INTRODUCTION
The great diversity of reproductive and developmental modes exhibited by marine invertebrates is a challenge to researchers seeking to understand the patterns and trends of their life histories (McHugh, Reference McHugh1993; McHugh & Rouse, Reference McHugh and Rouse1998; Ramirez-Llodra, Reference Ramirez-Llodra2002). The adaptive significance of reproductive and developmental modes in those groups has received some attention in the literature regarding the evolutionary constraints (e.g. Thorson, Reference Thorson1946; Schroeder & Hermans, Reference Schroeder, Hermans, Giese and Pearse1975; Strathmann, Reference Strathmann1985; McHugh & Rouse, Reference McHugh and Rouse1998). This challenge is especially true in polychaete annelids, which have been recognized for their high reproductive diversity (Rouse & Fitzhugh, Reference Rouse and Fitzhugh1994; McHugh & Fong, Reference McHugh and Fong2002; Qian & Dahms, Reference Qian, Dahms, Rouse and Pleijel2006). Wilson (Reference Wilson1991) and Giangrande (Reference Giangrande1997) have identified 17 modes of sexual reproduction in more than 500 species of polychaetes. Among these different reproductive and developmental modes, typical characteristics include the type of oogenesis, type of spermatozoon, maximum oocyte size, number of eggs per single spawning event, total female body size, development time, fecundity and individual growth rates (McHugh, Reference McHugh1993; Giangrande, Reference Giangrande1997; Nylin & Gotthard, Reference Nylin and Gotthar1998; Marshall & Keough, Reference Marshall and Keough2007; Moran & McAlister, Reference Moran and McAlister2009).
Among the polychaetes, the family Terebellidae contains approximately 70 genera and more than 500 species and is distributed worldwide (Hutchings, Reference Hutchings, Beesley, Ross and Glasby2000; Rouse, Reference Rouse, Rouse and Pleijel2001; Garraffoni et al., Reference Garraffoni, Nihei and Lana2006; Garraffoni & Lana, Reference Garraffoni and Lana2008). This high diversity contrasts with the small amount of data regarding their life history and reproduction, as reproductive and developmental traits have been investigated for only 29 terebellid species (Wilson, Reference Wilson1991; McHugh, Reference McHugh1993; Giangrande, Reference Giangrande1997).
Nicolea uspiana is a terebellid species which constructs mucous tubes covered by sand grains and shell fragments in the midst of aggregates of algae, ascidians, hydroids and bryozoans (Nogueira, Reference Nogueira2003; Garraffoni & Amaral, Reference Garraffoni and Amaral2009). It forms dense aggregations with individuals at different stages of development (Garraffoni & Amaral, Reference Garraffoni and Amaral2009). Although it is a common Brazilian rocky-shore terebellid from the States of São Paulo and Paraná (latitudinal range: 23o21′S 44o51 W–25o35′S 48o20 W) (Blankensteyn & Moreno, Reference Blankensteyn and Moreno1999; Nogueira, Reference Nogueira2003; Garraffoni & Amaral, Reference Garraffoni and Amaral2009; Santos et al., Reference Santos, Nogueira, Fukuda and Christoffersen2010), no reproductive studies have been performed; however, the relative growth and population structure of N. uspiana have been studied by Garraffoni et al. (Reference Garraffoni, Yokoyama and Amaral2010). Thus, the present study investigated the reproductive biology and gamete development of N. uspiana, to elucidate the diversity of reproductive and developmental patterns of the Terebellidae. Data on the mode of reproduction, body size, maximum oocyte size, maximum fecundity, mode of development and breeding strategy were analysed to examine the life history of this species. Furthermore, information about the reproductive patterns and life histories in other known terebellid species were compared in order to summarize the reproductive knowledge about this family.
MATERIALS AND METHODS
Specimens of Nicolea uspiana were collected monthly (May 2006–May 2007) in the intertidal zone along the rocky shore of Porchat Island on Itararé Beach (23o57′35″S 46o23′15″W, São Vicente, Brazil). Specimens were collected qualitatively by removing the sand-covered mucous tubes from the rocks using a scalpel.
Samples were kept on ice in an insulated container to relax the animals. The polychaetes were transported to the laboratory and kept in an aquarium with seawater to sort them alive. In the laboratory, the specimens were removed from their tubes using a stereomicroscope, and the sex was determined by visual examination of the coelomic gametes or, in the smallest individuals, with a microscope. Females were identified by their whitish-yellow body coloration and the presence of yolk in the oocytes. Males were off-white in colour and possessed sperm-morule structures. Juvenile specimens lacked visible sexual characteristics (Eckelbarger, Reference Eckelharger1974). After these procedures, the specimens were anaesthetized in a solution of seawater and magnesium chloride for approximately 1 h, fixed in 6% formalin for at least 48 h, and then stored in 70% ethanol.
The histological analysis of gametogenesis in 10 mature individuals (5 males and 5 females) was performed by obtaining longitudinal sections from individuals of different sizes (10–18 mm in length). The specimens were dehydrated in an ethanol gradient (80, 95 and 100%), infiltrated and embedded in glycol-methacrylate resin, and serially sectioned at a thickness of 3–5 µm with glass knives in a microtome. Sections were stained with toluidine blue and later analysed by light microscopy and photographed.
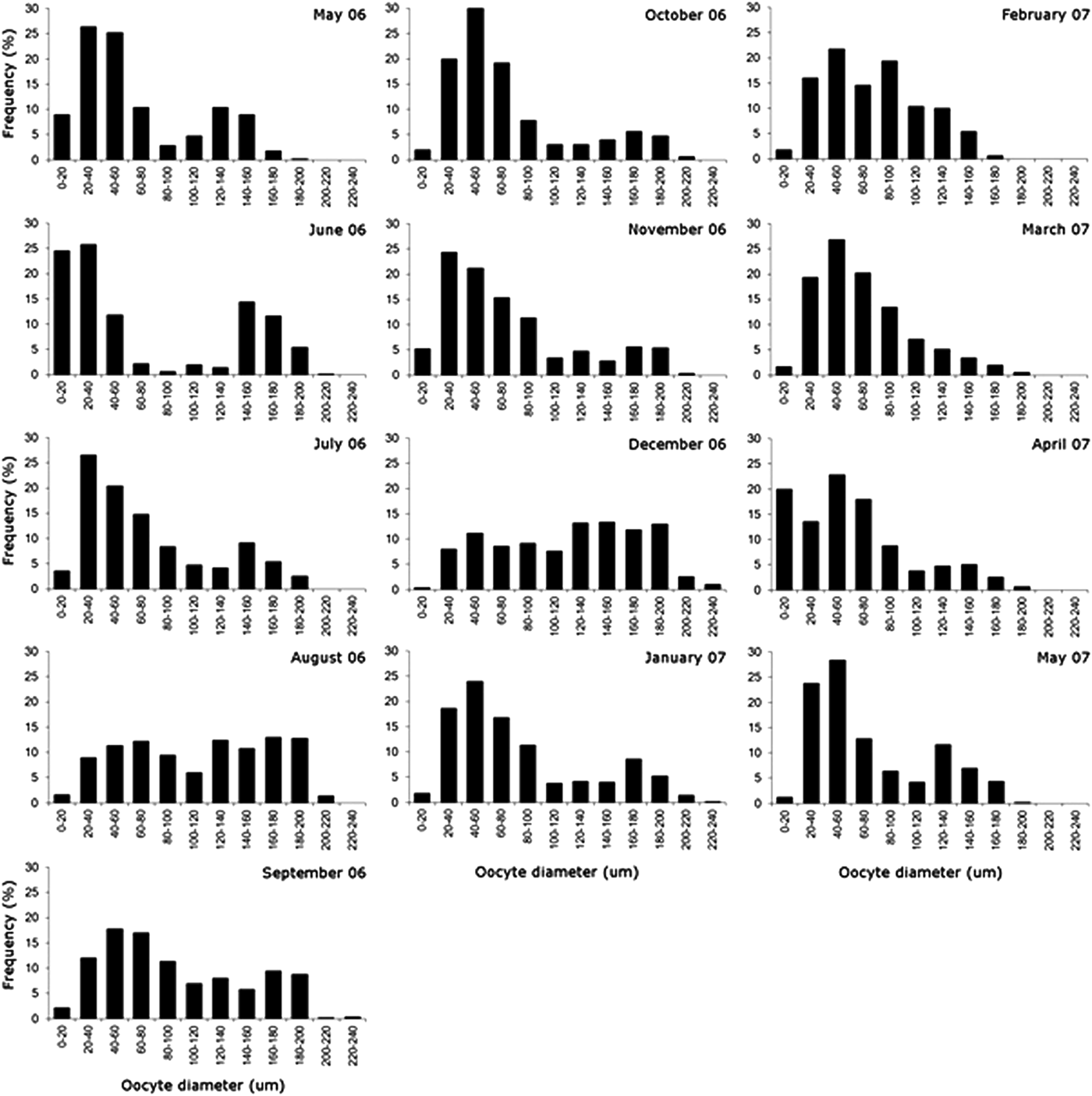
To determine the size and volume of the oocytes in the vitellogenic stage, the coelomic content was examined monthly from 10 females randomly selected from the total number of females collected each month. These females were placed on a slide and gently compressed with a cover slip to let the oocytes shed from the coelomic fluid. Thus, fifty oocytes from each female were squashed under a cover slip, and their diameters were measured. The oocyte volume was determined under the assumption that it was spherical (total oocyte volume = 3/4πr 3, where 2r is the diameter of the oocyte). A series of histograms of oocyte sizes were constructed to evaluate the pattern of oocyte maturation over the study period. The criteria used for identifying the stage of oogenesis followed those of Eckelbarger (Reference Eckelharger1974), allowing the growth cycles of oocytes to be divided into the gonadal, follicular and vitellogenic phases.
The frequency of the occurrence of sperm stages was determined from a random selection of 100 gametes from the coelomic content of 10 randomly selected males collected between October 2006 and May 2007. The criteria used for staging sperm followed those of Blake et al. (Reference Blake and Van Dover2005), and allowed the growth cycles of sperm to be classified as rosettes of spermatocytes, early spermatids and morulae composed of tailed spermatids.
RESULTS
General characteristics
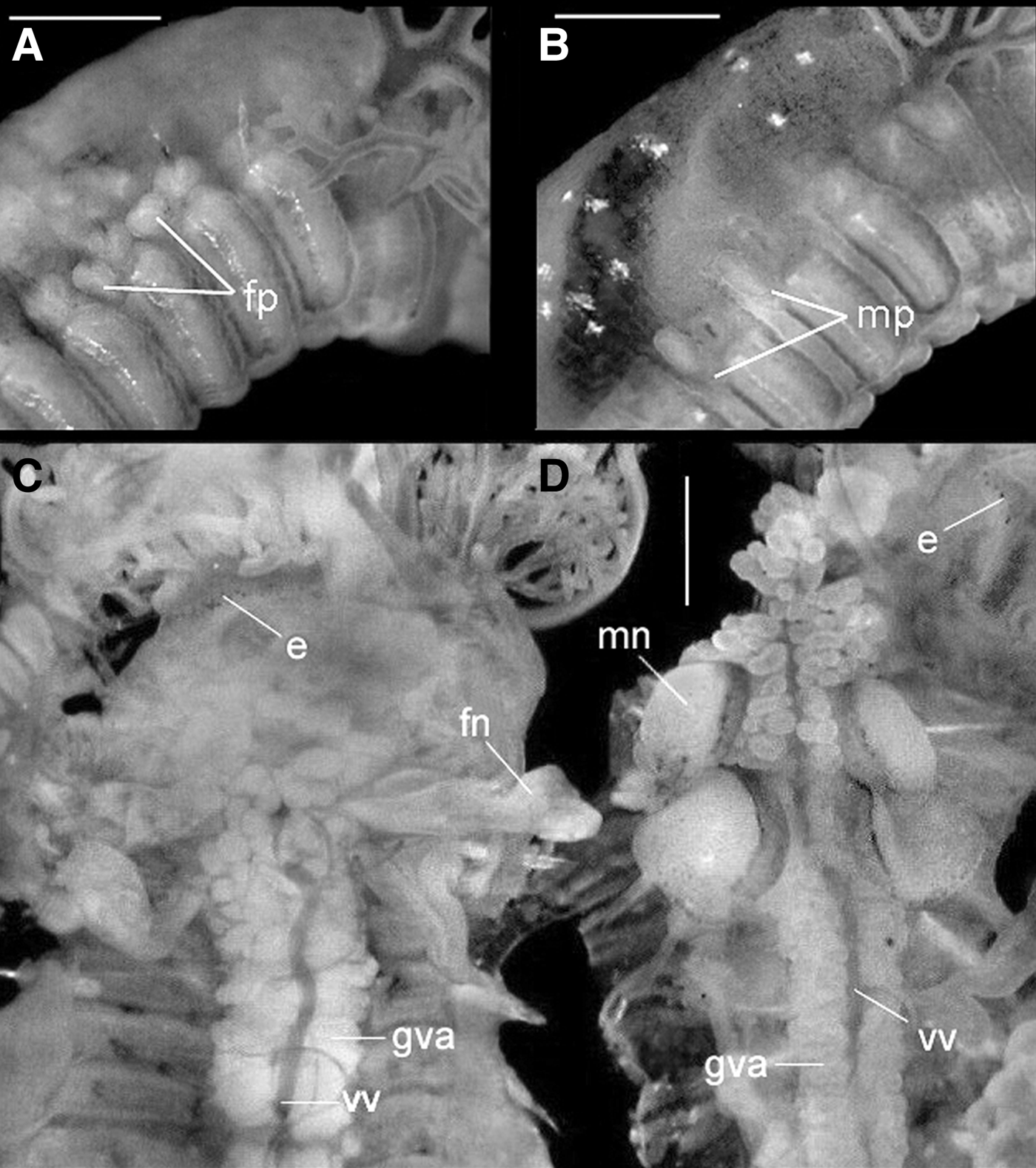
Nicolea uspiana is a gonochoric species with slight sexual dimorphism verified by the shape of the genital papillae, which are located dorsally and posterior to the notopodia (Figure 1A, B). The number and position of the genital papillae in the two sexes are identical; however, in the female, they are restricted to glandular walls and real papillae are not visible (Figure 1A), whereas papillae in the male are slender tubes or cones and are much more evident (Figure 1B). Both sexes have nephromixia composed of two inflated loops, one distal and the other proximal, and they are usually projected upward in the coelomic spaces around the gut. The excretory nephromixia of Nicolea uspiana occur on segment 3, and the reproductive nephromixia occur on segments 6 and 7. Internally, the distinction between the reproductive nephridial structure of males and females is evident (Figure 1C, D). However, both mature male and female specimens lack distinct ovaries or testes.

Fig. 1. Nicolea uspiana: size and shape of the nephridial papilae in female (A) and male (B) and internal morphology showing the female nephromixia (C) and male nephromixia (D). fp, female nephridial papilae; mp, male nephridial papilae; fn, female nephromixia; mn, male nephromixia; gva, glandular ventral area; e, eyesptos; vv, ventral vessel. Scale bars: A,B, 300 µm; C, 250 µm; D, 150 µm.
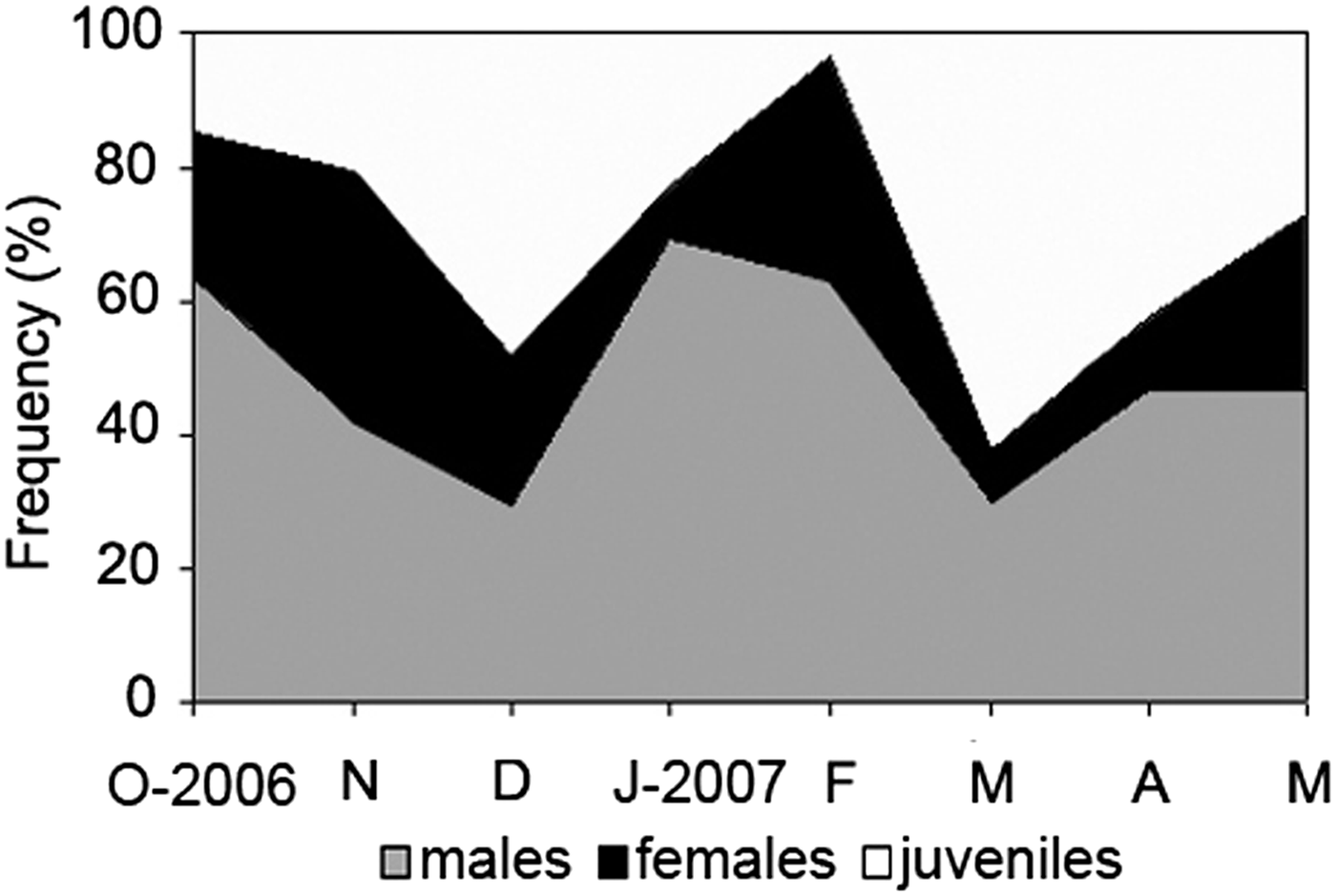
Of the 847 total specimens of Nicolea uspiana examined, 163 were females (19.3%), 391 were males (46.2%), and 293 were juveniles (34.6%). The sex-ratio of N. uspiana was 2.4:1 (male:female), which is significantly different from a 1:1 ratio (χ2 = 93.834; df = 1; P < 0.05). Males dominated the population in all samples (Figure 2).

Fig. 2. Nicolea uspiana: relative monthly frequency (%) of juveniles, females, and males from October 2006 to May 2007.
Oogenesis
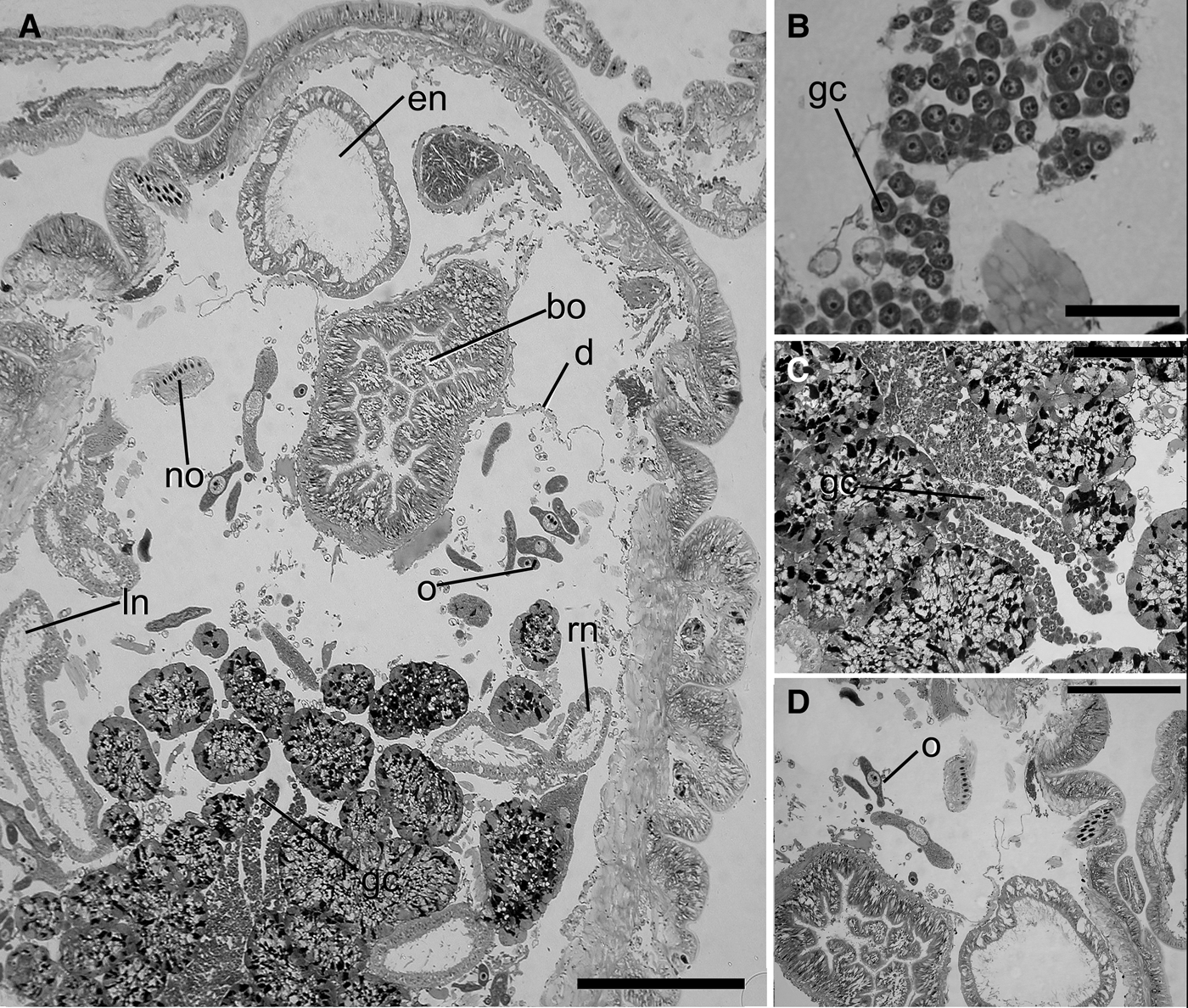
The oocytes of Nicolea uspiana change shape from flattened to spherical during oogenesis. Histological characterization of the follicular and vitellogenic phases is shown in Figures 3A–D and 4A–D. In the follicular stage of gametogenesis, large clumps of oocytes (approximately 25 µm in diameter) are surrounded by follicular cells bulging into the coelom. At this stage, it is possible to observe the germinal vesicle in a central position in the oocyte with an oval nucleus (7–8 µm in diameter) surrounded by a cytoplasmic component. In the next stage, the vitellogenic phase, the degeneration of the peritoneal membrane occurs, releasing oocytes that float freely in the coelomic fluid. During this phase, oocytes of various sizes, resulting from the different amount of cytoplasmic components in the oocytes that substantially increase during maturation, are observed in the coelom (Figures 3B–D, 4D).
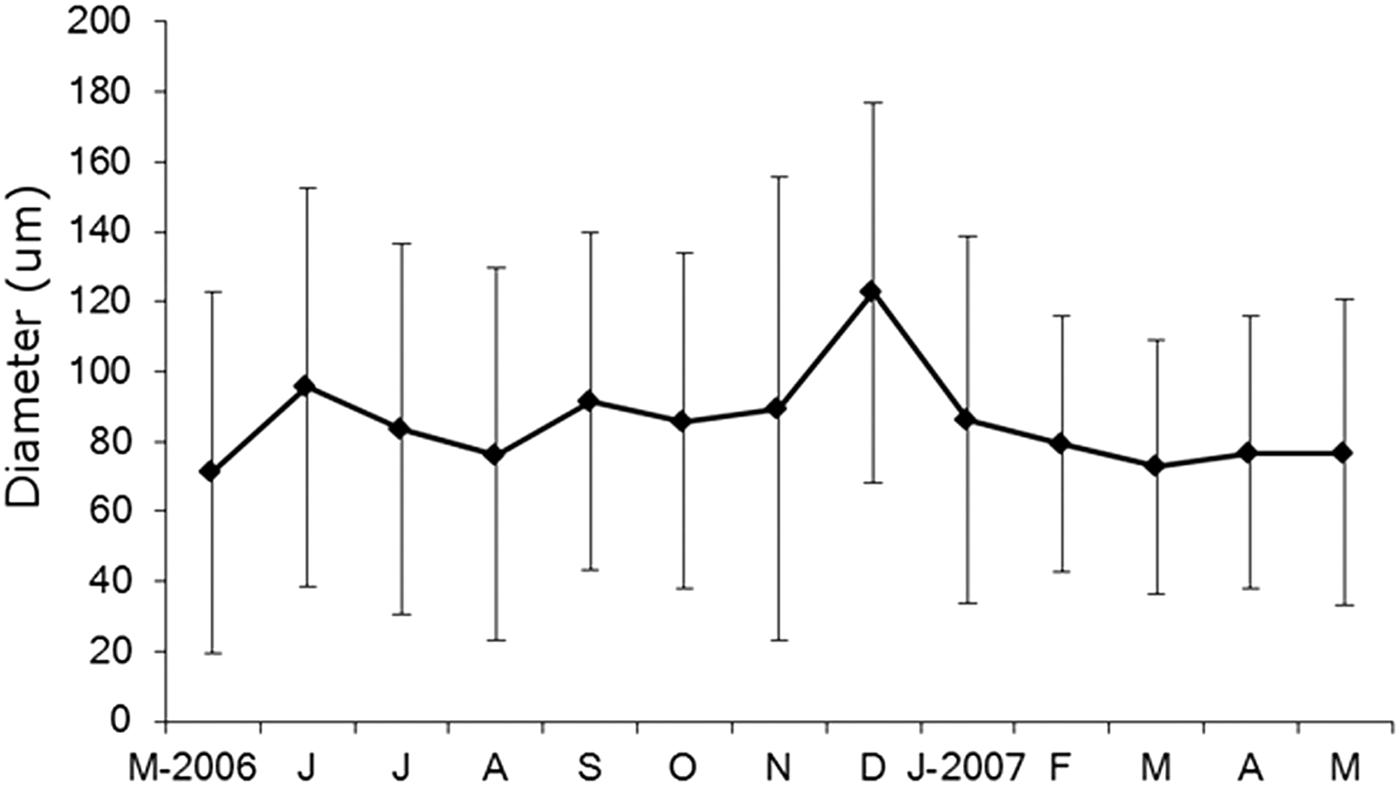
Oocyte development was not synchronized in the Itararé population, with individuals in different gonadal and vitellogenic phases and also some with no gametes. The analysis of the distribution of oocyte sizes of N. uspiana showed a wide range of oocyte sizes throughout the year (Figure 5), from less than 10 µm to greater than 240 µm (mean ± standard deviation: 85.2 ± 49.2 µm). The mean diameter was greatest in June 2006, September 2006 and December 2006, corresponding to the periods with fewer small oocytes (this pattern is clearest in December 2006). After each of these months, the mean diameter decreased and the number of small oocytes in the coelomic cavity increased.

Fig. 3. Nicolea uspiana: longitudinal histological section of the anterior region and first segments of the mature female showing the two pairs of nephridia and the mature oocytes (A). Details of the mature oocyte and large clumps of oocytes bulged into the coelom (B–D). bo, buccal organ; d, diaphragm; gc germinative cell; ln; left nephridia, en, nephromixia; no, notochaeta; o, mature oocyte; rn, right nephridia. Scale bars: A,D, 400 µm; C, 200 µm; B, 100 µm.

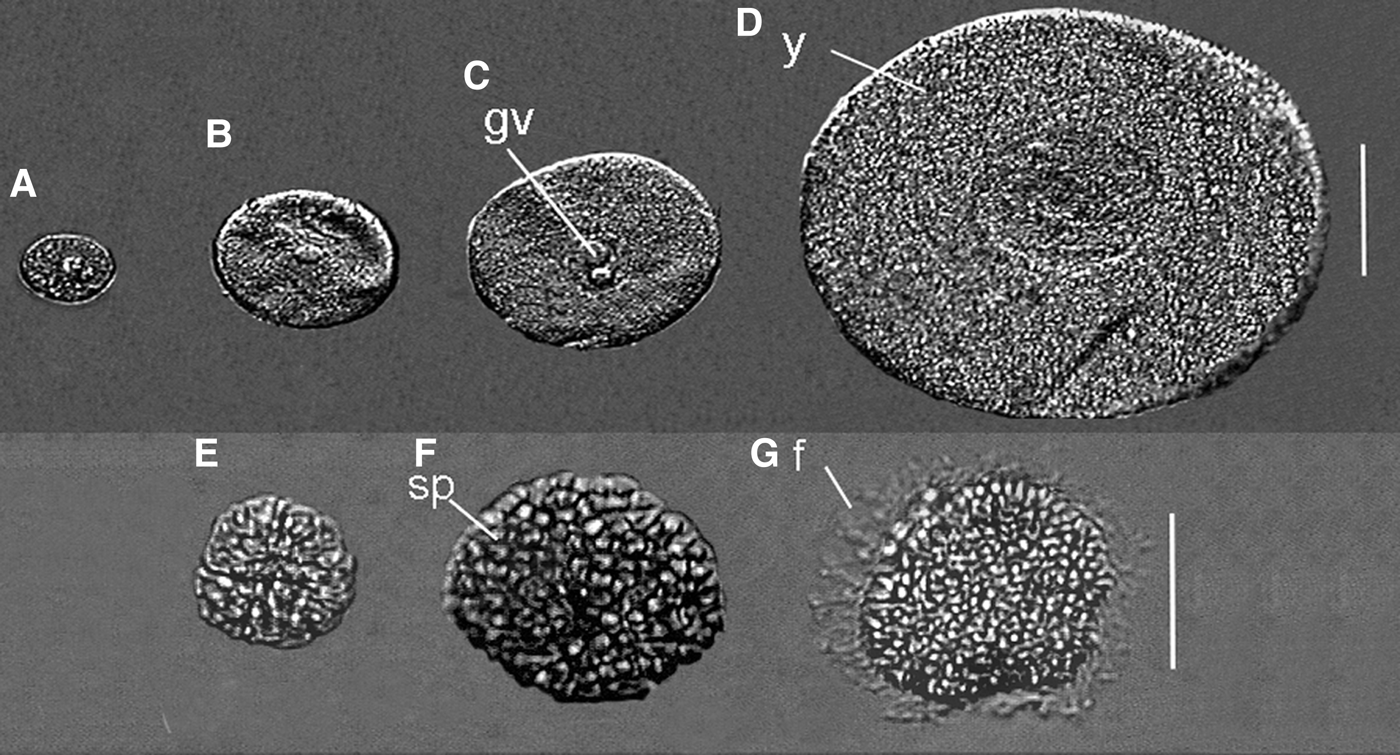
Fig. 4. Nicolea uspiana: distinct maturation stages of the oocytes (A–D) and spermatozoon (E–G). (A–C) immature oocyte; (D) mature oocyte; (E) spermatogonia; (F) spermatocyte; (G) spermatid. f, flagellum; gv, germinal vesicle; sp, spermatozoon; y, yolk. Scale bars: A–D, 50 µm; E–G, 30 µm.

Fig. 5. Nicolea uspiana: monthly size—frequency histograms of maximum oocyte diameter from May 2006 to May 2007. In each month, 50 oocytes were measured from each of the 10 females.
Generally, monthly samples had a bimodal pattern with many small oocytes and a small proportion of large (>200 µm in diameter) oocytes (Figure 6). These bimodal distributions occurred mainly within May, June, July, September and November of 2006 and January, February, March, April and May of 2007.
Spermatogenesis
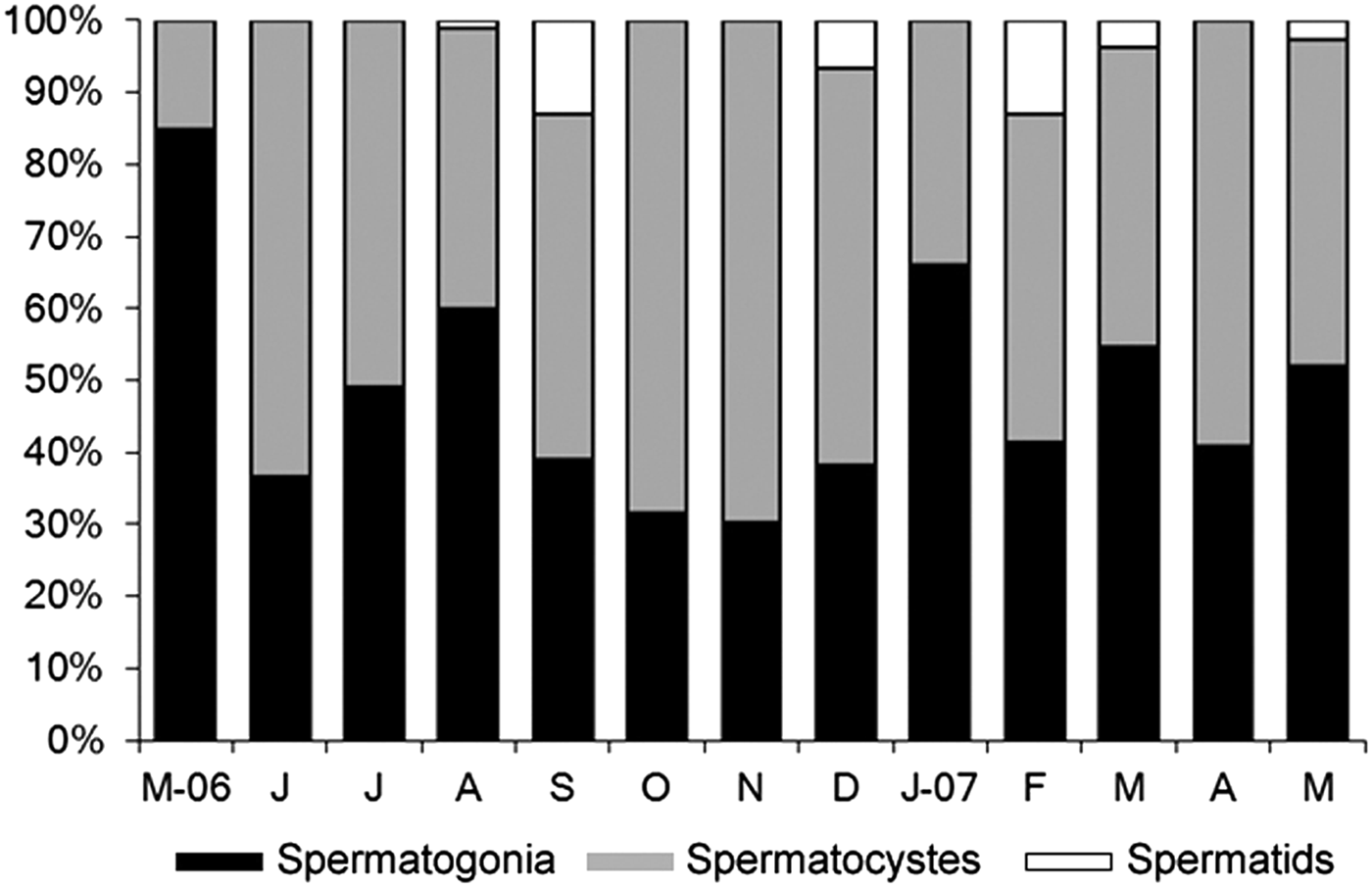
The histological characterization of the three reproductive stages of male specimens is shown in Figure 4. In the males examined in Itararé, the most common sperm stages were rosettes of spermatocytes (48.9%—Figures 4F, 7) and spheres of spermatogonia (48.1%—Figures 4E, 7). Morulae were much less common (3%—Figures 4G, 7).

Fig. 6. Nicolea uspiana: mean and standard deviation of egg sizes from May 2006 to May 2007.

Fig. 7. Nicolea uspiana: relative monthly frequency (%) of spermatogenic stages males into rosettes of spermatocytes, early spermatids, and morulae. In each month 100 spermatozoids were measured from 10 males.
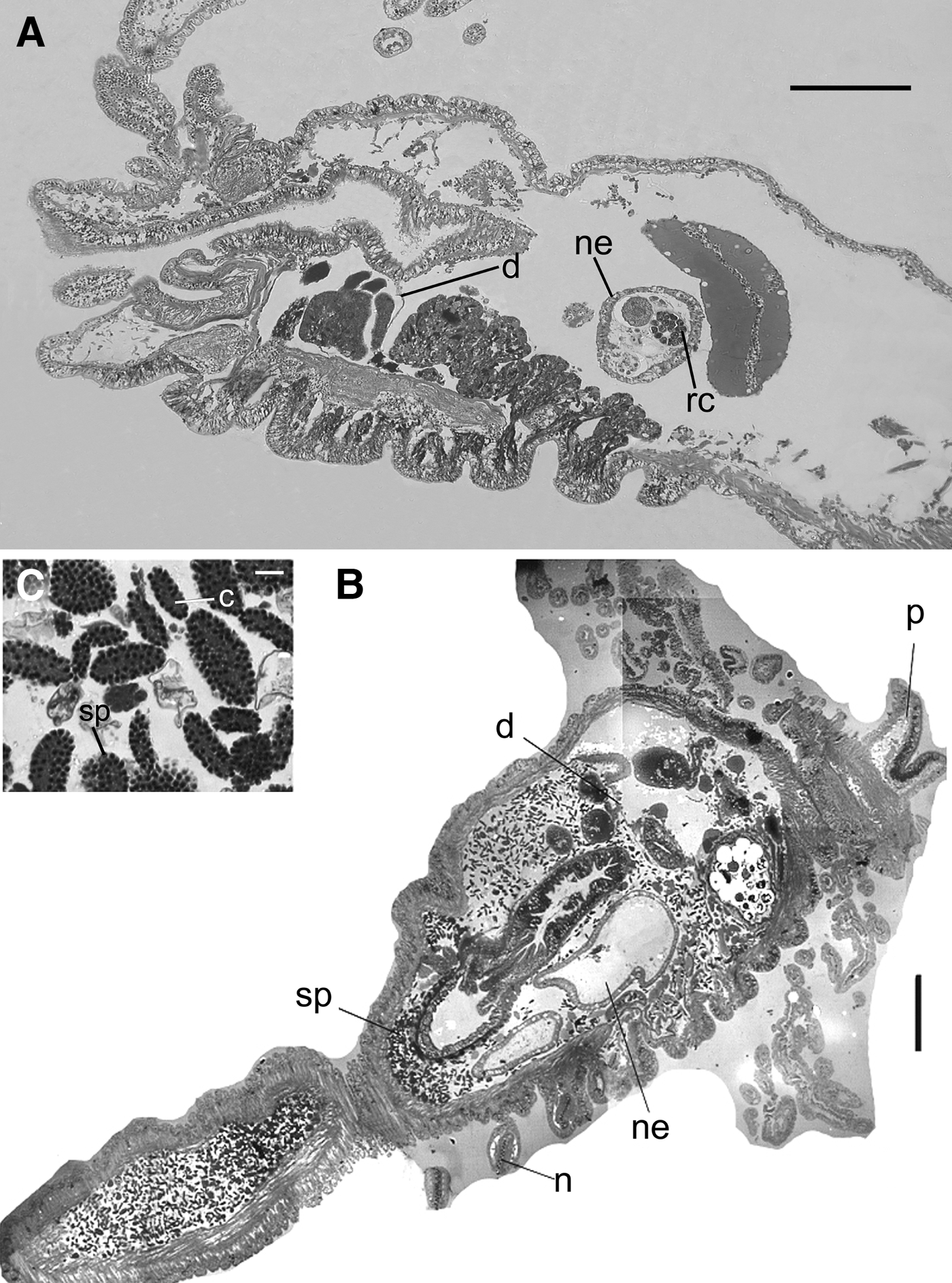
The spermatogonia are the initial stage of spermatozoon development and occur in the nephromixia structures (Figure 8A). After this stage, sperm platelets are found throughout the coelomic cavity as ovoid, flattened plaques of germinal cells. The anterior portion of each acrosome is positioned toward, and held together by, a cytoplasmic mass, the cytophore (Figure 4E, F). In these clusters, it is possible to observe distinct spermatozoon development stages as spheres of spermatogonia, rosettes of spermatocytes and morulae spermatids (Figure 4E, F). During this last stage, meiosis occurs and spermiogenesis results in elongate, tailed spermatids held together as morulae.

Fig. 8. Nicolea uspiana: transversal histological section of the anterior region of the mature male with cores in nephridial loop (A), anterior region showing the first pair of nephromixia (B), detail of the spermatogonia (C). c, cytophore; d, diaphragm; n, notopodia; ne, nephridia; p, prostomium; sp, spermatogonia; rc, residual core. Scale bars: A, 200 µm; B, 100 µm; C, 15 µm.
Without exception, males with non-motile sperm plates in the coelomic fluid had empty nephromixial ducts (Figure 8B, C). On the other hand, males in which the number of plates in the coelomic fluid was reduced or absent had nephromixial ducts filled with what appeared to be the ‘cores’ or cytophores of sperm plates.
Reproductive and developmental traits in Terebellidae
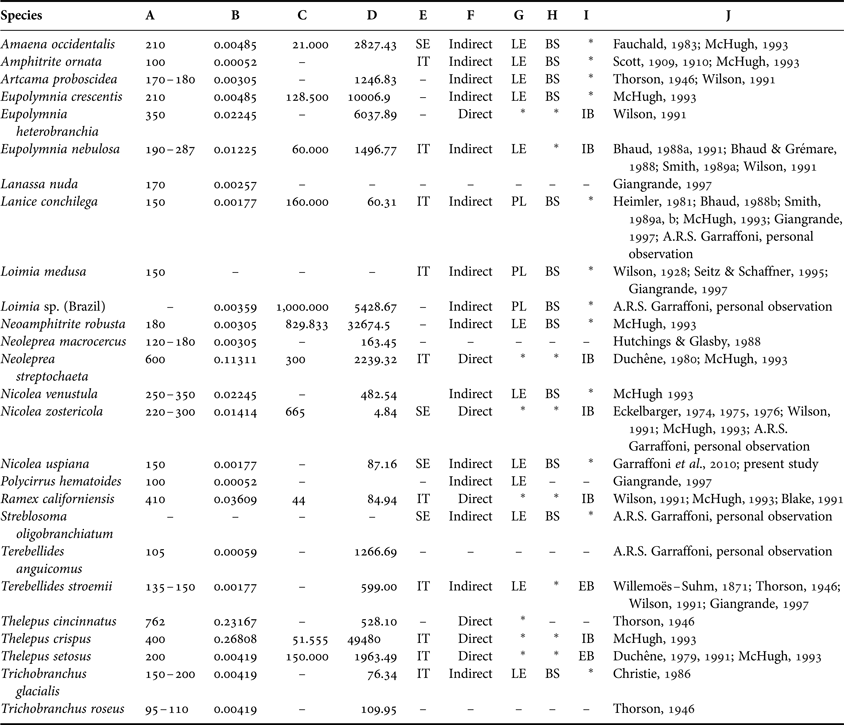
Table 1 summarizes the reproductive characteristics of 29 known terebellid species. Some species such as Artacama proboscidea, Eupolymnia crescentis, Amaena occidentalis and Amphitrite ornate are free spawning with lecithotrophic development. Lanice conchilega and Loimia medusa are free spawning with planktotrophic development. Nicolea zostericola deposits its gametes in gelatinous masses with direct development. On the contrary, Eupolymnia nebulosa deposits its gametes in gelatinous masses with lecithotrophic development, and Eupolymnia heterobranchia, Ramex californiensis, Thelepus crispus, Thelepus setosus, and Neoleprea streptochaeta brood inside the tube and show direct development. Regarding gametogenesis, only a few species have been as well studied as Nicolea zoostericola, Lanice conchilega and Eupolymnia nebulosa.
Table 1. Summary of reproductive characteristics available for the family Terebellidae. (-) missing information; (*) inapplicable information; A, maximum eggs diameter (mm); B, egg volume (calculated from maximum diameter (mm3); C, maximum fecundity; D, adult volume (mm3); E, breeding strategy; F, development type; G, larva type; H, sperm release; I, egg release; authors; BS, broadcast spawner; EB, extratubular brooder; IB, intratubular brooder; IT, iteroparous; LE, lecithotrophic; PL, planktotrophic; SE, semelparous.

DISCUSSION
Nicolea uspiana is one of the most abundant and widespread terebellid species on the south-eastern coast of Brazil with well-established populations occurring over a wide geographical range from the States of São Paulo to Paraná (Blankensteyn & Moreno, Reference Blankensteyn and Moreno1999; Nogueira, Reference Nogueira2003; Garraffoni & Amaral, Reference Garraffoni and Amaral2009; Santos et al., Reference Santos, Nogueira, Fukuda and Christoffersen2010).
Garraffoni & Amaral (Reference Garraffoni and Amaral2009) argued that N. uspiana has indirect development with a brief planktonic stage, characteristic of lecithotrophic larvae. This conclusion was based on the absence of intra- or extratubular brooding (an indication of direct development) and the presence of larvae with statocysts on the second segment (an indication of indirect development with planktonic larval stage). Moreover, N. uspiana can be considered an iteroparous species, as the mature individuals can breed several times during their lifetime (Garraffoni et al., Reference Garraffoni, Yokoyama and Amaral2010). This species exhibits extremely variable recruitment periods, with multiple spawning events and the settlement of new juveniles occurring in several different months (Garraffoni et al., Reference Garraffoni, Yokoyama and Amaral2010). Thus, because this species develops from relatively large eggs and has an abbreviated larval period without a feeding apparatus (Allen & Pernet, Reference Allen and Pernet2007), it can be assigned the reproductive and developmental traits of free spawning with lecithotrophic development.
Although individuals of N. uspiana have a maximum egg size (240 µm) close to that of other terebellid species (e.g. Eupolymnia crescentis—210 µm; Amaena occidentalis—210 µm; Thelepus crispus—220 µm; Nicolea zostericola—230 µm), maximum fecundity (i.e. maximum number of full-grown oocytes) differs among these species because of their different sizes. Nicolea uspiana has a smaller body length (ranges from 30 to 40 mm) than other species such as Amaena occidentalis (100 mm), Eupolymnia crescentis (130 mm) and Neoamphitrite robusta (250 mm). Thus, the reproductive output (percentage of total body volume given to a single brood or spawn) of individual N. uspiana will be lower than for individuals of other terebellid species.
Although N. uspiana and N. zostericola are in the same genus, oogenesis proceeds differently. Nicolea uspiana showed small cycles of oocyte production, with fluctuations on the oocyte diameter distributions over short periods of time. Eckelbarger (Reference Eckelharger1974, Reference Eckelharger1975) reported that N. zostericola is a semelparous species with annual growth cycles and with oogenesis divided into gonadal, follicular and vitellogenic phases. In the preliminary stages of oogenesis in N. zostericola, only small and flat oocytes (25–50 µm diameter) are present. During the follicular phase, the oocytes (50–75 µm diameter) are floating into the coelomic fluid. In the vitellogenic phase, the deposition of yolk increases and the diameter of the oocyte may reach a maximum of 300 µm (Eckelbarger, Reference Eckelharger1974, Reference Eckelharger1975). The sex-ratio of N. uspiana is distinctly different compared to Nicolea zostericola. Eckelbager (Reference Eckelharger1975) observed in the latter species that females comprised 44.8%, males 37.9% and juveniles 12.8%, with no significant difference in sex ratio, while here we determined that N. uspiana females comprised 19.3%, males 46.2%, and juveniles 34.6% with a significant difference in the sex-ratio.
In the classification system proposed by Rouse & Jamieson (Reference Rouse and Jamieson1987), there are three sperm types: (1) ect-aquasperm: spermatozoids are released into the water and fertilize similarly released eggs; (2) ent-aquasperm: spermatozoids are released freely into the ambient water but are gathered by or, in some other way, reach the female; and (3) introsperm: the spermatozoids have no contact with the water when passed from male to female (Rouse & Jamienson, Reference Rouse and Jamieson1987; Jamieson & Rouse, Reference Jamieson and Rouse1989; Rouse, Reference Rouse2005). The species studied in the present paper appears to fit the ect-aquasperm type (Thorson, Reference Thorson1946; Franzén, Reference Franzén1956; Eckelbarger, Reference Eckelharger1974, Reference Eckelharger1975; Jamieson & Rouse, Reference Jamieson and Rouse1989; Rouse & McHugh, Reference Rouse and McHugh1994; McHugh, Reference McHugh1995), as do most of the Terebellidae (N. zostericola, Ramex californiensis and Terebellides stroemii—only Streblosoma acymatum fits the ent-aquasperm type) because, this type of sperm is frequently correlated with species that have life history traits such as small egg size and external fertilization (Giangrande, Reference Giangrande1997). However, to confirm this hypothesis a sperm ultrastructure study needs to be conducted.
Comparative data of reproduction and development in terebellids
In terebellid species, male and female specimens are not easily distinguished by their external anatomy (Benham, Reference Benham1927). The only exception is the shape of the genital papillae or external gonopores, which are slender tubes in males and papillae in females (Benham, Reference Benham1927; Smith, Reference Smith1992, Reference Smith1994; present study). Regarding the position of the genital papillae, this structure can be found from segment 6 onward and can be located in three different positions: (a) dorsal to the notopodia; (b) aligned with the notopodia and posterior to them; and (c) inserted between the parapodial lobes (Nogueira et al., Reference Nogueira, Hutchings and Fukuda2010). The number of pairs of the external gonopores in the genera may vary from two in Terebellides, Lanicides, Nicolea, Pista, Thelepus and Streblosoma to three in Leaena, four in Amphitrite, eight in Terebella and nine in Neoamphitrite (Benham, Reference Benham1927; Smith, Reference Smith1992; Nogueira et al., Reference Nogueira, Hutchings and Fukuda2010; present study).
Regarding the internal anatomy in terebellid species, the body coelom is divided into two distinct parts by a septum or diaphragm: one anterior with excretory functions and nephridial papillae and a posterior one with reproductive functions and genital papillae (Rouse & Fauchald Reference Rouse and Fauchald1997; Zhadan & Tzetlin, Reference Zhadan and Tzetlin2002). The development and proliferation of the early stages of the gametes occurs only in the interior of the nephromixia, positioned posterior to the diaphragm, and gametes are then shed to the coelomic cavity where they are directly exposed to the coelomic fluid and can develop there for a long period (Eckelbarger, Reference Eckelharger1975; Smith, Reference Smith1992; McHugh, Reference McHugh1993). This type of gametogenesis was observed in N. uspiana, and is very similar to that reported by Smith (Reference Smith1989a, Reference Smithb, Reference Smith1992), Eckelbarger (Reference Eckelharger1975) and McHugh (Reference McHugh1993), who studied the terebellids L. conchilega, N. zostericola, Eupolymnia crescentis, Neamphitrite robusta, Ramex californiensis and Thelepus crispus.
Egg size is an important life history trait, as an indicator of the energy invested by the parental generation in its offspring (Giangrande et al., Reference Giangrande, Belmonte and Geraci1994; Giangrande, Reference Giangrande1997). This relationship between egg diameter and mode of development is well-established and delimited in marine invertebrates (Wray & Raff, Reference Wray and Raff1991; Jeffery & Emet, Reference Jeffery and Emlet2003). Thorson (Reference Thorson1950) and Schroeder & Hermans (Reference Schroeder, Hermans, Giese and Pearse1975) have reported that egg size is correlated with fecundity and development mode in several marine invertebrate groups, including polychaetes. Among terebellids, a huge range of egg diameter is observed (Table 1) from less than 100 μm in diameter (e.g. Polycirrus haematoides, Amphitrite ornata and Trichobranchus roseus) to larger than 762 μm in Thelepus cincinnatus (Fauchald, Reference Fauchald1983; Wilson, Reference Wilson1991; McHugh, Reference McHugh1993; Giangrande, Reference Giangrande1997). However, most of those terebellids have small eggs (15 species) with a planktonic larval phase, compared to a few species with large eggs (seven species) that develop directly into juveniles (Thorson, Reference Thorson1950; Schroeder & Hermans, Reference Schroeder, Hermans, Giese and Pearse1975). For this reason, Giangrande (Reference Giangrande1997) grouped the Terebellidae together with other families (e.g. Cirratulidae or Maldanidae) according to their different larval types as ‘coastal and deep benthic forms with a tendency for parental care and lecithotrophy’.
Regarding the terebellids that have small eggs and a planktonic larval phase, there are two types of larval development: lecithotrophic and planktotrophic, also called aulophore (Marcano & Bhaud, Reference Marcano and Bhaud1995; Garraffoni & Lana, Reference Garraffoni, Yokoyama and Amaral2010). The main difference between the lecithotrophic and planktotrophic larvae is that the former remain in the planktonic phase for a restricted period (a few hours to a week) and complete development without feeding because the larvae utilize the energy reserves of the yolk stored in the egg. On the other hand, aulophoric larvae (the only known larvae with a larval tube and a short planktonic feeding phase), even in the early stages of development when it has few segments, uses its ciliated tentacles to move to capture food particles. Moreover, this type of larvae is present in only two genera of the family Terebellidae, Lanice and Loimia (Bhaud, Reference Bhaud1888b; Smith, Reference Smith1989a; Marcano & Bhaud, Reference Marcano and Bhaud1995; Garraffoni & Lana, Reference Garraffoni and Lana2008, Reference Garraffoni, Yokoyama and Amaral2010), unlike what was reported by Strathmann (Reference Strathmann1993), and it appears as one of the shared putative exclusive synapomorphies between the two genera in the terebellid phylogenetic study of Garraffoni & Lana (Reference Garraffoni and Lana2008).
ACKNOWLEDGEMENTS
We thank three anonymous referees for offering suggestions that greatly improved the manuscript.
FINANCIAL SUPPORT
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for providing a postdoctoral fellowship (Process 05/59809-7) to the first author and FAEPEX/UNICAMP and CNPq (Process 308072/2006-5) for financial support for this Project.