Significant outcomes
∙ Brief intervention in traffic schools can reduce traffic risks and prevent accidents.
∙ DAT1 VNTR and 5-HTTLPR genotypes are associated with traffic behaviour.
∙ Efficacy of intervention may vary by genotype.
Limitations
∙ Reliability of stratified analyses would have benefited of larger sample than feasible in an intervention study.
∙ Questionnaire data were not available for all participants.
Introduction
Road injuries killed 1.3 million people in 2015, being the tenth leading cause of death in the world, and the first for people in ages 15–29 (1). Road traffic collisions, injuries and mortality in traffic are strongly related to risk-taking behaviour (Reference Ouimet, Pradhan, Brooks-Russell, Ehsani, Berbiche and Simons-Morton2–Reference Fuermaier, Tucha, Evans, Koerts, De Waard, Brookhuis, Aschenbrenner, Thome, Lange and Tucha6). Focussing on information on risk and on change of attitudes has been found to produce little behavioural change (Reference Maes and Boersma7,Reference Frank and Lee8). Teaching behavioural methods for controlling risky behaviour is a more effective approach to the prevention of crashes (Reference Makeham9), as is the personal video-based analysis and feedback (Reference Carney, Mcgehee, Lee, Reyes and Raby10).
Decision-making in everyday life, including the traffic-related situations, is influenced by personality traits, and as to the traffic situations, particular significance can be attributed to impulsivity (Reference Jonah11–Reference Alavi, Mohammadi, Souri, Kalhori, Jannatifard and Sepahbodi14). We have previously shown that a brief intervention, guided by the affective neuroscience concept (Reference Panksepp15) and focusing on the acknowledgement of personal risks of impulsive traffic behaviour, can have a positive effect: The intervention group had only half as many speeding violations in the year following the intervention as compared to control (Reference Paaver, Eensoo, Kaasik, Vaht, Mäestu and Harro16), and the diminishing effect on drunk driving and traffic accidents was present through four years following the intervention (Reference Eensoo, Paaver, Vaht, Loit and Harro17).
Inter-individual differences in impulsivity and decision-making derive from genetic and developmental differences in brain function. The capacity of the brain serotonergic system and several gene variants that shape its function are strongly associated with impulse control (Reference Evenden18–Reference Laas, Kiive, Mäestu, Vaht, Veidebaum and Harro22), and measures of serotonergic activity have indeed been associated with risky traffic behaviour (Reference Eensoo, Paaver, Pulver, Harro and Harro23–Reference Luht, Eensoo, Tooding and Harro25). At all serotonergic synapses the serotonin transporter plays a crucial role in the conduct of neurotransmission. The serotonin transporter gene promoter polymorphism (5-HTTLPR) (26) that has consequences to development in childhood (Reference Canli and Lesch27,Reference Brody, Beach, Philibert, Chen and Murry28) is associated with impulsivity (29,Reference Paaver, Kurrikoff, Nordquist, Oreland and Harro30), alcohol use (31–34), intent to drive while intoxicated (35) and actual speed limit exceeding and traffic accidents (Reference Eensoo, Paaver, Vaht, Loit and Harro17). The bulk of evidence suggests the 5-HTTLPR to be the ‘plasticity genotype’, the s-allele carriers being more adaptive to the environment (Reference Homberg and Lesch36). The plasticity genotype concept would suggest that the s-allele carriers may be violating traffic regulations more often if these are not universally respected in the community and enforcement is not rigorous, but less often if the social norms strongly adhere with the law or if the regulations are seen personally fitting.
Besides the serotonergic system, the dopaminergic system has a major role in impulse control and risk-taking behaviour (Reference De Wit, Enggasser and Richards37–Reference Congdon, Lesch and Canli41). The dopamine transporter (DAT) plays a critical role in terminating dopamine neurotransmission in the central nervous system (Reference Chen and Reith42), and the nine-repeat-allele (9R) of the DAT gene (SLC6A3) polymorphism [DAT1 variable number of tandem repeats (VNTR)] is linked to lesser transporter activity and higher synaptic neurotransmitter levels (Reference Fuke, Suo, Takahashi, Koike, Sasagawa and Ishiura43–Reference Vanness, Owens and Kilts45). Van de Giessen et al. (Reference Van De Giessen, De Win, Tanck, Van Den Brink, Baas and Booij46) and Faraone et al. (Reference Faraone, Spencer, Madras, Zhang-James and Biederman47) have shown that the DAT1 VNTR 9R allele carriers have higher striatal DAT availability than do 10-repeat (10R) allele homozygotes and this could be associated with increased risk-taking in experimental paradigms (Reference Heitland, Oosting, Baas, Massar, Kenemans and Böcker48). This is consistent with higher self-reported impulsivity in 9R allele carriers (Reference Forbes, Brown, Kimak, Ferrell, Manuck and Hariri49). Conclusively, the s-allele carriers of the 5-HTTLPR and 9R carriers of the DAT1 VNTR could differ in traffic behaviour, and might be differently responsive to interventions aimed at reduction of impulsivity-related behaviours.
Aims of the study
The aim of this study was to re-evaluate the effect of a brief psychological intervention as conducted by driving school teachers who received training in applying the brief intervention technique previously successfully used by trained psychologists (Reference Paaver, Eensoo, Kaasik, Vaht, Mäestu and Harro16,Reference Eensoo, Paaver, Vaht, Loit and Harro17). We also assessed the potential association of the risk candidate genotypes (5-HTTLPR, also examined in the previous study, and newly DAT1 VNTR) and their role as moderating factors to the eventual intervention effect.
Materials and methods
Participants
Twenty driving-schools agreed to participate in the study. After the initiation of the study, every first group formed of students applying for a passenger car driving license was assigned to the intervention condition, and every second group to the control condition. Out of 1746 subjects asked to participate in the study, in total 1441 (82.5%) (mean age 22.5±7.9 years) agreed, and of these 43.3% were males. The intervention group included 321 (44%) males and 416 (56%) females, and the control group 303 (43%) males and 401 (57%) females. The study was approved by the Research Ethics Committee of the University of Tartu.
Procedure
The study was introduced by team members to the participants at the driving schools, collected the signed informed consent forms, and the saliva samples. The intervention ’Reducing Impulsive Action in Traffic’ (Reference Paaver, Eensoo, Kaasik, Vaht, Mäestu and Harro16,Reference Eensoo, Paaver, Vaht, Loit and Harro17) consisted of a lecture (45 min) and group work (45 min) as previously described. This intervention was theoretically guided by the affective neuroscience concept (Reference Panksepp15) and aimed at acknowledgement of personal impulsive tendencies, so that subjects of intervention could build their own strategies to reduce personal risk. Lectures were carried out and the group work conducted by regular teachers of the driving schools, who had previously been trained in a tailor-made 2 European Credit Transfer and Accumulation System point course at the University of Tartu to carry out the intervention.
Questionnaires
After recruitment, the participants completed web-based self-report questionnaires. The Adaptive and Maladaptive Impulsivity Scale, based on the concept of functional and dysfunctional impulsivity (Reference Dickman50), was used to measure different facets of impulsivity: fast decision-making, thoughtlessness, excitement seeking and disinhibition (Reference Laas, Reif, Herterich, Eensoo, Lesch and Harro51). Each facet was measured by six items on five-point Likert scale. Subjects reported their relationship status, education and monthly income. The frequency of consuming strong and light alcoholic drinks during the previous year on a six-point scale (none to almost every day) was reported (Reference Eensoo, Paaver, Harro and Harro52). For assessing alcohol-related problems, five diagnostic and statistical manual of mental disorders-IV diagnostic criteria for alcohol abuse, relating to specific life events (e.g., ‘turned aggressive while drunk’, ‘had longer periods of alcohol use’) were used. Subjects were categorised dichotomously based on whether they had ever experienced any alcohol-related problems or not.
Genotyping
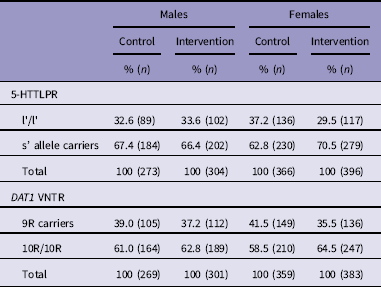
Saliva samples (2 ml) were obtained from 1341 subjects (93.1% of total sample) using the SalivaGene® Collection module II (STRATEC Molecular GmbH, Berlin, Germany). DNA was extracted from the samples using the NucleoSpin® Blood method (MACHEREY-NAGEL GmbH & Co KG, Düren, Germany) designed for extracting genomic DNA from various body fluids. Genotyping for the triallelic classification of the 5-HTTLPR polymorphism was performed according to Anchordoquy et al. (Reference Anchordoquy, Mcgeary, Liu, Krauter and Smolen53). Genotyping was done in two stages. First, all subjects were genotyped for the 5-HTTLPR VNTR polymorphism, then for single nucleotide polymorphism (SNP) rs25531 (A/G). The polymorphic region was amplified using the primers 5-HTTLPR-F: 5′-6FAM-ATG CCA GCA CCT AAC CCC TAA TGT-3′ and 5-HTTLPR-R: 5′-GGA CCG CAA GGT GGG CGG GA-3′. Then SNP rs25531 (A→G) was determined as described in detail elsewhere (Reference Tomson, Merenäkk, Loit, Mäestu and Harro54). Triallelic 5-HTTLPR genotypes were categorised into groups according to the effectiveness at the transcriptional level as follows: lG/lG, lG/s, and s/s were designated as s’/s’; lA/s and lA/lG as l’/s’; and lA/lA as l’/l’. Genotype frequencies were in the Hardy–Weinberg equilibrium. As the original experiments have shown that the long allele of the 5-HTT gene has a more efficient promoter than the short allele, and that the l/s or s/s genotype cells (26), do not differ in this regard, we compared the s’ allele carriers (s’/s’ and l’/s’; n=895; 66.8%) with the l’/l’ (n=444; 33.2%) homozygotes. This decision was also based on our previous study showing differences in traffic behaviour between the l’/l’ homozygotes and s’-allele carriers (Reference Eensoo, Paaver, Vaht, Loit and Harro17). Distribution of the 5-HTTLPR genotype in control group (n=639) and intervention group (n=700) by gender is shown in Table 1. Genotype frequencies were not statistically significantly different between the groups.
Table 1 Distribution of the participants by 5-HTTLPR and DAT1 VNTR genotypes, gender, and involvement in the intervention
Triallelic 5-HTTLPR genotypes were obtained for 1339 and DAT1 VNTR for 1341 subjects; 29 subjects who had a rare VNTR genotype (10R/11R, 6R/10R) were excluded from the analysis.
The DAT1 (SLC6A3) VNTR was genotyped following the analytical method by Anchordoquy et al. (Reference Anchordoquy, Mcgeary, Liu, Krauter and Smolen53) as described in detail by Maksimov et al. (Reference Maksimov, Vaht, Murd, Harro and Bachmann55). Polymorphic region were amplified using the primer rs28363170F: 5′ /56-FAM/TGT GGT GTA GGG AAC GGC CTG AG 3′ and rs28363170R: 5′ CTT CCT GGA GGT CAC GGC TCA AGG 3′ for DAT1 3′UTR VNTR. The VNTR repeat numbers range from 6 to 11, with 9 and 10-repeat alleles being the most common. Genotype frequencies were in the Hardy–Weinberg equilibrium. We compared the 9-repeat carriers (9R/9R and 9R/10R; n=502; 38.9%) and 10-repeat (10R/10R) homozygotes (n=810; 60.4%); subjects who had a rare VNTR genotype (10R/11R, 6R/10R) were excluded from the analysis. Distribution of the DAT1 VNTR is shown in Table 1. Genotype frequencies were not statistically significantly different between the groups.
Database search
Traffic offenses and crashes were monitored in the period of 1 January 2014 to 1 January 2017. Information about subjects obtaining the driving licence was derived from the Estonian Road Administration. Police and Border Guard Board database was used for collecting information about violations in traffic including drunk driving (penalties for drunk driving with an estimated blood alcohol level of 0.2‰ or more) and speed limit exceeding. Data on traffic accidents were received from the Traffic Insurance Fund database. Accidents in which the subject was at fault were classified as active and other accidents as passive. Subjects with occurrence of either recorded traffic offence or a collision were classified into the high general traffic risk group. From 278 subjects with high general traffic risk (occurrence of either a recorded traffic offence or a collision) 23 (8.3%) were also drunk drivers and 179 (64.4%) subjects with violations.
Statistical analysis
Data were analysed using IBM SPSS (version 22.0, Chicago, IL, USA) and SAS (version 9.4 SAS Inc., Cary, NC, USA) software. By survival analysis (Kaplan–Mayer estimates) probabilities of non-occurrence of traffic accidents and/or general traffic risk (survival probabilities) were compared between control and intervention groups. Cox regression analyses were used to investigate the effect of different variables upon the traffic accidents and on general traffic risk. By t-tests impulsivity was compared in groups by genotype. Pearson’s χ2 tests were used for comparison of distribution of traffic offences and accidents by participation in intervention, gender, and/or by genotype.
Results
Effect of intervention on traffic behaviour and accidents
Control and intervention groups did not differ by gender, age, education, income, or impulsivity measures (data not shown). According to the Road Administration database the control and intervention groups did not differ significantly in any respect with regard to the obtaining of driving license. By the survival analysis, participants of the intervention group were significantly less likely in the general traffic risk group (p=0.004 for the log-rank test, DF=1, χ2=8.49) and, specifically, less involved in traffic accidents (p=0.038 for the log-rank test, DF=1, χ2=4.30) compared to controls during the 3-year study period (Fig. 1).
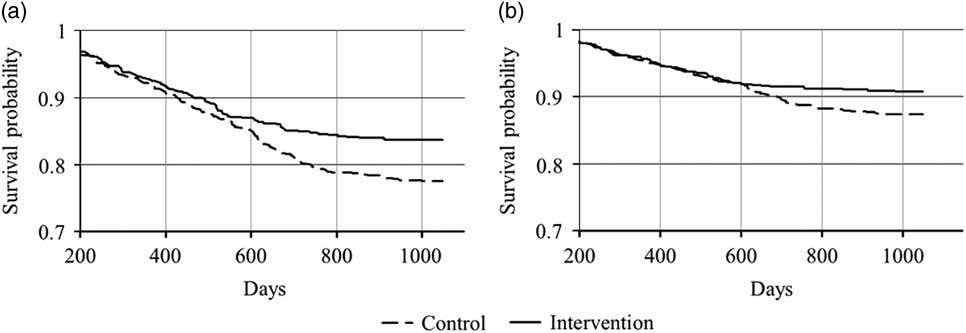
Fig. 1 Occurrence of indicators of high general traffic risk (a, occurrence of either recorded traffic offence or a collision) and of traffic accidents (b) during the 3-year study period.
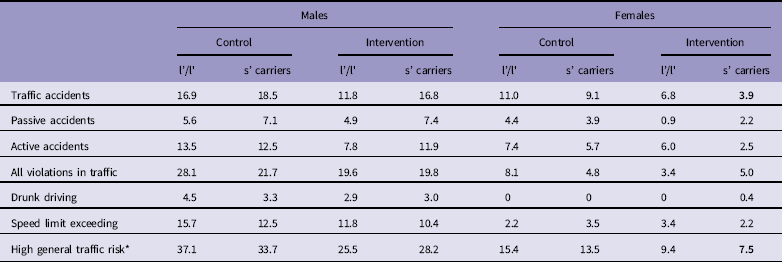
Table 2 presents the occurrence of traffic accidents and violations by intervention and gender. Both male and female intervention groups had significantly lower general traffic risk than control group, and intervention reduced the occurrence of both active and passive accidents in females.
Table 2 Traffic accidents and violations by gender and participation in the intervention
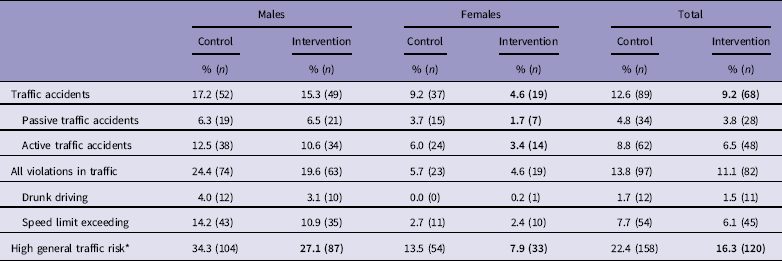
* Occurrence of either a recorded traffic offence or a collision.
Bold values represent statistical significance p<0.05, significant difference compared to respective control group.
Predicting traffic accidents and the general traffic risk
For further regression analyses we selected the two summary measures affected by the intervention, traffic accidents, and high general traffic risk, as the remaining measures in Table 2 are contained in one or another. Using Cox regression, traffic accidents and high general traffic risk were first independently predicted by selected variables one by one (Table 3). For traffic accidents, gender and participation in the intervention were significant predictors. High general traffic risk was also predicted by gender and participation in the intervention, but additionally by the DAT1 VNTR genotype, alcohol use, occurrence of alcohol-related problems, excitement seeking, fast decision-making, and educational level. For clarifying how these significant predictors together influence the occurrence of traffic accidents and high general traffic risk, additional models were composed. If all single statistically significant predictors were included in a common model predicting accidents, the effect of the intervention remained significant (n=1441; −2 LOG L without covariate = 2225.82; −2 LOG L with gender as a covariate = 2190.44). While significant predictors for high general traffic risk from univariate analysis were included in the multivariate model, significance of the effect of intervention decreased (n=824; −2 LOG L without covariates = 1846.49; −2 LOG L with covariates = 1771.32).
Table 3 Cox regression models predicting participation in the traffic accident and high general traffic risk
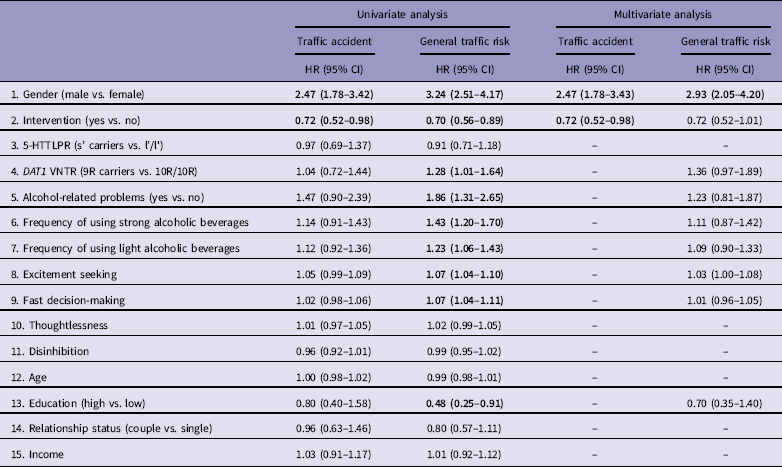
HR, hazard ratio with 95% confidence intervals (CI).
Bold values represent statistical significance p<0.05.
5-HTTLPR genotype, traffic behaviour, and the intervention effect
5-HTTLPR s’-allele carriers had significantly lower mean score in fast decision making than l’/l’ homozygotes (17.6, SD=4.5 vs. 18.1, SD = 4.0; p=0.008). This difference was more evident in females (16.8, SD=4.4 vs. 17.8, SD = 3.9; p=0.042). No other significant association between aspects of impulsivity and the genotype was found in this sample.
5-HTTLPR genotype had no significant predicting effect on general traffic risk and traffic accidents, neither in the total sample (Table 3) nor if stratified by gender (data not shown). However, the lowest proportion of traffic accidents or general traffic risk were observed in female s’ allele carriers after intervention: for the general traffic risk (χ2=(3)7.91; p=0.048) and for traffic accidents (χ2=(3)8.70; p=0.034) (Table 4).
Table 4 Proportion (%) of subjects with traffic offences and accidents in subgroups by, gender, intervention, and 5-HTTLPR
* Occurrence of either recorded traffic offence or a collision.
Bold values represent statistical significance p<0.05.
DAT1 VNTR genotype, traffic behaviour, and the intervention effect
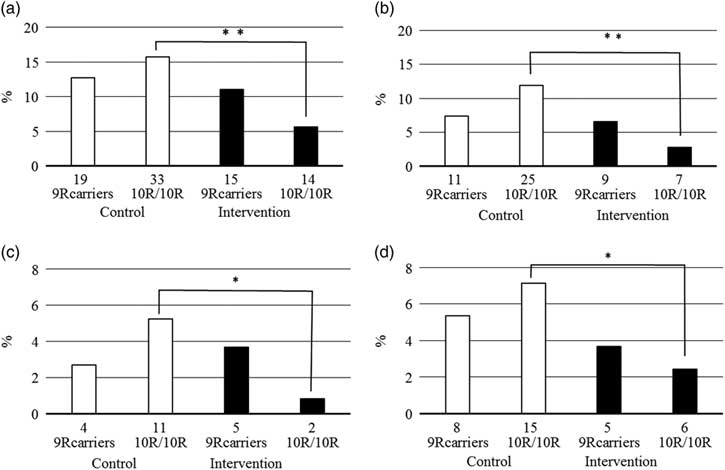
The DAT1 VNTR genotype was associated with traffic behaviour, but differently in males and females: while male 9R allele carriers had more frequently been driving drunk [odds ratio (OR) = 2.89; 95% confidence intervals (CI) = 1.12–7.47], and belonged more frequently to the high general traffic risk group (OR = 1.46; 95% CI = 1.02–2.10), in females the genotype was not significantly associated with traffic risk behaviour and there was rather a tendency for the 10R homozygotes to have higher traffic risk (Fig. 2). The intervention effect was independent of genotype in males, but in females largely on account of the 10R/10R genotype.
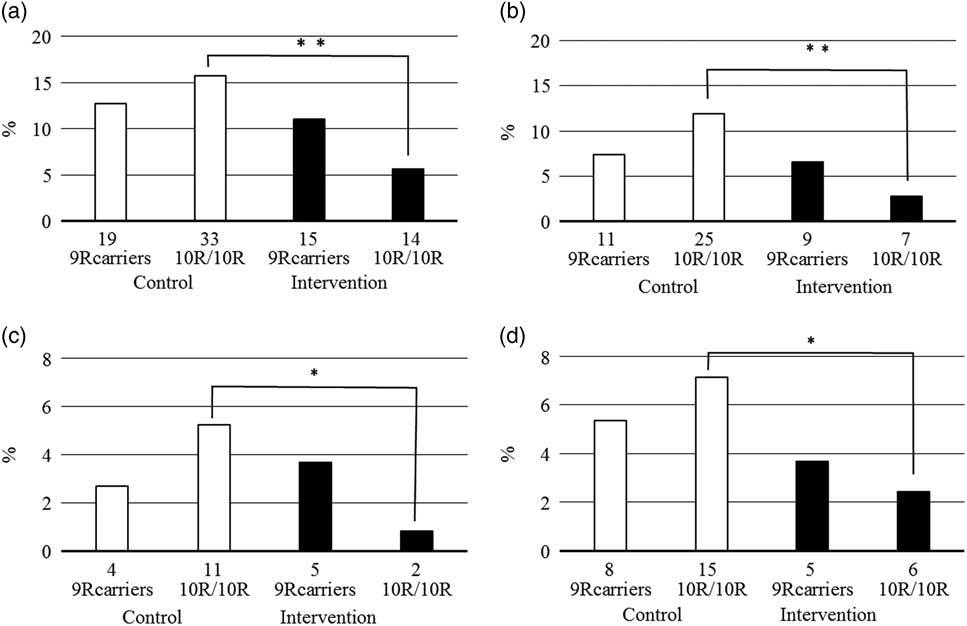
Fig. 2 Proportion of females with high general traffic risk (a, occurrence of either recorded traffic offence or a collision), traffic accidents (b), passive (c), and active traffic accidents (d) by participation in intervention and DAT1 VNTR. Number of the cases presented below each column. Total number of female 9R carriers in the control group 149 and in the intervention group 136; total number of female 10R homozygotes in control group 210 and in the intervention group 247. *p<0.05, **p<0.01, significant difference.
Discussion
We have previously reported that a brief intervention with focus on personal psychological risk factors, included in the driving education program but conducted by psychologists, had a significant impact on traffic safety 1 year after the intervention (Reference Paaver, Eensoo, Kaasik, Vaht, Mäestu and Harro16), and that this impact persisted throughout the following 4 years (Reference Eensoo, Paaver, Vaht, Loit and Harro17). The affective neuroscience concept (Reference Panksepp15) leads to the conclusion that much of everyday behaviour, in particular in situations with high cognitive demand, is guided by activity in evolutionally old emotive circuits that cannot be controlled in real time; nevertheless, cognitive/behavioural strategies can be constructed to mitigate their adverse outcomes. The brief intervention in driving schools aimed at enhancing awareness of impulsivity and the health risks that impulsive action can bring about, to help students to spot and acknowledge impulsive tendencies both in themselves and in others, and to guide students to monitor themselves and to encourage them to develop personal strategies for mitigation of risks borne by impulsivity in traffic (Reference Paaver, Eensoo, Kaasik, Vaht, Mäestu and Harro16). The study reported herein was meant to attempt independent replication in a new sample, but with one important difference from the previous study: according to the training the trainers dissemination approach, we trained the traffic school teachers to deliver the intervention session, because in everyday practice this should be much more convenient and less demanding of resources as compared to the arrangement of psychologists visiting the traffic schools. During the 3-year study period a significant impact of intervention on traffic safety was indeed present. Similarly to the previous investigation, the intervention had a positive effect on involvement in traffic offenses and accidents. At variance from the previous study the effect on traffic offences as analysed separately was not statistically significant. It should however be noted that this investigation was also conducted in a different overall traffic culture as it had significantly improved. This is well illustrated by changes in the most valid and robust measure, annual mortality by traffic injuries per one million inhabitants, which in 2007 (while the first intervention study started) was 146 but by the 2016 (while the monitoring period of the current intervention study ended) had decreased to 36. Increasing the likelihood of a floor effect the improved traffic climate may have increased the demand on statistical power. Nevertheless, the aggregate measure of traffic safety was significantly lower in the intervention group, suggesting that the training the trainers approach is feasible for the delivery of the impulsivity-focussed instrument. Further studies should address the feasibility of internet-based training instruments as drivers’ education varies in different settings.
The effect of intervention was not affected by consideration of a number of factors known to be associated with risk-taking in traffic. It is known that males take more risks in traffic (Reference Turner and Mcclure56), lower education level is associated with higher risk (Reference Harper, Charters and Strumpf57) and several studies, including our own, have reported higher general risks in association with alcohol-related problems (Reference Paaver, Eensoo, Pulver and Harro24,Reference Eensoo, Harro, Pullmann, Allik and Harro58,Reference Van Dyke and Fillmore59). The findings in the present study are thus consistent with earlier research. Facets of impulsivity increase the risk in traffic (Reference Barkley and Cox12,Reference Bicaksiz and Özkan13,Reference Luht, Eensoo, Tooding and Harro25), and the results of the present study highlight excitement seeking and fast decision-making. These two impulsivity facets were, amongst the studied impulsivity measures, also the most significant predictors of general traffic risk in our previous study on a different traffic school sample (Reference Paaver, Eensoo, Kaasik, Vaht, Mäestu and Harro16). Nevertheless, the association of intervention with reduction of traffic accidents and risk-taking was largely similar in multivariate analyses and apparently the acknowledgment of impulsivity in traffic can be broadly usable strategy.
It is, however, plausible that some subjects may be more and some less malleable to the intervention, and because our research strategy involves the aspect of precision medicine by consideration of common functional gene variants, we included two candidate genes into the study. We had previously studied the association of the 5-HTTLPR genotype with traffic behaviour and the effect of intervention (Reference Eensoo, Paaver, Vaht, Loit and Harro17): previously it had been reported that the 5-HTTLPR s’ allele carriers consumed more drinks at bars and the s’/s’ homozygotes expressed higher intention to drive a motor vehicle after drinking (35). Longitudinal population-representative studies have indeed described earlier and higher alcohol use in 5-HTTLPR s’/s’ homozygotes (31,Reference Vaht, Merenäkk, Mäestu, Veidebaum and Harro32). In general, lower prevalence of traffic incidents was found in the 5-HTTLPR s’ allele carriers in our previous study, and the effect of intervention was largely observed in l’/l’ homozygotes probably owing to the floor effect in the s’-allele carriers (Reference Eensoo, Paaver, Vaht, Loit and Harro17). In the present study the overall association of 5-HTTLPR with behaviour in traffic was not statistically significant. A likely explanation to this discrepancy could again be found in the overall improved traffic culture: For example, in the previous study the prevalence of speeding offences among females of the control group was 18% in the 5-HTTLPR l’/l’ homozygotes versus 4% in s’-allele carriers, but in the present study, several fold less (Table 4). However, consistently with the previous study, the female s’-allele carriers of the intervention group were the safest drivers in traffic. This also fits nicely with the notion that the 5-HTTLPR s’-allele is associated with higher social cohesion, and particularly so in females.
Among males the proportion of DAT1 VNTR 9R carriers was higher among drunk drivers and subjects with high general traffic risk. These results support the significant role of dopaminergic system in impulse control and risk-taking behaviour (Reference De Wit, Enggasser and Richards37–Reference Congdon, Lesch and Canli41) and the potentially higher risk in DAT1 VNTR 9R allele carriers (Reference Vanness, Owens and Kilts45,Reference Van De Giessen, De Win, Tanck, Van Den Brink, Baas and Booij46,Reference Guo, Cai, Guo, Wang and Harris60), including in traffic (Reference Tokko, Eensoo, Vaht, Lesch, Reif and Harro61). In females, carrier status of the DAT1 VNTR 9R allele had no significant association with traffic behaviour at baseline, but prevented the intervention effect to occur. This suggests that under different environmental conditions the 9R allele might appear as a risk allele even in females, and that other type of interventions may be more adequate for this group.
Limitation of the study to be borne in mind is the sample size dictated by the feasibility for an intervention study. Especially the genetic analyses should be regarded as exploratory for this reason. Moreover, we used several self-administered questionnaires and the more uncomfortable questions (e.g., alcohol usage) were missed by a number of participants. Larger studies should be conducted to allow for more reliable stratified analyses.
In conclusion, the brief intervention conducted by driving school teachers had a significant impact on traffic safety and could be a part of curricula at driving schools. An important aspect in planning interventions is to recognise the differences, in part genetic, within the target population that suggest the necessity of combining a variety of measures.
Acknowledgements
The authors are grateful to the EPSTB participants, and the whole study team. This work was supported by the Estonian Research Council (3.2.1002.11-0002, TerVE VIGA and IUT20-40], the EC FP7 project Aggressotype (FP7-Health-2013-Innovation-1, 602805), the EC Horizon 2020 projects CoCA (H2020-PHC-2015-667302) and Eat2beNICE (H2020-SFS-2016-728018), and the Estonian Road Administration. Authors’ Contribution: J.H. and D.E. designed the study and coordinated data collection. M.V. peformed genotyping genotyping. K.L., T.T., and D.E. contributed to the literature searches, and conducted statistical analysis. K.L. wrote the first draft of the manuscript. All authors contributed to the acquisition and analysis of the data, critically reviewed the content, edited the draft manuscript, and approved the final version for publication.
Financial Support
Financial support was received from the Estonian Research Council (3.2.1002.11-0002, TerVE VIGA and IUT20-40], the EC FP7 project Aggressotype (FP7-Health-2013-Innovation-1, 602805), the EC Horizon 2020 projects CoCA (H2020-PHC-2015-667302) and Eat2beNICE (H2020-SFS-2016-728018), and the Estonian Road Administration.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.