedition was organised in 1937 by L. A. Zilber to determine the cause of the disease. Virus isolates were obtained from the blood of patients and from Ixodes persulcatus ticks. The disease has several names, including Russian spring-summer encephalitis (RSSE), Far Eastern encephalitis, and forest spring encephalitis (Zilber & Soloviev, 1946). In 1948, a similar though less severe form of encephalitis affected humans residing in central Bohemia, Czech Republic. The virus recovered from the blood of a patient and from I. ricinus ticks was related to the isolates from RSSE cases (Rampas & Gallia, 1949). Similar or milder forms of the disease, called biphasic meningoencephalitis, were observed in other central and eastern European countries. TBEV is endemic over a wide area – covering Europe, northern Asia and China. Several thousand human cases are recorded annually, with considerable variation from year to year. In general, the Far Eastern subtype of TBEV cause severe disease in humans with a mortality that can be 50% in some outbreaks; disease associated with the European subtype is less severe and mortality is usually under 5% (Gresíková & Calisher, 1988). Based on genome analyses, recently a third Siberian subtype has been established. Members of this subtype are known to cause chronic infections in humans.
Louping ill virus (LIV) derives its name from the disease of sheep (louping ill), which has been recognized in southern Scotland for at least two centuries (Smith & Varma, 1981). ‘Louping’ refers to the characteristic behaviour of ‘leaping’ shown by sheep infected with LIV. The disease is found throughout much of the upland sheep farming areas of Scotland, northern and southwestern England, Ireland and Wales affecting sheep and red grouse (Lagopus scoticus), with other species of domestic animals and humans affected less frequently. The main vector is I. ricinus (Reid, 1984). Now two subtypes of LIV are recognized in the British Isles (Irish and British subtypes), as well as in Norway. In Turkey and Spain, the disease has been attributed to infections with Spanish and Turkish subtypes of LIV. The virus can cause severe encephalitis in humans; however, there are only about 35 recorded cases and they have been confined mostly to laboratory workers, veterinary surgeons, farmers and abattoir workers (Smith & Varma, 1981).
Powassan virus (POWV) is the North American member of the TBE subgroup although there are several records of POWV from ticks and mosquitoes collected also in Russia. The virus was originally isolated from a pool of Dermacentor andersoni ticks collected in 1952 in Colorado (Thomas, Kennedy & Eklund, 1960) and derives its name from the town in Northern Ontario where the first fatal case of the encephalitic disease was recognized. About 20 cases of disease associated with POWV infection have been reported in North America and there was one fatal case recorded in Russia (Artsob, 1988). Deer tick virus, is a newly recognized relative of POWV isolated from Ixodes and Dermacentor spp., and from human brain (Telford et al. 1997).
Omsk haemorrhagic fever (OHF) was first reported in 1941 when physicians recorded sporadic cases in the forest steppes of Omsk Region-Siberia among muskrat hunters. Though closely related to TBEV, Omsk haemorrhagic fever virus (OHFV) is unique with respect to both the clinical features of the disease (haemorrhagic symptoms contrasting with the encephalitis) and the ecology of the virus. The primary tick vector of OHFV is Dermacentor reticulatus and the hosts are voles, particularly the narrow-skulled vole, Microtus gregalis. Another tick species, Ixodes apronophorus, plays a role together with its main host, the water vole. D. marginatus and I. persulcatus ticks play secondary roles in the transmission cycles of OHFV (Lvov, 1988).
Kyasanur Forest disease virus (KFDV) is another highly pathogenic member of the tick-borne flaviviruses producing haemorrhagic disease in infected humans. Since the first record of the disease in 1957 in Karnataka State, India, epidemics have occurred every year in the affected region. The highest recorded incidence was in 1983 with 1555 cases and 150 deaths. Preceding the first human epidemic, a large number of sick and dead monkeys had been noticed in the nearby forest area. KFDV was isolated from dead monkeys, sick patients and from Haemaphysalis and other tick species. Clinically, the disease is particularly interesting because, in contrast to most of the other tick-borne flaviviruses that cause encephalitis, KFDV causes a haemorrhagic disease even more severe than that associated with OHFV (Banerjee, 1988). Alkhurma virus, a close relative of KFDV, was first isolated in 1995 from patients with haemorrhagic fever in Saudi Arabia (Zaki, 1997; Charrel et al. 2001). Little is known of the ecology and epidemiology of this virus.
Langat virus, which is transmitted by Ixodes granulatus, is the least pathogenic representative of the mammalian tick-borne flavivirus group. This virus was isolated in Malaysia (Karabatsos, 1985). Because of its naturally low virulence and serological cross-reactivity with TBEV it was assessed as a candidate vaccine against TBE in Russia with disastrous consequences.
The remaining members of the group, namely Kadam virus, Karshi virus, Royal Farm virus and Gadgets Gully virus, are not considered human pathogens and consequently knowledge of their ecology and epidemiology is limited (Karabatsos, 1985).
Ticks are potential reservoirs of at least two mosquito-borne flaviviruses. The evidence is based on the isolation of YFV from the eggs and emergent larvae of an ixodid tick species collected in the field (Germain et al. 1979). West Nile virus (WNV) is included in Table 4 because, although clearly a mosquito-borne virus, it has been isolated repeatedly from bird-infesting argasid and ixodid ticks in arid regions of Russia and from Hyalomma detritum in a desert region of Turkmenistan (Lvov et al. 1975). The five isolates of WNV from O. maritimus ticks collected in the nest site of gulls (Larus argentatus) were from Glinyanyi Island in the Caspian Sea, where mosquitoes are reported to be absent. For WNV, long-term survival and co-feeding transmission of the virus has been demonstrated in laboratory studies with Ornithodoros ticks (Vermeil, Lavillaureix & Reeb, 1959; Lawrie et al. in press).
The surprisingly broad spectrum of ecological conditions occupied by tick-borne flaviviruses is worthy of further comment. As we have seen, they range from the seabird colonies occupied by Ixodes uriae as the dominant tick species, to the typically forested habitats of the Northern Hemisphere with I. ricinus and I. persulcatus as the dominant tick species. In addition, representatives of the TBEV serogroup have dispersed across Asia and Europe in a cline (continuous or gradual evolution across a geographic area). The most divergent lineages (often incorrectly referred to as the most ancient viruses) appeared in the east and the more recent lineages emerged in the west, with LIV at the extreme west of this geographical distribution (Zanotto et al. 1995).
Unassigned viruses
A significant number of viruses have not yet been assigned to a recognized virus family or genus. Some of these appear to be tick-borne viruses (Table 5).
Nyamanini virus was first isolated from cattle egrets (Bulbulcus ibis) collected in 1957 in South Africa. Subsequent isolations have been from cattle egrets and ticks in Africa. It is antigenically related to (though clearly distinct from) Midway virus, which has been isolated from ticks collected in seabird colonies. Virions are enveloped and contain RNA.
Quaranfil virus was isolated from Argas arboreus ticks collected near Cairo, Egypt in 1953. The virus has also been isolated from humans with febrile illnesses, from birds (ibis and pigeons) and ticks. The virus has an envelope and preliminary studies suggested a polypeptide profile in infected cells similar to that of arenaviruses. Little more is known about the related Johnston Atoll virus, which was isolated from seabird ticks. This virus has an envelope together with three major and two minor proteins, two of which appear to be glycoproteins. Both Quaranfil virus and Johnston Atoll virus have morphological and morphogenetic characteristics similar to members of the Arenaviridae (Zeller et al. 1989b). No members of this family are currently recognized as arboviruses.
Seven of the 8 ungrouped viruses listed in Table 5 have been isolated from bird-feeding ticks. Aride virus was recovered from ticks collected from dead roseate terns (Sterna dougallii) found on Bird Island in the Seychelles. The birds breed on nearby Aride Island, hence the virus name. Caspiy virus is found in seabird colonies of the Caspian Sea basin. One isolate was from a clinically sick gull (Larus sp.). Slovakia virus was isolated from a single pool of engorged female ticks collected during an investigation of the cause of sickness and deaths among domestic chickens heavily infested with Argas persicus.
Runde virus was isolated from two pools of I. uriae collected from a puffin (Fratercula arctica) colony on the island of Runde, Norway, and antibodies detected (Traavik, 1979). The two isolates appeared to be antigenically identical but were unrelated to any major arbovirus group. Electron microscopy revealed morphological features similar to those of members of the Coronaviridae, a virus family that has no recognized arbovirus members to date. However, no antigenic relationship to infectious bronchitis virus (an avian coronavirus) was detected. The virus appears to have a single-stranded RNA genome. Røst Islands virus (NorAr V-958) showed a similar coronavirus-like morphology when examined by electron microscopy.
Jos virus was originally isolated in 1967 from the blood of a cow during slaughter at the abattoir in Jos, north central Nigeria (Lee et al. 1974). The 42 additional isolates were from ticks collected at cattle markets in southern Nigeria. The virus has also been isolated from A. variegatum collected in Senegal and Ethiopia. Although the virus replicated in Vero cell culture, attempts to characterize the virus by electron microscopy were unsuccessful (Zeller et al. 1989a).
CONCLUSIONS
Most tick-borne viruses were isolated during the 1950s, '60s and '70s when many American, Russian, and European scientists went out into the field, in many parts of the world, to collect specimens that were then screened for arboviruses in specialist laboratories. Inevitably, the distribution of these viruses reflects the scientists' activities and the accessibility of their published results. For example, many viruses have been isolated from ticks collected in seabird colonies where the permanence of the breeding sites, and the near guarantee of a blood-meal, provide an ideal habitat for ticks and an attraction for tick collectors. Comparatively few of the viruses listed in Tables 1 to 5 are from China, which is most likely a misrepresentation of the true picture. There are surely many more tick-borne viruses yet to be discovered. Likewise, there are exciting discoveries to be made about how tick-borne viruses infect and replicate in both vertebrate and tick cells, and what determines whether the infection is pathogenic or benign. Advances in applying reverse genetics, together with site directed mutagenesis and interference RNA techniques, will provide insights into the molecular basis of the tick-borne virus life cycle. However, there will still be the need for field studies to understand how these viruses survive in nature.
References
REFERENCES

*Table 1. Tick-borne viruses of the families Asfarviridae, Rhabdoviridae and Orthomyxoviridae

*Table 2. Tick-borne viruses of the family Reoviridae

*Table 3. Tick-borne viruses of the family Bunyaviridae

*Table 4. Tick-borne viruses of the family Flaviviridae, genus Flavivirus

*Table 5. Unassigned tick-borne viruses
- 185
- Cited by


