INTRODUCTION
Marine scuticociliates are histophagous protozoa that cause parasitic diseases in farmed fish worldwide. New species of parasites and new aquatic hosts are being reported every year. Little is known about their route of entry, infection mechanisms and how they evade host immune responses. Uronema marinum and Miamiensis avidus are the most common pathogenic scuticociliates in farmed finfish such as turbot (Scophthalmus maximus) and flounder (Paralichthys olivaceus) (Iglesias et al. Reference Iglesias, Parama, Alvarez, Leiro, Fernandez and Sanmartin2001; Jee et al. Reference Jee, Kim and Park2001; Jung et al. Reference Jung, Kitamura, Song and Oh2007; Song et al. Reference Song, Sasaki, Okada, Sakashita, Kawakami, Matsuoka, Kang, Nakayama, Jung, Oh and Kitamura2009) and they have recently been associated with moribund groper (Polyprion oxygeneios) and kingfish (Seriola lalandi) (Smith et al. Reference Smith, Mcveagh, Hulston, Anderson and Gublin2009). Scuticociliatosis is characterized by the appearance of skin lesions due to the grazing of the histophagous parasites on the host's skin. Subsequently, parasites penetrate into the underlying muscle causing bleeding ulcers and gaining access to other internal organs. Among the clinical signs of this disease there is a high production of cutaneous mucus (Harikrishnan et al. Reference Harikrishnan, Balasundaram and Heo2010) and anaemia due to ingestion of red blood cells (Azad et al. Reference Azad, Al-Marzouk, James, Almatar and Al-Gharabally2007).
Acid phosphatases (AcP) are ubiquitous enzymes that hydrolyse phosphomonoesters and phosphoproteins at acidic pH. They are putative virulence factors in both bacteria and parasites (Baca et al. Reference Baca, Roman, Glew, Christner, Buhler, Adam and Aragon1993; Volety and Chu, Reference Volety and Chu1997; Aguirre-García and Okhuysen, Reference Aguirre-García and Okhuysen2007; Mohapatra et al. Reference Mohapatra, Balagopal, Shilpa, Schlesinger and Gunn2007; Escalona-Montaño et al. Reference Escalona-Montaño, Pardavé-Alejandre, Cervantes-Sarabia, García-López, Gutiérrez-Quiroz, Gutiérrez-Kobeh, Becker-Fauser and Aguirre-García2010). Acid phosphatase and other parasite proteases could act in concert to cause tissue degradation and to ease the passage of parasites through various tissue and matrix environments (Lonsdale-Eccles and Grab, Reference Lonsdale-Eccles and Grab2002). Acid phosphatases can be classified into type 1 and type 2, according to their cellular localization and substrate specificity. Moreover, their resistance to tartrate treatment further divides them into TRAPs (tartrate-resistant acid phosphatases) and TSAPs (tartrate-sensitive acid phosphatases). The optimal pH at which different AcPs function also varies among the different members of the AcP enzyme family. Tyrosine phosphorylation and dephosphorylation modulate various functions of the host–pathogen interactions and are regulated by the concerted action of protein tyrosine kinases and protein tyrosine phosphatases (PTPs) (Ghosh and Chakraborty, Reference Ghosh and Chakraborty2002). PTPs are used by different microbial pathogens to infect host cells and counteract host defences (Bliska and Black, Reference Bliska and Black1995; Hamid et al. Reference Hamid, Gustavsson, Andersson, Mcgee, Persson, Rudd and Fallman1999).
Parasites of aquatic hosts are known to possess a variety of proteases that may contribute to their pathogenicity. For instance, Perkinsus marinus, a parasite infecting American oyster, secretes acid phosphatases (Volety and Chu, Reference Volety and Chu1997). On the other hand, Philasterides dicentrarchi, a synonym of Miamiensis avidus, (M. avidus will be used in this study hereafter) infecting farmed turbot in Spain (Paramá et al. Reference Parama, Castro, Arranz, Sanmartin, Lamas and Leiro2007) as well as Tetrahymena spp. infecting farmed guppies (Leibowitz et al. Reference Leibowitz, Ofir, Golan-Goldhirsh and Zilberg2009) both have cysteine proteases. However, very little is known regarding the biochemical characteristics and cellular localization of AcPs in ciliated parasites. Additionally, how AcPs interact with different host's factors has not been studied to date.
The objective of this study was to characterize the acid phosphatases present in 3 species of marine scuticociliates that infect marine farmed fish in New Zealand and to investigate how their activity is modulated when encountering their host.
MATERIALS AND METHODS
Parasite isolates and in vitro culture
Miamiensis avidus strain A2 and Uronema marinum strain Pro-3 were obtained from the National Institute of Water and Atmospheric Research (NIWA, Wellington, New Zealand) ciliate collection. A description of the initial isolation, identification and preservation of both species can be found elsewhere (Anderson et al. Reference Anderson, Hulston, Mcveagh, Webb and Smith2009; Smith et al. Reference Smith, Mcveagh, Hulston, Anderson and Gublin2009). Parauronema virginianum was isolated from adult groper that had skin lesions typical of scuticociliatosis. DNA from groper skin scrapings (collected with sterile cell scrapers) was purified and the small subunit ribosomal RNA gene (SSU rRNA) was sequenced as explained elsewhere (Smith et al. Reference Smith, Mcveagh, Hulston, Anderson and Gublin2009). The obtained sequence was blasted using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) Parauronema virginianum being the closest match (99% homology, accession number AY392128).
Parasites were initially grown in 24-well plates containing 1 ml of filtered seawater (fsw) until enough numbers were achieved to be transferred into culture flasks. Flask cultures were fed with 5 μl of a groper tissue homogenate per ml of fsw. The tissue homogenate was obtained from liver, brain and kidney of healthy groper. A sample of 25 g of tissue was placed in 50 ml of complete L-15 medium (Gibco). Tissues were mashed and passed through a 100 μm cell strainer. After autoclaving, this solution was used to feed the parasite cultures once every 72 h. Unless otherwise stated, all ciliates were obtained from cultures 72 h post-feeding. All experiments were conducted with cultures that were maintained in vitro between 4 and 10 weeks.
Acid phosphatase activity: light and electron microscopy
For light microscopy studies, parasite suspensions were washed twice in PBS. Pellets were fixed in a 10% methanol, 60% acetone mixture in 0·03 m citrate buffer (pH 5·2) for 15 min at room temperature. After washing in distilled water, samples were stained for acid phosphatase using Naphthol AS-BI phosphoric acid solution diazotized fast garnet GBC (Sigma acid phosphatase procedure, Kit 387A). Negative controls contained no GBC. Samples were incubated in this solution for 1 h at room temperature followed by 3 washes in distilled water. Some samples were incubated with the complete staining solution to which L-tartrate had been added.
For ultrastructural localization of AcP, samples were processed using a modified Gomori procedure (Gomori, Reference Gomori1952) as explained elsewhere (Volety and Chu, Reference Volety and Chu1997). Briefly, parasite cultures were washed twice and centrifuged to pellet and fixed in cold 10% formaldehyde (4°C for 20 min). Following fixation, the cells were centrifuged and washed twice in distilled water (10 min each time) and transferred to the substrate medium containing acetate buffer (0·6% acetic acid (v/v) 300 ml (pH 5·0), 0·2 m sodium acetate 700 ml, distilled water 100 ml, lead nitrate (0·12 g), and 10 ml of 3% (w/v) b-glycerophosphate) for incubation (1 h at 37°C). Two control samples were run in parallel. One control treatment was incubated in the medium without the substrate, and the secondary control was incubated in the complete substrate medium with the addition of 200 μ m ammonium molybdate, a specific acid phospatase inhibitor. Following incubation, the cells were fixed in 2·5% (v/v) glutaraldehyde in freshly prepared PBS for 10 min at room temperature. The fixed parasite cells were then centrifuged to pellet and resuspended in PBS (3 times, 10 min) and then in 1% osmium tetroxide (w/v) in PBS for 2 h at 4°C. The fixed cells were then washed in PBS (3 times, 10 min), dehydrated in a graded series of ethanol (10–100%) through changes of propylene oxide. Post-fixed parasites were then embedded in Epon resin, sectioned and stained with uranyl acetate and lead citrate before being examined in a PHILIPS TECNAI 12 transmission-electron microscope. Acid phosphatase activity appears as dense black lead deposits.
Fish host samples
Skin mucus from 3 groper and 3 kingfish were obtained from adult individuals using a sterile cell scraper. Mucus was diluted 1:1 (v/v) in sterile PBS and stored at 4°C overnight. After vortexing, samples were filtered through a 0·22 μm nylon filter and protein concentration was calculated using the Bradford assay (Sigma). Mucus samples were adjusted to 1 mg total protein/ml and stored at −80°C until use.
Healthy groper (mean weight 1·5 kg) were anaesthetized and bled from the caudal vein. One ml of blood was placed in 7 ml of RPMI-1640 culture medium (Gibco) containing 100 IU/ml penicillin (Flow), 100 mg/ml streptomycin (Flow), 10 IU/ml heparin (Sigma). The suspension was carefully layered onto 51%–34% Percoll density gradients and centrifuged (30 min, 400 g, 4°C). Peripheral blood leucocytes (PBLs) were obtained from the buffy coat interphase whereas red blood cells (RBCs) were collected from the pellet. Cells were washed twice in RPMI medium and counted in a Neubauer chamber. Preliminary tests revealed that at the 20:1 cell per parasite ratio, all RBCs and PBLs were ingested by the parasites after 3 h (data not shown).
Experimental infections with Miamiensis avidus
In vitro infections consisted of skin explants obtained from healthy groper. Briefly, sections of skin and underlying muscle (mean weight 0·5 g) were dissected and surface-sterilized in 70% ethanol for 1 min followed by 2 washes in sterile PBS. In 24-well plates, the explants were placed in 1 ml of fsw containing 100 IU/ml penicillin (Flow) and 100 mg/ml streptomycin (Flow) and 3000 M. avidus cells per well. After 72 h, skin explants were removed and parasites were collected, washed extensively in PBS and adjusted to 2×106 cells/ml.
For in vivo experiments, 3 groper received 2×105 M. avidus cells by intramuscular injection near the dorsal fin. Two of the 3 fish became infected and developed skin lesions 7 days post-injection. Fish lost appetite and the lesions became more extensive showing a strong inflammatory response and reddening 30 days post-injection. At this point, fish were euthanized and M. avidus was recovered from skin explants and placed in 24-well plates as detailed above. The next day the tissue was removed and the parasites were recovered from the bottom of the wells. Following extensive washing they were transferred to culture flasks containing 5 ml of fsw without any food supplement and they were collected 5 days later, when host cells had been depleted.
Acid phosphatase in gel assays
Parasites grown in culture flasks were collected, washed twice in PBS and adjusted to 2×106 cells/ml. Lysates were obtained by sonication (4 cycles, 30 sec/cycle, 10% amplitude). Lysates were stored at −80°C until used. Protein samples were re-suspended in Laemmli buffer (Laemmli, Reference Laemmli1970) without 2-mercaptoethanol and run in native polyacrylamide gels (4%–10%) at 200 V. Positive controls consisted of 0·4 mU of potato acid phosphatase (Sigma) in PBS. After rinsing in double-distilled water, gels were incubated for 2 h in acetate buffer (0·5 m, pH 5·2) containing 1 mg/ml of naphthyl alpha phosphate (Sigma) and 1 mg/ml GBC (Sigma). A second gel was developed in the same solution to which L-tartrate had been added (Sigma procedure, Kit 387A). Reaction was stopped by rinsing gels in double-distilled water. Gels were immediately scanned at 300 days p.i.
Acid phosphatase plate assay and effect of inhibitors
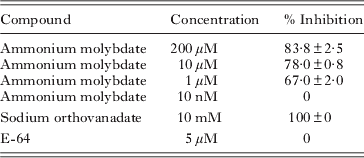
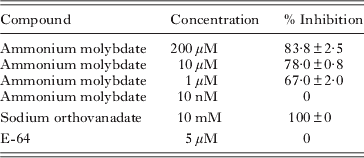
Parasites were collected from culture flasks, washed twice in PBS and adjusted to 105 parasites/ml. Fifty μl of the suspension was pippeted onto black flat-bottomed 96-well plates. The effect of ammonium molybdate, sodium orthovanadate (specific inhibitors of tyrosine protein phosphatases) and E-64 (a specific inhibitor of cysteine proteases) on AcP activity was studied. All inhibitors were obtained from Sigma. Fifty μl of ammonium molybdate (200 μ m, 10 μ m, 1 μ m and 10 nm), sodium orthovanadate (10 mm) or E-64 (5 μ m) were added to the wells containing the parasite suspensions.
Then 50 μl of PBS, groper or kingfish mucus (adjusted to 1 mg/ml of total protein in PBS) were added to the wells in triplicate. Additionally, freshly isolated groper RBCs and PBLs were added to some of the wells containing the ciliate suspensions at a ratio of 20:1 groper cells per parasite. Plates were incubated for 30 min at 18°C.
Acid phosphatase activity was measured fluorometrically using 4-methylumbelliferyl phosphate (Fluka) as the substrate. AcP hydrolyses this compound, releasing fluorescent methylumbelliferone. Thus 100 μl of 0·5 m sodium acetate buffer (pH 5·2) and 4-methylumbelliferyl phosphate were added to each well. A standard curve using methylumbelliferone (MUF) dilutions ranging from 20 nmol to 0 nmol was used to calculate the amount of fluorescence released per min. Plates were read at 18°C for 4 h in a Modulus microplate fluorometer (Turner Biosystems) (excitation, 375 nm; emission, 455 nm).
Each experiment was conducted 3 independent times (n=3). Results are expressed as mean AcP (nmol/min/104 parasites)±standard error.
Immunofluorescence and flow cytometry
Immunofluorescence and flow cytometry assays were carried out using the mouse monoclonal antibody anti-human placenta PTP1b (Merck KGaA, Darmstadt, Germany). For IF, parasite suspensions were washed twice in fsw and layered over glass slides. After air drying, slides were fixed for 10 min with ice cold 100% methanol or for 3 min with 4% paraformadehyde (PFA) for antibody-labelling tests. Best results were achieved with PFA and therefore it was used thereafter for all experiments. Slides were blocked with Starter Protein Blocker (Pierce) for 15 min and incubated with 0·2 μg/ml of mouse anti-human PTP1b monoclonal antibody in PBS containing 0·1% BSA and 0·1% Triton X-100 (PBT) (overnight, 4°C). After washing, the secondary antibody Cy3-conjugated donkey anti-mouse IgG (Jackson Scientific, USA) was added (2 h, room temperature). Prior to mounting, nuclei were stained with Hoescht dye (Invitrogen). Stained samples were observed under an Olympus BX51 fluorescence microscope coupled to an Olympus digital camera DP70 and analySIS LS Research software. Negative controls consisting of slides with either no primary antibody or an isotype control (mouse anti-human IgG2a) were included.
For flow cytometry, we compared M. avidus suspensions from in vitro cultures (used for the experimental infection) and M. avidus isolated from skin lesions of infected groper. Samples of 105 parasites (in triplicate) were PFA-fixed and stained with 0·2 μg/ml of anti-human PTP1b Mab or the same amount of the isotype control in PBT. The secondary antibody was an FITC-conjugated goat anti-mouse IgG (Jackson Scientific, USA). Samples were analysed in a FACScan flow cytometer (Becton Dickinson). A total of 20 000 events were collected and M. avidus cells were gated in order to record only the PTP1b-positive parasites.
Statistical analysis
Data were analysed using Statistica 8.0 (StatSoft, Inc., USA). ANOVA and Tukey HSD post-hoc tests were conducted when necessary. Differences were statistically significant when P<0·001.
RESULTS
Identification of AcP in marine scuticociliates by light and electron microscopy
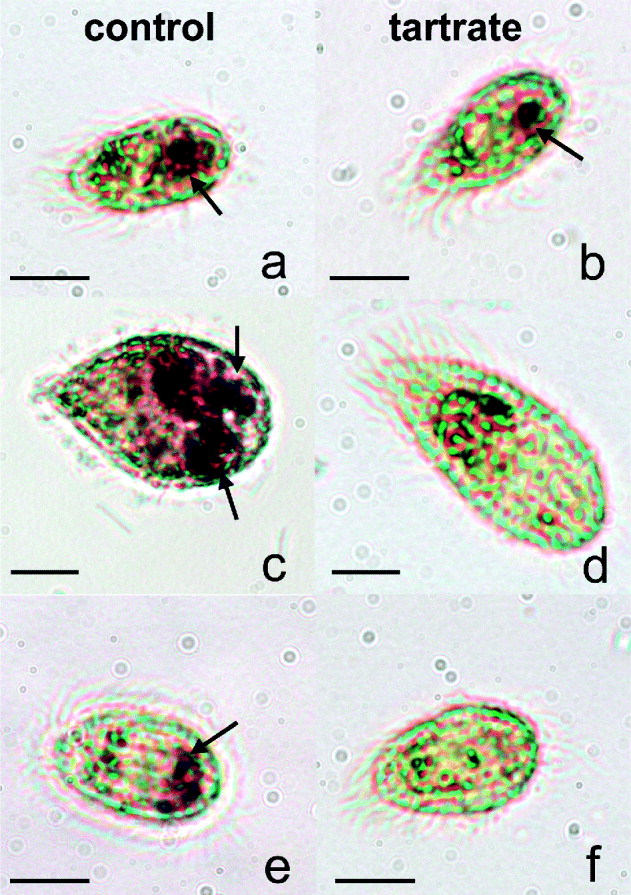
We first identified the presence of AcP in the 3 scuticociliate species by light microscopy. Figure 1a shows the differential distribution of the AcP activity in U. marinum, M. avidus and P. virginianum. Whereas in U. marinum and P. virginianum AcP was mainly localized at the posterior end of the cell (Fig. 1a and e), M. avidus possessed AcP-positive vacuoles that occupied up to two thirds of the cell (Fig. 1c). The addition of L-tartrate resulted in the inhibition of AcP brown deposits in M. avidus and P. virginianum (Fig. 1d–f). However, in the presence of L-tartrate U. marinum retained 1 AcP-positive circular vesicle at the posterior end (Fig. 1b).
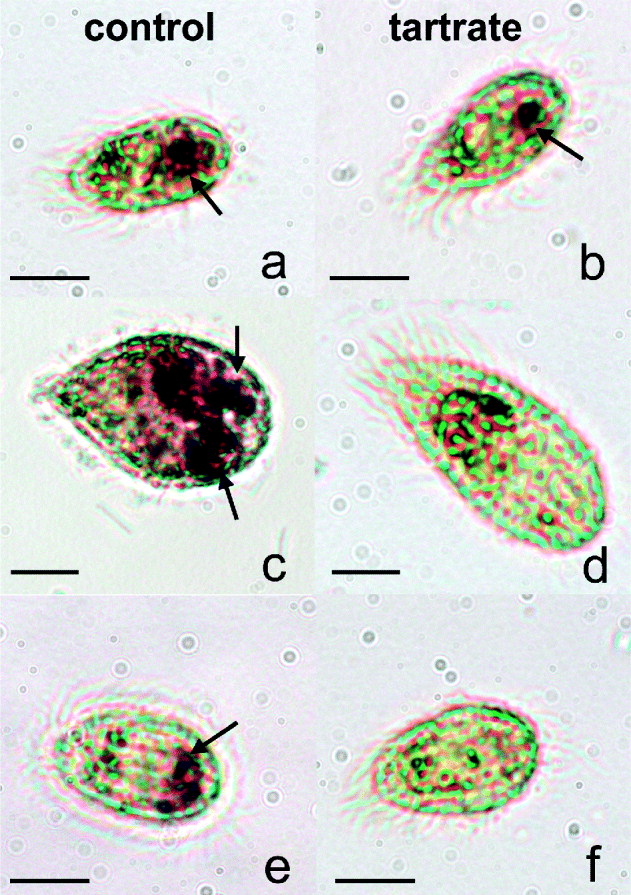
Fig. 1. Cytochemical localization of acid phosphatase (AcP) from scuticociliate species cultured in vitro. Uronema marinum (a), Miamiensis avidus (c), Parauronema virginianum (e). L-tartrate treatment showed that U. marinum had some tartrate-resistant AcP (b) whereas the activity was inhibited in M. avidus (d) and P. virginianum (f). Arrows indicate AcP-positive areas.
The ultrastructural distribution of AcPs was studied by transmission electron microscopy. Whilst none or very little reaction was observed in the control M. avidus and P. virginianum cells (Fig. 2a and b), some electron-dense deposits of lead phosphate indicative of acid phosphate activity were found in U. marinum. Small vesicles located in the peripheral region of the cytoplasm and food vacuoles located throughout the cytoplasm had discrete dotted granules of AcP activity (Fig. 2c). No lead nitrate deposits were detected in other organelles, e.g. endoplastic reticulum or mitochondria. No precipitation of lead nitrate was detected in either control sample, i.e. control without substrate and control with substrate and inhibitor (not shown).

Fig. 2. Ultrastructure of scuticociliate AcP stained in Gomori medium (seen as electron-dense lead nitrate deposits) in Miamiensis avidus control cultures (a), Parauronema virginianum control cultures (b), Uronema marinum control cultures (c). Ultrastructure of AcP in U. marinum following incubation with groper skin mucus (d). Ultrastructure of AcP in M. avidus following ingestion of groper peripheral blood leucocytes (e and f). M, macronucleus; Ci, cilia; pm, plasma membrane; FV, food vacuole; V, vesicle; ba, buccal apparatus. Arrows indicate electron-dense deposits of AcP activity.
AcP in-gel assays
The study of AcP at the protein level by using native gels further confirmed that all 3 scuticociliate species possess AcP activity. In-gel assays showed that AcPs are nevertheless very different in each species. Miamiensis avidus has only 1 isoform with AcP activity at pH 5·2. The band appeared just under the potato AcP-positive control, which has a molecular weight of 69 kDa. We consider this AcP to be high molecular weight in order to differentiate it from those found in the other species. When M. avidus was exposed to groper skin explants for 72 h, a darker band appeared in the same position as that found in the lysates from cultures grown in flasks (Fig. 3a). No additional activity bands were observed. In the presence of L-tartrate, M. avidus AcP was absent (Fig. 3b).

Fig. 3. AcP activity bands from native polyacrylamide gels of scucticociliate lysates developed with control substrate (a) or control substrate with L-tartrate added (b). Lane 1. Miamiensis avidus after exposure to groper skin explants for 72 h; Lane 2. M. avidus in vitro culture; Lane 3. Parauronema virginianum in vitro culture; Lane 4. Uronema marinum in vitro culture; Lane 5. Positive control, potato AcP 0·4 mU. Diamond arrows indicate a low molecular weight isoform present in P. virginianum. Arrow heads indicate the low molecular weight isoforms identified in U. marinum.
U. marinum had 2 clear AcP isoforms (Fig. 3a) of much lower molecular weight than that observed in M. avidus. L-tartrate treatment reduced but did not eliminate the presence of these two activity bands. A possible third faint band, also with low molecular weight, could be seen above the other two and it was no longer visible following L-tartrate treatment (Fig. 3b).
Finally, P. virginianum lysates possessed 1 single low molecular weight AcP isoform (Fig. 3a). This AcP was resistant to L-tartrate (Fig. 3b).
Plate assays and effect of inhibitors
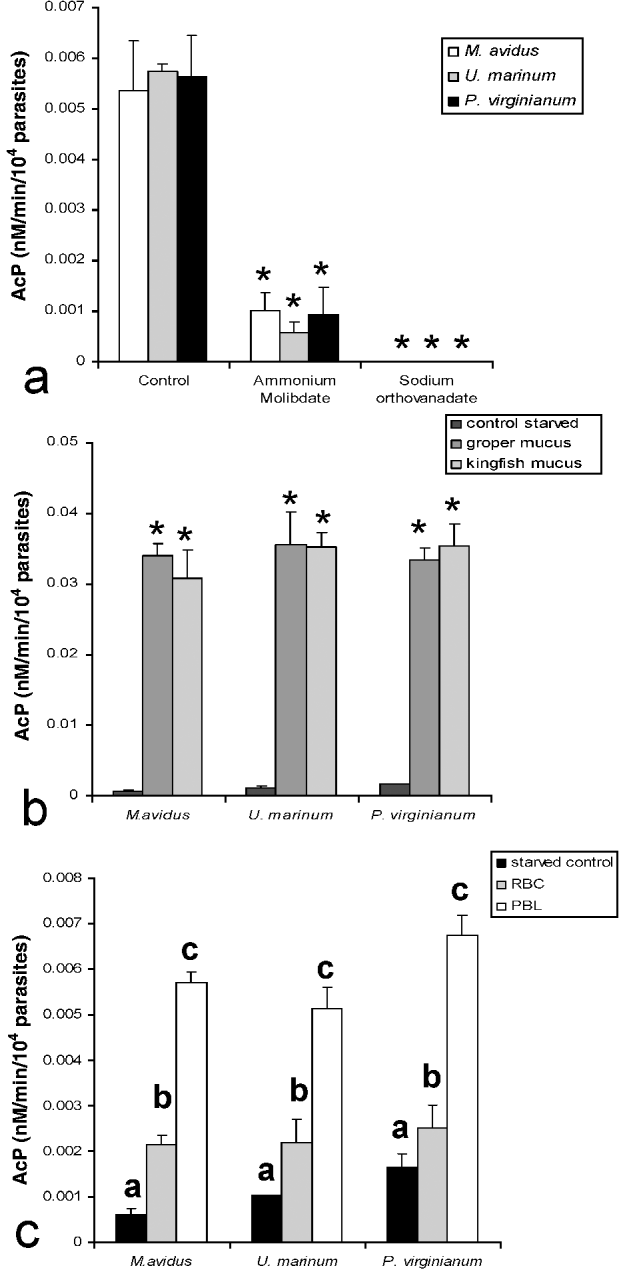
Plate assays showed that all 3 species have similar total AcP contents (Fig. 4a). Table 1 summarizes the effect of the 3 inhibitors tested on the total AcP activity of M. avidus. Ammonium molybdate inhibited AcP in a dose-dependent manner. E-64, in turn, did not inhibit M. avidus AcP. In order to corroborate these results, we measured total AcP following incubation with ammonium molybdate (200 μ m) or sodium orthovanadate (10 mm) in the 2 other scuticociliate species. Both protein-tyrosine phosphatase inhibitors markedly decreased AcP activity in all 3 parasite species (P<0·001) (Fig. 4a).

Fig. 4. (a) Quantification of AcP activity from whole scuticociliate cells cultured in vitro and AcP sensitivity to protein tyrosine phospahatase inhibitors, ammonium molybdate (200 μ m) and sodium orthovanadate (10 mm). (b) Effects of starvation and re-exposure to fish skin mucus on scuticociliate AcP. (c) Modulation of scuticociliate AcP following ingestion of groper RBCs or PBLs. AcP activity is expressed as nmol/min/104 parasites. Asterisks denote statistically significant differences compared to the control group. Different letters indicate significant differences among groups; P<0·001. Results are representive of 3 independent experiments.
Table 1. Effect of inhibitors on Miamiensis avidus acid phosphatase activity
Host factors influencing AcP activity in scuticociliate parasites
In this experiment, we aimed to evaluate whether AcP re-activation occurs after a starvation period of 7 days. We exposed parasites to either groper or kingfish skin mucus for 30 min and then measured AcP activity via 4 h MUF plate assays. As shown in Fig. 4b, all 3 scuticociliates dramatically increased their AcP activity both in the presence of groper and kingfish skin mucus. Control wells containing skin mucus only had no detectable AcP activity (data not shown).
We further investigated this activation by TEM using only U. marinum exposed to groper mucus. An increment in the size and intensity of lead phosphate deposits was observed (Fig. 2d). Greater numbers of AcP-positive small vesicles were found in the periphery compared to control cultures. Additionally, larger and denser deposits were seen within food vacuoles in the cytoplasm. The plasma membrane and cilia had no AcP activity.
In order to investigate the modulation of total AcP activity by host cells we challenged 7-day starved parasites to either RBCs or PBLs (1:20) readily isolated from healthy groper. By the end of the plate assay, all target cells had been consumed. RBCs or PBLs alone had no measurable AcP activity in these plate assays. Results revealed a similar trend in all 3 species. Ingestion of RBCs resulted in a 1·5 to 3·5-fold increase in the total AcP depending on the species (P<0·001). In the presence of PBLs, scuticociliate AcP activity was significantly higher than in controls and RBC group with ratios ranging between 4 and 9·6 (P<0·001) (Fig. 4c). Control wells containing PBLs or RBCs only had no detectable AcP activity (data not shown).
We also evaluated whether the increased AcP activity induced by host cells could be explained by a protein-tyrosine phosphatase by adding specific inhibitors to the control, RBC or PBL treatments. The increase in AcP activity observed in Fig. 4c was abrogated by the inhibitors ammonium molybdate and sodium orthovanadate (not shown).
The ultrastructure of M. avidus incubated with groper PBLs (Fig. 2e) showed an important increment in the amount of AcP deposits compared to controls (Fig. 2a). Food vacuoles had dense deposits of AcP activity or small discrete vesicles close to the vacuole periphery (Fig. 2f). More importantly, a product reaction appeared on the plasma membrane and, in many cases, it co-localized with the buccal apparatus of this species (Fig. 2e). In some membrane areas, AcP appeared to be secreted to the surrounding medium. Moreover, cilia also had AcP deposits on them (Fig. 2f) not observed in controls (Fig. 2a). No precipitation of lead nitrate was detected in either control sample, i.e. control without substrate and control with substrate and inhibitor.
Miamiensis avidus has a PTP1b homologue: in vitro activation by host cells
All our previous data show the presence of protein tyrosine phosphatases and the activation of a membrane form in M. avidus following ingestion of host PBLs. We further investigated this hypothesis by studying the one particular protein tyrosine phosphatase PTP1b in M. avidus.
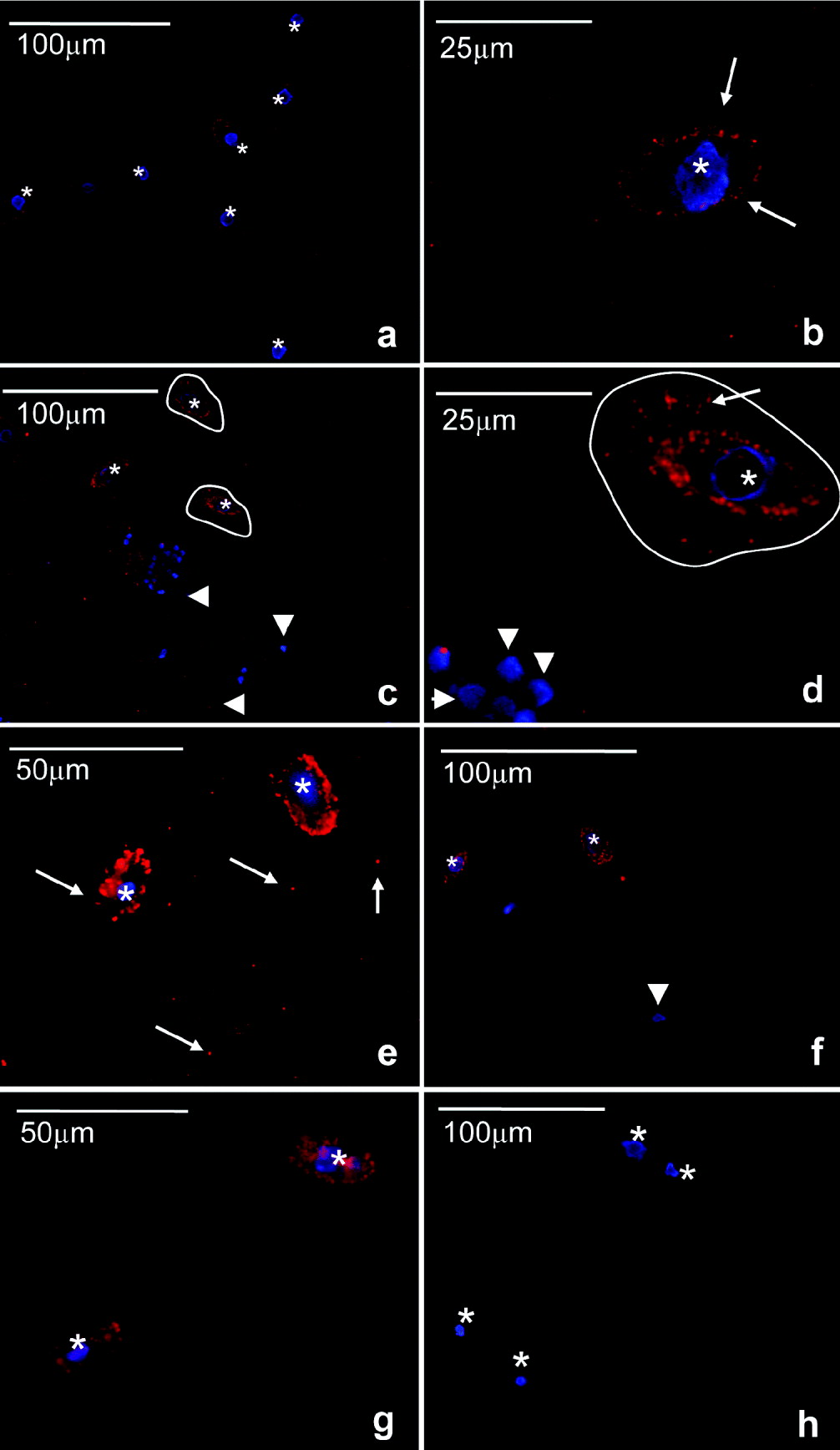
Miamiensis avidus control cultures were positively stained with the anti-human PTP1b antibody showing a typical punctuated but not very intense staining on the cellular membrane (Fig. 5a and b). Samples incubated with the isotype control did not show such stain although some faint background was observed in the cytoplasm (Fig. 5h). In the presence of groper PBLs and after 30 min of incubation, the staining became brighter and thicker on the parasite membrane compared to controls (Fig. 5c). Morevover, some M. avidus cells had a ‘corolla’ of positive staining in the immediate surrounding medium (Fig. 5d). At this point, the parasites had commenced ingestion of PBLs but the majority of the cells were still seen externally. Discrete staining of groper PBLs was observed at this point (arrowhead Fig. 5d). After 3 h, all cells were consumed and M. avidus displayed the brightest staining on the membrane (Fig. 5e). After both 30 min and 3 h, some positive staining appeared in the cytoplasm but it was never as intense as that observed in the membrane.
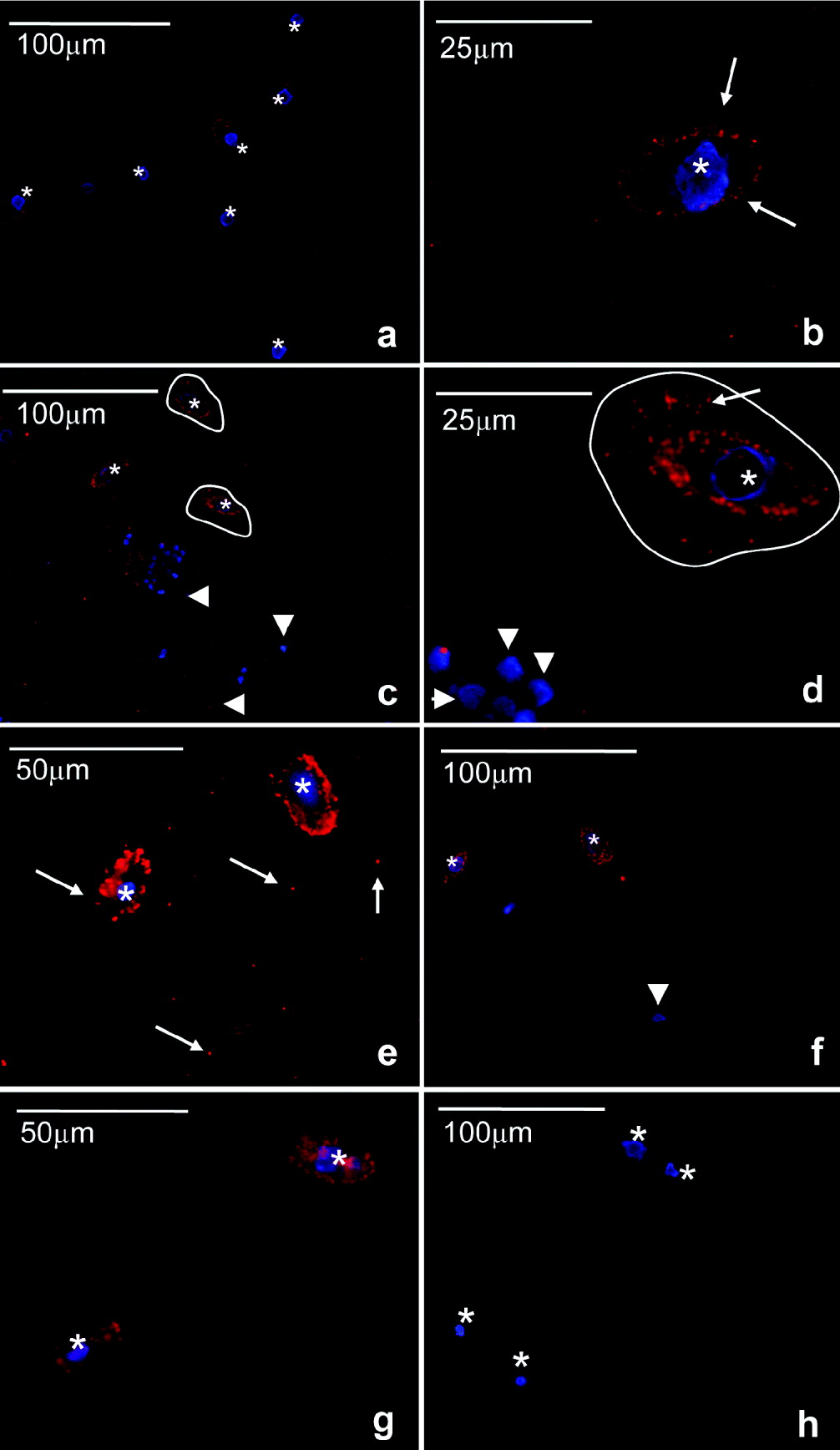
Fig. 5. Modulation of scuticociliate PTP1b by host's cells. Immunofluorescence studies of Miamiensis avidus using anti-human PTP1b antibody (red). Nuclei (blue) are stained with the DNA intercalating dye Hoechst. Control M. avidus culture (a,b); incubated for 30 min with groper PBLs (c,d); incubated for 3 h with groper PBLs (e); incubated for 30 min with groper RBCs (f); incubated for 3 h with groper RBCs (g), isotype control (h). Arrows indicate positive PTP1b staining. Arrowheads indicate groper PBL or RBC nuclei stained in blue. Asterisks denote M. avidus nuclei stained in blue. Circled lines show the area surrounding M. avidus with PTP1b activity. Results are representative of 4 independent experiments.
The same experiment was conducted with RBCs. As shown in Fig. 5f, when M. avidus was exposed to groper RBCs for 30 min, the cytoplasmic staining of some parasites increased compared to controls (Fig. 5a) but intense staining of the plasma membrane like that observed in the presence of PBLs did not occur. Moreover, the appearance of PTP1b-positive areas surrounding the parasite plasma membrane and forming a ‘corolla’ as described for the PBL treatment (Fig. 5c and d, circled lines) was not detected. After 3 h, when all the RBCs were consumed by the parasite, the staining pattern was very similar, the cytoplasm being positive compared to the controls but the membrane clearly less intensely stained than in the PBL group (Fig. 5g). The nuclei of the groper cells and the parasite cells were stained in blue with the Hoescht dye. Groper PBLs and RBCs nuclei were rounded and had a typical staining pattern. In M. avidus, the spherical or slightly elliptical macronucleus (approximate diameter 8μm) was located in the centre of the cell. The blue staining sometimes appeared as a ring rather than a full sphere. The micronucleus was rarely visible.
PTP1b is activated in M. avidus during in vivo infection
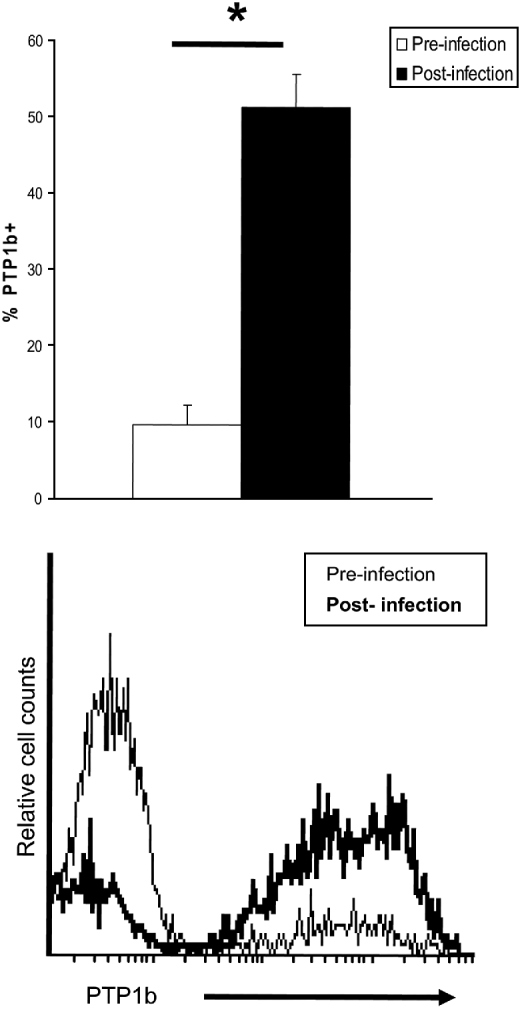
The role of PTP1b during in vivo infections was studied by flow cytometry. In vitro M. avidus suspensions used to infect the fish consisted of 9·8±2·4% PTP1b+ cells. When we recovered parasites from skin lesions of experimentally infected fish, we found that 51·5±4·3% of the cells were positively stained for PTP1b (P<0·01) (Fig. 6).
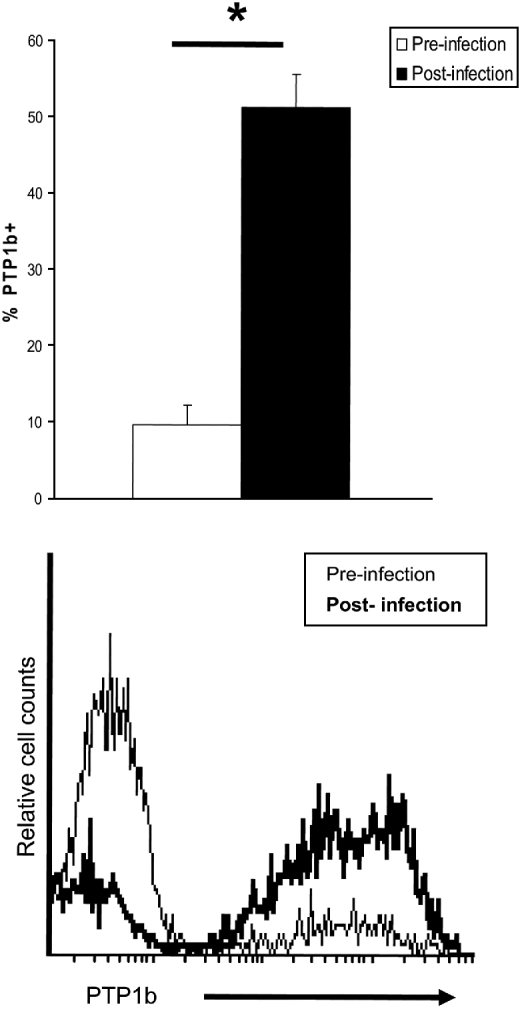
Fig. 6. Miamiensis avidus PTP1b is activated in vivo during infection. Flow cytometry studies on M. avidus PTP1b prior to infection (in vitro culture) and during the course of infection (isolated from groper skin lesion) (n=5). Histogram shows the relative number of PTP1b-positive M. avidus prior to infection (thin line) and after infection (bold line). Negative controls consisted of M. avidus stained with a mouse IgG2a (isotype control). Asterisk denotes statistically significant differences between groups; P<0·001.
DISCUSSION
Marine scuticociliates are facultative extracellular parasites that cause mortality in farmed fish worldwide. These ciliated protozoans are ubiquitous in aquatic environments and they initiate infection. Acid phosphatases are not only virulence factors in a number of microorganisms including bacteria and parasites but they are also critical in many biological processes such as general protein catabolism, cell division and life stage differentiation (Szöor et al. Reference Szöor, Wilson, Mcelhinney, Tabernero and Matthews2006).
Some studies have reported the presence of proteinases in marine scuticociliates (Paramá et al. Reference Paramá, Iglesias, Alvarez, Leiro, Ubeira and Sanmartín2004; Marzouk and Azaid, Reference Marzouk and Azad2007). Moreover, AcP activity was observed in the vicinity of young Ichthyophthirius multifiliis trophonts, a ciliate that causes white spot disease in many fish species (Matthews, Reference Matthews2005). More recently, the attenuation of Tetrahymena spp. and its AcP by long-term in vitro culture was shown to decrease this parasite's pathogenicity in farmed guppies (Leibowitz et al. Reference Leibowitz, Ofir, Golan-Goldhirsh and Zilberg2009). Since nutrients such as nucleotides and co-factors are mostly phosphorylated in the host cell cytosol they need to be dephosphorylated by the parasite. Thus, AcPs may facilitate the lysis of host tissue in the pathogenic process.
The first aim of this study was to characterize the AcP isoforms, from 3 different genera of scuticociliates. Strinkingly, we found great diversity in the AcP activity at the protein level, their resistance to tartrate and their cellular distribution in each of the species studied. Humans possess 9 AcP isoforms, isoform 5 being tartrate resistant which, when found in elevated levels, is used as a marker for prostate cancer. In parasites such as Leishmania donovani 2 distinct acid phosphatases are present; a tartrate-resistant enzyme localized to the external surface of the plasma membrane, and a tartrate-sensitive enzyme that is secreted into the growth medium (Saha et al. Reference Saha, Das, Robert, Glew and Gottlieb1985). M. avidus contained only a high molecular tartrate-sensitive AcP that was present in the membrane organelles and occupied most of the parasite cytoplasm. On the other hand, U. marinum possessed only low molecular weight AcPs, 2 of them tartrate-resistant forms. Electrophoresis results were confirmed by histochemistry under light microscopy. Parauronema virginianum is a species that has never been associated with marine fish mortalities in the past. We first isolated it from moribund adult groper maintained in sea cages. Interestingly, this species showed only 1 faint AcP activity band very similar to one of the bands observed in U. marinum. It is worth noting that Uronema spp. and Parauronema spp. are phylogenetically closer than Miamiensis spp. From our results we can conclude that marine scuticociliate parasites may have differential pathogenesis and mechanisms of fish tissue destruction as reflected by the diversity of biochemical and cellular features of their AcPs.
All the ciliate AcPs that we found were inhibited by specific inhibitors of tyrosine protein phosphatases but not by cysteine protease inhibitors. In this assay, we used intact scuticociliate cells to measure total AcP. Similarly, live Trypanosoma cruzi trypomastigotes and amastigotes possess ecto-protein tyrosine phosphatase activity (Furuya et al. Reference Furuya, Zhong, Meyer-Fernandes, Lu, Moreno and Docampo1998). Vanadate derivatives are effective in controlling visceral and cutaneous leishmaniasis (Olivier et al. Reference Olivier, Romero-Gallo, Matte, Blanchette, Posner, Tremblay and Faure1998). Therefore, our results suggest that PTP inhibitors represent a novel target for therapeutic intervention against scuticociliatosis in fish.
The next purpose of this work was to investigate the activation of scuticociliate AcPs by different host's products (fluids and cells). It is believed that scuticociliates first start grazing on fish skin and therefore skin mucus is the first substance that they encounter. Here we have proven rapid activation of total AcP activity of the 3 parasites studied in the presence of fish mucus. We tested mucus from 2 different marine fish species, both of them known to suffer from scuticociliatosis (Smith et al. Reference Smith, Mcveagh, Hulston, Anderson and Gublin2009). Skin mucus composition is quite variable across teleosts (Shephard, 1994), and therefore more research is needed to address whether scuticociliate AcPs are activated or not by skin mucus of other teleost species. TEM studies further confirmed our results and revealed more intense lead deposits (AcP activity) associated with small vesicles and food vacuoles in the cytoplasm of U. marinum.
Following skin grazing, scuticociliates may reach the underlaying skeletal muscle and cause severe bleeding and ulcers. We investigated how AcP from U. marinum, M. avidus and P. virginianum respond in the presence of teleost fish RBCs or PBLs. Whereas some stimulation of the AcP activity occurred in the presence of RBCs, an even higher increase was observed when PBLs were added. A previous study had evaluated how cysteine proteinases isolated from M. avidus modulate the innate immune response of turbot head-kidney leucocytes (Paramá et al. Reference Paramá, Iglesias, Alvarez, Leiro, Ubeira and Sanmartín2004). Instead, we focused on the modulation of the parasite enzyme by the host. PBLs alone had no detectable levels of AcP in our plate assays. However, we could not rule out a possible stimulation of leucocyte AcP due to the parasites. Since the AcP activity recorded was fully inhibited by protein tyrosine phosphatase inhibitors, and in order to gain further knowledge in this respect, we specifically studied PTP1b.
Microbial and mammalian protein tyrosine phosphatases have highly conserved amino acid residues and are thought to share a common catalytic mechanism (Guan and Dixon, Reference Guan and Dixon1990). It is therefore not surprising that anti-human PTP1b antibodies cross-react with protozoan PTPs as demonstrated in Cryptosporidium parvum (Aguirre-García and Okhuysen, Reference Aguirre-García and Okhuysen2007). We used the same antibody to study PTP1b and found the presence of a PTP1b homologue in M. avidus. M. avidus PTP1b localized in the plasma membrane. Plasmodium falciparium, an intracellular parasite that causes malaria, secretes acid phosphatases to acquire host nutrients (Müller et al. Reference Müller, Knöckel, Eschbach, Bergmann, Walter and Wrenger2010). Secreted (Escalona-Montaño et al. Reference Escalona-Montaño, Pardavé-Alejandre, Cervantes-Sarabia, García-López, Gutiérrez-Quiroz, Gutiérrez-Kobeh, Becker-Fauser and Aguirre-García2010) and membrane-bound (Aguirre-García et al. Reference Aguirre-García, Escalona-Montaño, Bakalara, Pérez-Torres, Gutiérrez-Kobeh and Becker2006), PTP1b from L. mexicana and L. major, respectively, have been reported. T. brucei infectious stages possess an acidic ectoprotein phosphatase (TryAcP115) that greatly resembles human prostatic acid phosphatase. Immunofluorescence studies with the living parasites using a specific antibody against TryAcP115 showed that it localized to the surface of the parasite (Bakalara et al. Reference Bakalara, Santarelli, Davis and Baltz2000). This is in close agreement with what we observed in M. avidus. When M. avidus cells were exposed to fish PBLs or RBCs differential PTP1b stimulation could be observed. PTP1b staining markedly increased in the plasma membrane and the immediate area surrounding M. avidus suggesting that PTP1b may be secreted to the external media early after exposure to groper PBLs. Later on, the intensity of PTP1b staining continued to increase in the parasite plasma membrane. RBCs, on the other hand, produced increased staining of the parasite cytoplasm but neither secretion nor plasma membrane activation was observed.
TEM studies helped us identify significant AcP activity, not only inside the food vacuoles and small vesicles but also in the plasma membrane, cilia and surrounding extracellular space, in M. avidus that had ingested host PBLs but not in control parasites. Also, AcP was observed where the buccal apparatus of M. avidus is located and that phenomenon only occurred when the parasites had ingested fish PBLs. Thus, it seems that this particular area, where phagotrophy starts and the parasite first encounters the host cell, has specific relevance in M. avidus. A similar observation was made in Tetrahymena following phagotrophy (Seaman, Reference Seaman1961). Additionally, AcP has been reported in discharging mucocysts of T. thermophila (Tiedtke and Görtz, Reference Tiedtke and Görtz1983), and Shenberg (Reference Shenberg2003) reported high levels of mucocysts and their discharged material in the gills of guppies systemically infected with T. corlissi. In this study, M. avidus was not seen to produce mucocysts but, instead, the plasma membrane of this species appeared to have a pivotal role for PTP1b.
In terms of the transport route, it may be possible that, upon activation, membrane-associated lysosomal PTPs are transported to the plasma membrane and subsequently reach the lysosomes by endocytic membrane flow (Braun et al. Reference Braun, Waheed and Von Figura1989). On the other hand, our data point towards the presence of membrane-bound PTP1b homologue (low staining found in ‘resting’ control parasites). The latter appears specifically activated when M. avidus encounters host leucocytes and may be secreted extracellularly as a result of this interaction. The differential response to RBCs and PBLs observed in vitro may be explained as follows. Whereas RBCs are only ingested by the parasite, host PBLs are likely to respond to the presence of M. avidus prior to being ingested by releasing defence molecules into the medium. Parasite ecto-PTPs are known to interact with some host innate immune factors such as reactive oxygen species, as is the case in other parasitic models (Cosentino-Gomes et al. Reference Cosentino-Gomes, Russo-Abrahão, Fonseca-De-Souza, Rodrigues-Ferreira, Galina and Meyer-Fernandes2009). Thus, activation of M. avidus AcP in vesicles and food vacuoles could be the result of phagotrophy whereas membrane-bound PTP1b may represent a different AcP form that is activated in the presence of the fish host leucocytes. Further studies will shed light into this question.
We were able to demonstrate that M. avidus PTP1b expression increases during the course of infection. These parasites are facultative extracellular (histophagous) parasites and therefore we propose that the secretion of acid phosphatases to the surrounding medium may have a 2-fold role: (i) to facilitate the uptake of nutrients, i.e. skin mucosal secretion and (ii) to degrade immune factors released by the host leucocytes. Thus, both intracellular and extracellular protozoan parasites share common pathogenic mechanisms by which PTPs favour their progression, survival and success within their parasitic hosts. Collectively, our data show that high mucus production and bleeding ulcers are clearly advantageous scenarios for ciliate AcP activation. Moreover, induction of an inflammatory response at the skin lesion site would also be advantageous since greater numbers of host leucocytes would migrate from the main lymphoid organs to the skin, undermining the total immune defences of the host.
In conclusion, AcPs from 3 members of the Order Scuticociliatida were studied at the protein and cellular level. Major differences in AcP profiles were observed between M. avidus and the other 2 species. In all cases, AcP activity could be explained by ecto-protein tyrosine phosphatase/s. In vitro, fish host skin mucus is a potent activator of total AcP activity. PBLs and, to a lesser extent, RBCs also increase scuticociliate AcP. M. avidus possesses a PTP1b homologue in the plasma membrane which is activated following ingestion of fish PBLs. Importantly, upregulation of M. avidus PTP1b occurs during the course of infection. Altogether, our results reveal that PTPs play a key role in the interaction between scuticociliates and host defences and they may be one of the mechanisms by which histophagous parasites survive within their fish hosts.
ACKNOWLEDGEMENTS
Dr Salinas thanks Fundacion Seneca for a Post-doctoral Fellowship. We wish to thank Dr W. Fletcher for the advice on the in-gel assays and the staff at NIWA Mahanga Bay, for the fish husbandry and Erika Mackay for her assistance with the figure edits. Matt Voyles and Dr S.A. Anderson assisted with the identification of Parauronema spp. by PCR. This work was supported by the capability fund project CF113351 funded by NIWA.