Introduction
A substantial proportion of patients with chronic medical conditions report depressive symptoms and approximately 10% meet criteria for a diagnosis of major depression (Moussavi et al. Reference Moussavi, Chatterji, Verdes, Tandon, Patel and Ustun2007). This co-morbid major depression severely impairs patients' quality of life, reduces their compliance with medical treatments and increases the costs of medical care (Katon & Ciechanowski, Reference Katon and Ciechanowski2002).
The importance of co-morbid depression should make its effective management a priority; unfortunately, management is often inadequate in practice, with shortcomings in both identification and treatment (Greenberg, Reference Greenberg2004; Cepoiu et al. Reference Cepoiu, McCusker, Cole, Sewitch, Belzile and Ciampi2008; Mitchell et al. Reference Mitchell, Vaze and Rao2009; Coventry et al. Reference Coventry, Hays, Dickens, Bundy, Garret, Cherrington and Chew-Graham2011; Fann et al. Reference Fann, Ell and Sharpe2012). Depression is frequently unidentified in the medical consultation because the relevant symptoms are normalized or are simply not discussed, in part because patient and clinician focus on the management of the medical condition (Cape & McCulloch, Reference Cape and McCulloch1999; Nutting et al. Reference Nutting, Rost, Smith, Werner and Elliot2000). When identified, depression is often inadequately treated with insufficient patient education, failure to prescribe minimally effective doses of antidepressant drugs, inadequate provision of psychological treatment and failure to monitor outcomes and adjust treatment accordingly (Fann et al. Reference Fann, Ell and Sharpe2012).
New approaches have been developed to address these shortcomings in care. Systematic identification of cases by screening medical clinics is an important first step (NICE, 2009b ; U.S. Preventive Services Task Force, 2009). However, it is ineffective in improving patient outcomes if used alone (Gilbody et al. Reference Gilbody, House and Sheldon2001, Reference Gilbody, Sheldon and Wessely2006). Case identification therefore needs to be linked with systematic treatment integrated with medical care that typically includes: a multi-disciplinary approach, a structured management plan, a proactive approach to patient follow-up and enhanced interprofessional communication (Gunn et al. Reference Gunn, Diggens, Hegarty and Blashki2006). This approach has been called ‘collaborative care’ (Katon, Reference Katon2012).
Although the systematic treatment for cases identified by screening has been found to be cost-effective for depression co-morbid with some chronic medical conditions, including cancer, heart disease and diabetes, the cost-effectiveness evaluations have not adequately addressed the cost-effectiveness of the screening component. Hence we lack data on the cost-effectiveness of systematic depression management comprising both systematic identification and treatment.
Two of the authors of this paper (J.W. and M.S.) have developed a systematic approach to the identification and treatment of major depression for cancer out-patients that combines systematic case identification by a two-stage screening system in cancer clinics with a systematic collaborative care type treatment integrated with cancer care, known as ‘Depression Care for People with Cancer’ (DCPC; Walker & Sharpe, Reference Walker and Sharpe2009). The treatment was evaluated in the Symptom Management Research Trials in Oncology-1 (SmaRT Oncology-1) and found to be effective (Strong et al. Reference Strong, Waters, Hibberd, Murray, Wall, Walker, McHugh, Walker and Sharpe2008). However, as with other similar studies, the cost-effectiveness of the whole depression management system, including both systematic screening and systematic treatment, has not been determined.
In the current study we aimed to achieve the best estimate of the cost-effectiveness of systematic integrated depression management, including both systematic case identification and systematic treatment, when compared with usual practice for patients with major depression attending specialist cancer services by using multiple sources of data to supplement the data from SMaRT Oncology-1.
Method
Design
We conducted a cost-effectiveness analysis, using a decision analytic model and taking the perspective of a budget-constrained health-care system, to compare systematic depression identification and treatment (as an addition to usual practice) with usual practice alone. We focused on major depression because this denotes a severity and persistence of depressive symptoms that is generally considered to require treatment. We used data from our screening system, individual patient data from our clinical trial of DCPC and data from other published reports (Table 1 contains a full list of data sources). Outcomes were quality-adjusted life years (QALYs) achieved and costs incurred to the health-care system.
Table 1. Parameter estimates used in the model

PSA, Probabilistic sensitivity analysis; HRQoL health-related quality of life; PCP, primary care physician; HADS, Hospital Anxiety and Depression Scale; SMS, Symptom Monitoring Service; DCPC, Depression Care for People with Cancer.
Population
Our study sample comprised adult patients diagnosed with cancer (any type) attending specialist cancer out-patient services at any stage of treatment or follow-up, with an estimated life expectancy of ⩾1 year. The analysis was limited to adult patients with relatively good cancer prognosis because we considered that younger patients and those near the end of life require different types of treatment for depression.
Depression identification and treatment (Fig. 1)
Usual practice
-
(1) Identification of major depression by the patient's primary care physician (PCP) using their clinical skills (assisted by a standardized screening questionnaire if necessary).
-
(2) Treatment of depression based on the PCP's clinical judgement; any combination of ‘watchful waiting’, prescription of antidepressant medication and referral for psychological treatment.
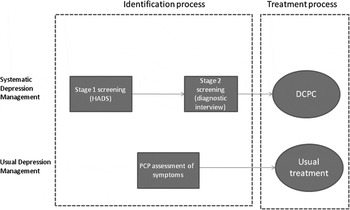
Fig. 1. Components of depression management. HADS, Hospital Anxiety and Depression Scale; DCPC, Depression Care for People with Cancer; PCP, primary care physician.
Systematic identification and treatment
-
(1) Identification of major depression using a two-stage screening system in specialist cancer clinics. In stage 1, screening staff assist patients to complete the Hospital Anxiety and Depression Scale (HADS) while waiting for their clinic appointment (Zigmond & Snaith, Reference Zigmond and Snaith1983). In stage 2, patients whose total HADS score is ⩾15 are telephoned at home, soon after their clinic appointment, and a brief diagnostic interview for major depression is administered (First et al. Reference First, Gibbon, Spitzer and Williams1996). (A HADS total score of ⩾15 has been found to be optimal for identifying cancer patients at risk of major depression; Walker et al. Reference Walker, Postma, Mchugh, Rush, Coyle, Strong and Sharpe2007.) At the end of the call, patients with major depression are advised to see their PCP or oncology clinician, both of whom receive a report from the screening service informing them of the diagnosis of major depression.
-
(2) Treatment of major depression using DCPC (Walker & Sharpe, Reference Walker and Sharpe2009). We have described DCPC in detail elsewhere (Walker & Sharpe, Reference Walker and Sharpe2009). In summary, DCPC is a multicomponent, systematic, team-delivered treatment programme integrated with the patient's cancer care. The treatment team comprises specially trained cancer nurses, consultation-liaison psychiatrists and the patient's PCP. The nurses provide education about depression and its treatment, deliver brief evidence-based psychological interventions (problem-solving therapy, behavioural activation) and monitor the patient's progress using the Patient Health Questionnaire nine-item depression scale (PHQ-9; Kroenke et al. Reference Kroenke, Spitzer and Williams2001; Hopko et al. Reference Hopko, Lejuez, Ruggiero and Eifert2003; Mynor-Wallis, Reference Mynor-Wallis2005). The psychiatrists supervise treatment with the aim of achieving and maintaining treatment targets, advise PCPs about prescribing antidepressant medication and provide direct consultations to patients who are not progressing. The initial treatment phase comprises a maximum of 10 sessions with the nurse, given over 4 months. The patient's PHQ-9 scores are then monitored monthly by telephone and additional sessions are provided for patients who do not meet the treatment targets.
Costs and outcomes
Costs, from the perspective of the UK National Health Service (NHS) and Personal Social Services (PSS), were expressed in pounds sterling at 2010 prices. Outcomes were measured in QALYs, a generic measure that combines any effect of interventions on both life expectancy and health-related quality of life (HRQoL; Drummond et al. Reference Drummond, Sculpher, Torrance, O'Brien and Stoddart2005). QALYs are the most widely used generic measure of outcome in health care.
Model structure
We constructed a model consisting of two linked parts: the first represented the process of identification of depressed patients from the cancer population; and the second captured patient outcomes and costs over a time horizon of 5 years including the effects of depression treatments. The model structure was based on reviews of other models in the literature and discussions with clinicians (Valenstein et al. Reference Valenstein, Vijan, Zeber, Boehm and Buttar2001; Sobocki et al. Reference Sobocki, Ekman, Agren, Jonsson and Rehnberg2006; Paulden et al. Reference Paulden, Palmer, Hewitt and Gilbody2009; NCCMH, 2010). These models are shown in online Supplementary Figs S1 and S2.
In the first part of the model, patients entered the identification processes as either ‘depressed’ or ‘not depressed’. They emerged from these in one of three states: ‘depressed-identified’, ‘depressed-not identified’ and ‘not depressed’. The proportion of patients in each of these states was dependent on the prevalence of depression in people with cancer and on the performance and uptake of the identification systems.
Patients then entered the second part of the model in one of these three states. They could remain in these states or move to other states, each of which had an associated HRQoL score and cost. For example, depressed patients could remain depressed or move into remission. The probabilities of making transitions between states differed by treatment, reflecting differential effectiveness. It was assumed that patients with unidentified depression did not receive any treatment. Patients incorrectly diagnosed with depression, which was assumed to only be possible through identification within usual practice because the diagnostic interview used in the second stage of systematic screening was a ‘gold standard’ instrument and therefore assumed to have 100% specificity (i.e. no false positives), were included in the ‘not depressed’ state but were assumed to receive treatment and therefore incur costs but derive no benefits.
Data sources
Identification
We obtained parameter estimates for identification of depression from the screening system and from published literature. Table 1 contains a list of data sources and parameter estimates. We estimated the number of PCP consultations using published UK data, which indicated that patients with chronic medical conditions attended an average of nine PCP appointments per year (Department of Health, 2011b ). We assumed that the PCP assessed patients for depression at each visit; the sensitivity and specificity of this assessment in diagnosing major depression was estimated from a meta-analysis (Mitchell et al. Reference Mitchell, Vaze and Rao2009). We assumed that the resource use involved in making a diagnosis of depression in usual practice was 2 min of a PCP's time to ask initial screening questions, plus a whole appointment (12-min duration) for the further assessment of those identified as having probable depression (Curtis, Reference Curtis2011). This assumption was based on clinical advice. The sensitivity and specificity of the HADS, used in the first stage of the screening system to identify patients at risk of major depression, was estimated from published data (Walker et al. Reference Walker, Postma, Mchugh, Rush, Coyle, Strong and Sharpe2007). We obtained data from the screening system to estimate the proportion of patients who complete each stage of screening and the associated costs based on resource use data. Estimates of the incidence and prevalence of major depression in people with cancer were taken from the published literature (NICE, 2009a ; Sharpe et al. Reference Sharpe, Strong, Allen, Rush, Postma, Tulloh, Maguire, House, Ramirez and Cull2004).
Treatment
We derived parameter estimates (probabilities of remission and relapse, costs and HRQoL) of outcomes for depression treatment (in usual practice and DCPC) from an efficacy trial, SMaRT Oncology-1, that compared the outcomes of patients allocated to DCPC with those allocated to usual depression treatment (with the PCP informed of the major depression diagnosis) up to 12 months (Strong et al. Reference Strong, Waters, Hibberd, Murray, Wall, Walker, McHugh, Walker and Sharpe2008). We supplemented the trial data with data from published literature (where possible, estimates were taken from systematic reviews and meta-analyses). Treatment costs included all PCP and cancer clinic visits along with in-patient stays and out-patient appointments (medical and psychiatric). Costs were based on resource use measured in SMaRT Oncology-1 and valued using national unit costs (BMA/RPS, 2010; Curtis, Reference Curtis2011; Department of Health, 2011a ). Where appropriate, unit costs included estimates for overheads and indirect time, such as training and administration. The costs of specific anti-cancer treatments (e.g. radiotherapy and chemotherapy) were excluded as we assumed these would be unchanged by depression management. The probability of relapse was assumed to be the same regardless of which treatment was received, based on SMaRT Oncology-1 data (Strong et al. Reference Strong, Waters, Hibberd, Murray, Wall, Walker, McHugh, Walker and Sharpe2008). Estimates of mortality rates were obtained from UK gender- and age-specific cancer survival rates (Walters et al. Reference Walters, Nur, Rachet, Gordon, Jakomis, Edgar and Coleman2010).
Patients with unidentified depression were assumed not to have received treatment. The outcomes for these patients were estimated by combining the outcomes observed in the ‘usual care’ arm of SMaRT Oncology-1 with a meta-analysis that compared depression outcomes for those receiving antidepressant drug treatment with those receiving no treatment (Strong et al. Reference Strong, Waters, Hibberd, Murray, Wall, Walker, McHugh, Walker and Sharpe2008; Arroll et al. Reference Arroll, Elley, Fishman, Goodyear-Smith, Kenealy, Blashki, Kerse and MacGillivray2009). We estimated the costs associated with no treatment from the health-care resource use recorded in the ‘usual care’ arm of SMaRT Oncology-1, minus depression-specific treatment costs (Strong et al. Reference Strong, Waters, Hibberd, Murray, Wall, Walker, McHugh, Walker and Sharpe2008).
Analysis
Base-case analysis
Although patients attend cancer clinics at varying intervals, it was assumed that they were formally screened for depression in the clinic only once a year. This assumption was based on expert advice of the logistical arrangements and the acceptability of screening. We used a time horizon of 5 years, chosen because this is the usual time after which patients successfully treated would be considered ‘cured’ and would no longer be followed up by cancer services (Parkin et al. Reference Parkin, Bray, Ferlay and Pisani2001). The base case considered the case of a 63-year-old woman, reflecting the mean age of patients attending the depression screening service.
Sensitivity and scenario analysis
To reflect uncertainty in the inputs to the model, we conducted probabilistic sensitivity analysis, where distributions are placed on uncertain parameters. This analysis generates the probability of the alternative options being cost-effective for a given cost-effectiveness threshold. To reflect uncertainty in modelling assumptions, we also considered other scenarios including alternative assumptions about the sex and age of the patients, the time horizon and the sensitivity and specificity of PCP assessments. The estimate of the incidence of depression was based on the general population and can be considered to be conservative, as depression incidence would be reasonably expected to be higher in cancer patients; we therefore increased the incidence as a scenario analysis. As our estimate of treatment effectiveness was based on data from a single trial, we also explored the effect of using an alternative estimate of effectiveness derived from a meta-analysis of systematic treatment interventions for depression in primary care (Archer et al. Reference Archer, Bower, Gilbody, Lovell, Richards, Gask, Dickens and Coventry2012).
Economic analysis
We discounted both costs and outcomes at 3.5% per annum in accordance with current UK guidance from the National Institute for Health and Care Excellence (NICE, 2008). Standard decision rules were used to identify the most cost-effective interventions in each analysis. Specifically, we calculated incremental cost-effectiveness ratios (ICERs), which express the additional cost per QALY gained from an intervention compared to the next most effective. We then applied the current range of the UK NICE cost-effectiveness threshold (£20 000–£30 000 per QALY) and defined the cost-effective intervention as the most effective option with an ICER below this threshold (NICE, 2008).
Results
Tables 1 and 2 show the key parameters and the results of modelling. In the base-case analysis, the addition of systematic depression identification and treatment generated more QALYs than usual practice alone (3.094 v. 3.085) but at an additional cost (£464 v. £365). This resulted in an ICER of £11 765 per QALY gained. The probability of systematic depression management being cost-effective at a threshold of £20 000 per QALY was more than 99%.
Table 2. Results

QALY, Quality-adjusted life year; ICER, incremental cost-effectiveness ratio.
The results were consistent across sex and age; the ICER for a 63-year-old man was £13 418 per QALY gained whereas the ICERs for a 50-year-old woman and a 70-year-old woman were £11 502 and £11 794 per QALY gained respectively.
Varying the estimated incidence of major depression had little effect on cost-effectiveness; doubling the incidence to 4.2% only slightly reduced the ICER to £11 278 per QALY gained.
Changing the time horizon from 5 to 10 and 20 years, by which time the majority of patients would be expected to have died, resulted in ICERs of £12 443 and £8871 per QALY respectively. The probability of systematic management being cost-effective remained in excess of 99%, regardless of the time horizon considered.
In the scenario analysis we considered what the sensitivity and specificity of usual depression identification by PCPs would have to be for the addition of systematic identification not to be cost-effective. We found that even if the estimated sensitivity and specificity of usual identification were increased to an improbable 100%, usual practice was still not cost-effective at commonly accepted thresholds. In this scenario all patients with depression will be identified in both arms and the result shows that DCPC is more effective and cost-effective than usual depression treatment.
Using the estimate of treatment effectiveness from other trials of collaborative care treatment of depression in primary care did not significantly change the results and generated an ICER of £10 546 per QALY.
Discussion
We found that a combined systematic approach to the identification and treatment of co-morbid major depression that is integrated with cancer care is likely to be cost-effective, when compared with usual practice. This finding was robust to variation in key parameters.
As far as we are aware, the analysis presented here is the first to adequately address the cost-effectiveness of a depression management system combining integrated case identification and treatment for major depression co-morbid with a medical condition. Few previous studies of the systematic identification of co-morbid depression have used methods appropriate to inform resource allocation decisions (Pignone et al. Reference Pignone, Gaynes, Rushton, Mills Burchell, Orleans, Mulrow and Lohr2002). Those that have used QALYs suggest that systematic screening for depression, without systematic treatment, is not cost-effective (Paulden et al. Reference Paulden, Palmer, Hewitt and Gilbody2009; Valenstein et al. Reference Valenstein, Vijan, Zeber, Boehm and Buttar2001).
Previous evaluations of the cost-effectiveness of systematic treatment of co-morbid depression have reported improved outcomes with increased costs in cancer patients and patients with other chronic conditions (Strong et al. Reference Strong, Waters, Hibberd, Murray, Wall, Walker, McHugh, Walker and Sharpe2008; NICE, 2009b ), and improved outcomes with reduced overall costs for depression co-morbid with several other medical conditions (Simon et al. Reference Simon, Katon, Lin, Rutter, Manning, Von Korff, Ciechanowski, Ludman and Young2007; Katon et al. Reference Katon, Russo, Lin, Schmittdiel, Ciechanowski, Ludman, Peterson, Young and Von Korff2012; Ladapo et al. Reference Ladapo, Shaffer, Fang, Ye and Davidson2012). One of these studies also included a simple estimate of screening costs (Simon et al. Reference Simon, Katon, Lin, Rutter, Manning, Von Korff, Ciechanowski, Ludman and Young2007). The results of our study show that a more systematic and structured approach to identification should be more explicitly considered in future evaluations and service developments.
This study has both strengths and limitations. Its strengths are: the use of estimates based on the best available data from clinical trials, other published data and real clinical services; the use of conservative assumptions (that is, in favour of usual practice); and the use of sensitivity analyses to address areas of uncertainty. There are some limitations: first, the estimate of sensitivity and specificity of depression identification by PCPs was taken from a systematic review that included studies from several countries. However, when the sensitivity and specificity parameters for usual practice were increased to 100% in a scenario analysis, systematic depression management was still cost-effective. Second, there is little evidence to inform our estimate of the incidence of depression in cancer patients; we therefore used the general population incidence rate of 2.1% (NICE, 2009a ). As the incidence in cancer patients is likely to be higher than this, we also determined the effect of a rate twice that of the general population and found that it enhanced, rather than lessened, the case for systematic management. Third, our analysis was simplistic in assuming that screening is simply conducted annually; it did not consider when screening should best be conducted in relation to clinical events such as diagnosis and relapse and more work is needed to assess the best times to screen individuals for depression. Fourth, estimates of the relative effectiveness of usual and systematic treatment of major depression were taken from a clinical trial that may not necessarily generalize to routine care. The outcome of depression with usual treatment taken from the trial may be superior to that achieved in practice because patients in the trial were told of their diagnosis of depression and encouraged to see their doctors for treatment. However, if that was the case it would only make our estimates of the relative effectiveness of DCPC more conservative. Fifth, not all parameters in the model were estimated from exhaustive systematic reviews. However, those parameters to which cost-effectiveness is particularly sensitive were either identified using such methods, or subject to extensive scenario analysis. Sixth, the analysis only applies to patients attending specialized cancer clinics. We acknowledge that some people with cancer will not attend such clinics and the results may not apply to non-ambulant populations. Finally, the model assumes that depression does not have an effect on mortality. There is some evidence that depression may influence life expectancy in cancer patients, possibly by influencing adherence to cancer treatment (Katon & Ciechanowski, Reference Katon and Ciechanowski2002; Pinquart & Duberstein, Reference Pinquart and Duberstein2010). However, our assumption of no effect is conservative with respect to the cost-effectiveness of systematic management and relaxing it would be unlikely to change our conclusions.
We have studied depression management in adult patients with good prognosis cancers attending specialist cancer out-patient clinics. The findings may generalize to younger cancer patients and also to those near the end of their life. Similarly, this may have important implications for the management of depression co-morbid with other chronic medical conditions in which systematic collaborative care treatment has already been found to be both effective and cost-effective (Simon et al. Reference Simon, Katon, Lin, Rutter, Manning, Von Korff, Ciechanowski, Ludman and Young2007; Katon et al. Reference Katon, Russo, Lin, Schmittdiel, Ciechanowski, Ludman, Peterson, Young and Von Korff2012; Ladapo et al. Reference Ladapo, Shaffer, Fang, Ye and Davidson2012). However, in both cases further research is needed to determine if this is the case. Although randomized controlled trials of the whole process of systematic identification and treatment would be informative, the cost and difficulty of organizing such trials may be prohibitive. Research, therefore, may most suitably take the form of clinical trials to inform the effectiveness and cost-effectiveness of systematic treatment for co-morbid depression, and other types of study design to generate evidence on the systematic identification component (e.g. diagnostic accuracy studies to generate evidence on sensitivity and specificity and costs of identification processes). These data could then be combined by modelling so as to fully consider the effectiveness and cost-effectiveness of a systematic approach to the management of co-morbid depression.
Conclusions
Health systems should consider the implications of our findings when deciding on the balance of services they commission for cancer patients. Although there is an inevitable focus on surgical, radiological and pharmaceutical interventions to improve cancer prognosis, the cost of generating health improvement through a systematic approach to both identifying and treating patients with co-morbid major depression is likely to be appreciably lower than that associated with some of these treatments. We have studied depression management in patients with cancer. It is reasonable to hypothesize that our findings may apply to other chronic medical conditions, although further research is required to test this.
The most cost-effective precise specification of the systematic management system may depend on local circumstances. Some of the parameters could be varied, including frequency of screening and the number and content of treatment sessions.
We conclude that combining the systematic identification of major depression with systematic integrated collaborative care treatment is cost-effective. The combined uncertainty in the available evidence results in a very small probability that this conclusion is not valid at the cost-effectiveness thresholds used by NICE. More work is required to determine the optimal specifications of this service, including the frequency of screening, choice of screening instrument and cut-off score, the intensity and duration of depression treatment and the extent to which this approach is also cost-effective for patients with major depression co-morbid with other chronic diseases.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713002079.
Acknowledgements
This work was funded by the charity Cancer Research UK (grant no. C5547/A7375).
Declaration of Interest
None.





