B-type natriuretic peptide is a neurohormone that is isolated in ventricular cardiomyocytes and affects sodium and water balance by inducing natriuresis, diuresis, and vasodilatation, counteracting the renin–angiotensin–aldosterone system.Reference Baughman1,Reference Mair, Hammerer-Lercher and Puschendorf2 Bioactive B-type natriuretic peptide is composed of 32 amino acids, and the precursor molecule is cleaved into the bioactive B-type natriuretic peptide and an N-terminal fragment. N-terminal pro-B-type natriuretic peptide (NT-proBNP), which consists of 76 amino acids, has no bioactivity. B-type natriuretic peptide is secreted primarily from the ventricles in response to increased left or right ventricular pressure and volume loads.Reference Baughman1,Reference Maisel, Krishnaswamy and Nowak3
In children, the normal levels of NT-proBNP change, with elevated levels after birth that gradually decrease after the neonatal period.Reference Soldin, Soldin, Boyajian and Taskier4,Reference Nir, Lindinger, Rauh and Bar-Oz5 However, NT-proBNP reference levels and intervals in the paediatric population have not yet been fully evaluated.
B-type natriuretic peptide levels correlate with the severity as well as prognosis of left ventricular dysfunction and congestive heart failure.Reference Oremus, McKelvie and Don-Wauchope6,Reference Clerico, Passino, Franzini and Emdin7 This is particularly relevant when echocardiography is not readily available.Reference Hammerer-Lercher and Ralf8 NT-proBNP levels are elevated in adult patients with systolic and diastolic dysfunction in both ventricles, ischemic heart disease, and hypertrophic cardiomyopathy.Reference Mair, Hammerer-Lercher and Puschendorf2,Reference Maisel, Krishnaswamy and Nowak3 In children, NT-proBNP levels are also used as markers of heart disease, such as heart failure, coronary lesions in Kawasaki disease, and prognosis of cardiac surgery.Reference Cantinotti, Walters, Crocetti, Marotta, Murzi and Clerico9–Reference Kawamura, Wago, Kawaguchi, Tahara and Yuge13 However, a few studies have shown that NT-proBNP levels may be elevated in acute infections, such as sepsis and other non-cardiac diseases in children.Reference Nevo, Erlichman, Algur and Nir14–Reference Hammerer-Lercher, Geiger, Mair and Url16 Furthermore, the concentration of NT-proBNP in patients with CHD and other haemodynamic conditions has not been completely understood in the paediatric field, and analyses have produced conflicting results.Reference Rudiger and Gasser17
Therefore, we compared NT-proBNP levels in children with cardiac diseases, infectious diseases, and non-cardiac, non-infectious diseases. And we identified the clinical statuses of patients that affect the NT-proBNP levels.
Materials and methods
Patient population
This study was performed at Keimyung University Dongsan Medical Center, Korea, between July 2014 and December 2016. The patients were divided into three groups: a cardiac disease group, an infectious disease group, and a non-cardiac, non-infectious disease group. The cardiac disease group was divided into four categories: (1) acute myocarditis, dilated cardiomyopathy, and restrictive cardiomyopathy; (2) congenital heart anomaly with/without heart failure or with/without pulmonary hypertension; (3) Kawasaki disease; and (4) cardiac arrhythmias. Open-heart surgery patients were excluded. The infectious disease group included patients with upper and lower respiratory infection, lymphadenitis, gastroenteritis, urinary tract infection, sepsis, and simple febrile illness. The non-cardiac, non-infectious disease group included patients with apnea, anemia, gastroesophageal reflux, headache, convulsion, and malignancy. The non-cardiac, non-infectious disease group consisted of patients with no history or signs of heart disease.
This study was approved by the Institutional Review Board of the Keimyung University Dongsan Medical Center (approval number: DSMC 2018-01-047).
Clinical and laboratory data
Patient data were retrospectively collected from medical records, and the NT-proBNP levels were compared according to diagnoses, clinical findings, such as symptoms and signs, management modalities, and outcome and laboratory data.
Based on the patient data, we assessed the systemic inflammatory response syndrome according to the systemic inflammatory response syndrome criteria for children.Reference Goldstein, Giroir and Randolph18 Systemic inflammatory response syndrome was defined as the presence of at least two of the following criteria: (1) temperature over 38.5°C or under 36°C, (2) tachycardia or bradycardia, (3) tachypnea or mechanical ventilation, and (4) leukocytosis or leukopenia.
The highest NT-proBNP level during admission was collected. The NT-proBNP level was measured using a commercial laboratory system: SIEMENS (Germany/Supplier: Korea) Dimension EWL integrated chemistry system, a one-step sandwich electrochemiluminescence immunoassay based on luminescent oxygen channelling immunoassay technology. Luminescent oxygen channelling immunoassay reagents include two synthetic bead reagents and a biotinylated monoclonal antibody fragment that recognises an epitope located in the N-terminal part of proBNP. Samples are incubated with Chemibeads and biotinylated antibody to form a particle/NT-proBNP/biotinylated antibody sandwich. Sensibeads are then added and bind to the biotin to form a bead-aggregated immunocomplex. Illumination of the complex by light at 680 nm generates singlet oxygen from the Sensibeads, which diffuses to the Chemibeads and triggers a chemiluminescent reaction. The resulting chemiluminescent signal is measured at 612 nm and is directly proportional to the concentration of NT-proBNP in the sample.
Statistical analysis
Because the data were not normally distributed, all values are reported as frequencies and medians including the range. Categorical variables were compared using the chi-square test and continuous variables were compared using the unpaired t-test or the Mann–Whitney U-test. A receiver operating characteristic curve analysis was used to determine the optimal cut-off value of NT-proBNP for cardiac disease. The Spearman correlation test was used to analyse the relationship between the NT-proBNP levels and laboratory findings. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Co., Armonk, NY, USA).
Results
Patient characteristics
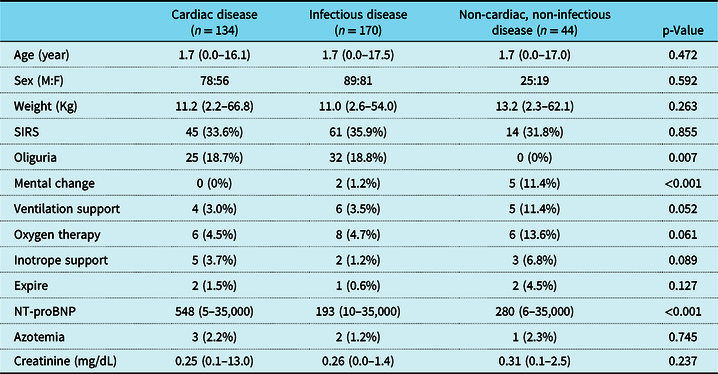
A total of 348 children were enrolled, including 134 patients (38.5%) with cardiac disease, 170 patients (48.9%) with infectious disease, and 44 patients (12.6%) with non-cardiac, non-infectious disease. The median age of the patients was 1.7 years (range: 1 month to 17.5 years), and the median body weight was 11.1 kg (range: 2.2–66.8 kg). There was no significant difference in age, sex, or body weight among groups (Table 1).
Table 1. Clinical and laboratory data of patients
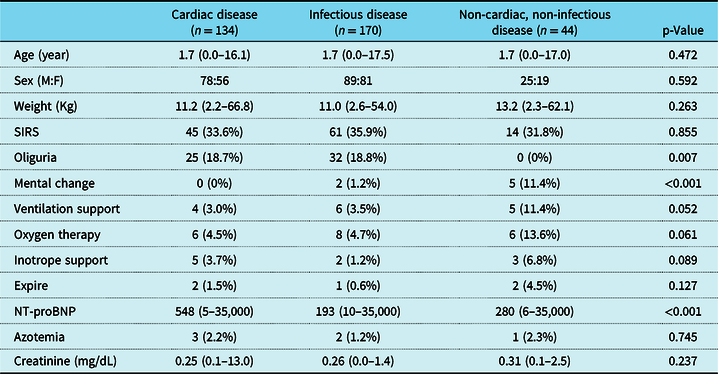
The values are presented as number (%) or median (range)
SIRS = systemic inflammatory response syndrome
Comparison of NT-proBNP levels among disease groups
The NT-proBNP level of the cardiac disease group (median: 548 pg/mL; range: 5–35,000 pg/mL) was significantly higher than that of the infectious disease group (median: 193 pg/mL; range: 10–35,000 pg/mL, p < 0.001) and the non-cardiac, non-infectious disease group (median: 280 pg/mL; range: 6–35,000 pg/mL, p = 0.004) (Fig 1). There was no significant difference between the infectious disease group and the non-cardiac, non-infectious disease group.
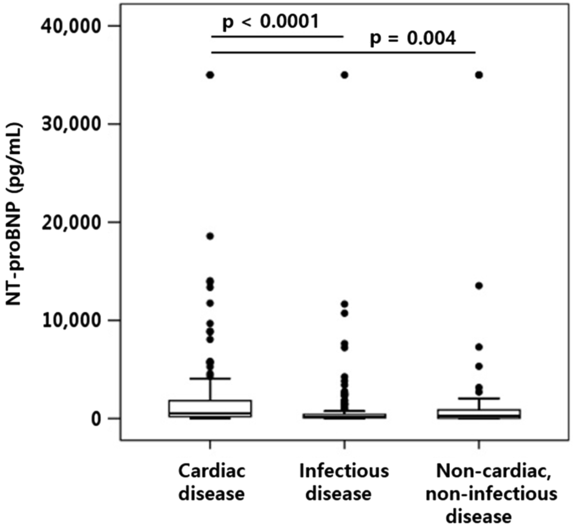
Figure 1. NT-proBNP levels among disease groups. The NT-proBNP level of the cardiac disease group was significantly higher than that of the infectious disease group (p < 0.001) and the non-cardiac, non-infectious disease group (p = 0.004). NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Among the four categories of the cardiac disease group, the value of the median NT-proBNP was 5,828 pg/mL (62–14,036 pg/mL) in category 1,3,631 pg/mL (5–35,000 pg/mL) in category 2, 484 pg/mL (19–13,894 pg/mL) in category 3, and 400 pg/mL (36–2,444 pg/mL) in category 4. The NT-proBNP levels of categories 1 and 2 were significantly higher than that of categories 3 and 4, respectively (p < 0.05).
When comparing the NT-proBNP level between the cardiac disease group and the infectious disease group or the non-cardiac, non-infectious disease group, the area under the receiver operating characteristic curve was 0.699 with a cut-off of 337.5 pg/mL. However, the diagnostic accuracy was low, with 64.9% sensitivity and 65.0% specificity.
Comparison of NT-proBNP levels according to clinical findings
The clinical findings and laboratory data among groups were presented in Table 1. In total, 120 patients (34.5%) were diagnosed with systemic inflammatory response syndrome, and the incidence of systemic inflammatory response syndrome was not significantly different between groups. The incidences of mechanical ventilation support, oxygen therapy, inotropic medication use, and mortality were not significantly different between groups. Furthermore, the incidence of oliguria was significantly lower, while the incidence of mental change was significantly higher in non-cardiac, non-infectious disease group than the others (p = 0.007 and p < 0.001).
In the cardiac disease group, ventilation support was required in three patients with CHD and heart failure and in one patient with pulmonary hypertension. Oxygen therapy was administered in four patients with ventilation support, two patients with acute myocarditis, and one patient with dilated cardiomyopathy. Three patients with acute myocarditis used inotropic medication, and one of each group with acute myocarditis and dilated cardiomyopathy died. In the infectious disease group, ventilation support was required in three patients with pneumonia and three with sepsis and disseminated intravascular coagulation, and oxygen therapy was administered to five patients with pneumonia and three patients with sepsis and disseminated intravascular coagulation. One patient with sepsis and disseminated intravascular coagulation needed inotropic medication, and one patient with pneumonia and pulmonary haemorrhage needed inotropic medication and died. In the non-cardiac, non-infectious disease group, ventilation support was required in three patients with status epilepticus, one with apnea, and one with gastroesophageal reflux, and oxygen therapy was administered in five patients with ventilation support and one with a malignancy. Three patients with status epilepticus needed inotropic medication, and one patient with status epilepticus and one with a malignancy died. Mental status changes were observed in seven patients; five patients with status epilepticus in the non-cardiac, non-infectious disease group and two with central nervous system infections in the infectious disease group. Among them, ventilation support was required in four patients, and oxygen therapy and inotropic medication were administered to three patients.
There was no significant difference in NT-proBNP levels between the patients with and without systemic inflammatory response syndrome. Regarding patients’ clinical findings, the patients who needed mechanical ventilation support, oxygen therapy, or inotropic medication had significantly higher NT-proBNP levels than those who did not (p < 0.001). Patients with a change in mental status also showed significantly higher NT-proBNP levels than those who did not (p = 0.001). However, there was no significant difference in NT-proBNP levels between the patients who died or had symptoms of oliguria and those who survived and did not have symptoms of oliguria (Fig 2).

Figure 2. Comparison of NT-proBNP levels according to clinical factors. NT-proBNP, N-terminal pro-B-type natriuretic peptide, SIRS, systemic inflammatory response syndrome.
Comparison of NT-proBNP levels according to laboratory findings
The incidences of azotemia (serum creatinine over 1.0 mg/dL) and the serum creatinine levels were not significantly different among groups. However, the patients with azotemia had significantly higher NT-proBNP levels than the patients without azotemia (p = 0.006). There was no significant correlation between NT-proBNP and creatinine levels. Correlation analysis revealed a negative correlation of NT-proBNP level with age (r = −0.381, p < 0.001), height (r = −0.407, p < 0.001), and body weight (r = −0.428, p < 0.001). It also had a positive correlation with body mass index (r = 0.213, p < 0.001), white blood cell count (r = 0.213, p < 0.001), and procalcitonin (r = 0.269, p < 0.001).
Discussion
We found that the patients with cardiac disease had significantly higher NT-proBNP levels than the patients with infectious disease and those with non-cardiac, non-infectious disease. NT-proBNP levels differed significantly according to clinical conditions such as the need for mechanical ventilation support, oxygen therapy, or inotropic medication or a change in mental status, as opposed to systemic inflammatory response syndrome and mortality.
The natriuretic peptide family consists of three peptides: atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Brain natriuretic peptide was originally identified in extracts of porcine brain, but its level is considerably higher in cardiac ventricles.Reference Baughman1,Reference Levin, Gardner and Samson19 Brain natriuretic peptide is released from the ventricles of the heart in response to haemodynamic stress, and blood levels of brain natriuretic peptide may be useful in the diagnosis of heart failure.Reference Mair, Hammerer-Lercher and Puschendorf2,Reference Maisel, Krishnaswamy and Nowak3 Because of its longer plasma half-life and higher stability, the measurement of NT-proBNP has been introduced into routine clinical diagnostics.Reference Rudiger and Gasser17 The NT-proBNP level is a suitable diagnostic tool to distinguish cardiac disease from other disease entities in both adult and children.Reference Clerico and Emdin20 However, it may be elevated in acute infection and other non-cardiac diseases. Several studies have shown an elevation in NT-proBNP levels in acute infection, sepsis, and septic shock in adults and children.Reference Nevo, Erlichman, Algur and Nir14–Reference Rudiger and Gasser17 Nevo et alReference Nevo, Erlichman, Algur and Nir14 reported that NT-proBNP levels were elevated in children with acute non-cardiac disease but were lower than those in children with acute cardiac disease. They also suggested that an NT-proBNP level over 1,000 pg/mL is related to acute cardiac disease, and an NT-proBNP level below 400 pg/mL is related to acute non-cardiac disease in children.Reference Nevo, Erlichman, Algur and Nir14 However, these cut-off values of NT-proBNP are not applicable to infants. Fried et alReference Fried, Bar-Oz and Algur15 also reported elevated NT-proBNP levels in the sepsis group, but these were not higher than those in the left ventricular dysfunction group in children. However, 50% of the patients with sepsis showed left ventricular dysfunction, consistent with reports in adults. They also suggested a threshold NT-proBNP level of 11,000 pg/mL as a marker for cardiac disease.Reference Fried, Bar-Oz and Algur15 In our study, we also found that NT-proBNP levels in patients with cardiac disease were significantly higher than those in patients with infectious disease as well as in patients with non-cardiac, non-infectious disease. However, the NT-proBNP levels in patients with infectious disease were not significantly higher than those in patients with non-cardiac, non-infectious disease.
There are several hypotheses for increased NT-proBNP levels in acute infectious disease.Reference Fried, Bar-Oz and Algur15 First, stress hormones, which are elevated during a febrile state (shock) can induce the secretion of the natriuretic peptides. Second, pro-inflammatory cytokines also induce brain natriuretic peptide synthesis and stimulate peptide release. Third, fever can increase cardiac output, and sepsis/septic shock is associated with left ventricular dysfunction. Fourth, intravenous fluid therapy may cause cardiac distension. Fifth, patients with renal failure showed high levels of brain natriuretic peptide and NT-proBNP because of slower elimination of the peptide.Reference Fried, Bar-Oz and Algur15
In contrast to previous studies, which have shown an elevation in NT-proBNP levels in acute infection, sepsis, and septic shock, the NT-proBNP levels in patients with infectious disease were not significantly higher than those in patients with non-cardiac, non-infectious disease in our study. It might be caused by the diversity of disease and severity of patients in the infectious disease group. So, we assessed the patient’s clinical status related to NT-proBNP levels and found that the NT-proBNP levels were not significantly different in systemic inflammatory response syndrome, which represented the inflammatory status of patients. However, the NT-proBNP levels were significantly higher in the patients who needed mechanical ventilation support, oxygen therapy, or inotropic medication, which was administered considering the patients’ clinical deteriorations as a state of shock. It could be that the patients who needed mechanical ventilation or oxygen therapy had high oxygen demand, resulting in high-output heart failure. Additionally, the patients who needed inotropic medication, such as acute myocarditis and status epilepticus, may have had left ventricular systolic dysfunction. The patients of severe pneumonia (with pulmonary haemorrhage) or sepsis with disseminated intravascular coagulation in the infectious disease group who needed the mechanical ventilation, oxygen therapy, or inotropic medication might have increased stress hormones owing to the pro-inflammatory cytokines. To assess these mechanisms, further studies will be needed with echocardiograms and cytokine level assessments.
The NT-proBNP levels were significantly higher in patients with a change in mental status than in the patients without such change in our study. We must consider that the patients with a change in mental status included those with numerous convulsive disorders, indicating that convulsive disorders were related to high NT-proBNP levels, possibly due to left ventricular dysfunction or stress hormones owing to a state of shock. Some other studies also reported that patients with convulsions showed left ventricular systolic dysfunction and elevated NT-proBNP levels.Reference El Amrousy, Abd El-Hafez, Nashat and Hodeib21,Reference Alehan, Erol, Cemil, Bayraktar, Ogüs and Tokel22
Our study has several limitations. First, the study population was relatively small, and the range of age and body weight of patients was wide. Second, the disease entities were very heterogeneous among the groups. Third, because an echocardiogram was not performed in all subjects in this study, we could not compare the objective parameters of left ventricular function and NT-proBNP levels.
In conclusion, we found that NT-proBNP levels were significantly higher in cardiac diseases than non-cardiac diseases among heterogeneous groups of children. Furthermore, the NT-proBNP levels were related to patients’ clinical deteriorations such as shock rather than the inflammatory status of patients.
Acknowledgements
None
Financial Support
None
Conflicts of Interest
The authors declare that they have no conflicts of interest with respect to this work.