INTRODUCTION
Oceanic islands are productive ecosystems, often supporting a large number of species, including a high level of endemism (Allen, Reference Allen2008; Clark et al., Reference Clark, Rowden, Schlacher, Williams, Consalvey, Stocks, Rogers, O'Hara, White, Shank and Hall-Spencer2010; Rowden et al., Reference Rowden, Dower, Schlacher, Consalvey and Clark2010). These insular environments may also be part of larger-scale processes (e.g. tectonism, ocean circulation) and thus have connections with other geological environments (de Forges et al., Reference de Forges, Koslow and Poore2000). Furthermore, continental and island shelf systems often consist of expansions of sedimentary environments that can support diverse and functionally important benthic marine communities such as micro-, meio- and macrobenthos (Snelgrove, Reference Snelgrove1999; Gray, Reference Gray2002).
Intertidal meiofaunal communities are affected by many environmental factors including salinity, temperature and sediment grain size (Gourbault, Reference Gourbault1981; Bouwman, Reference Bouwman1983); in turn, these factors might vary along different climatic periods and among intertidal zones. Across small spatial scales, meiofauna is strongly influenced (horizontally and vertically) by grain size (Fricke & Flemming, Reference Fricke, Flemming, McLachlan and Erasmus1983). Depending on the granulometry, meiofauna may occur throughout the entire intertidal region and in different depths into the sediment; for example in sandy environments the organisms might penetrate into depths of a few centimetres (dissipative beaches) to metres (reflective beaches), whereas in mud-rich estuarine environments, these organisms are restricted to the first centimetres of the sediment. In the case of volcanic oceanic islands, due to their geographic isolation, little is known about the characteristics of sediments (endemic soils; Guo et al., Reference Guo, Gong and Amundson2003; Bockheim, Reference Bockheim2005). Consequently, knowledge with respect to the distribution of meiofauna in these environments is still lacking.
Meiofauna, and particularly free-living marine nematodes, comprise an important component of the benthic biota both in abundance and biomass. They are also closely related to other organisms, thus playing a key role in trophic food webs (see Giere, Reference Giere2009 for a review). Meiofauna of sandy beaches is generally dominated by harpacticoid copepods and nematodes, with the dominance of one group over the other depending on the sediment grain size. Although the presence of nematodes is independent of sediment composition (Vanaverbeke et al., Reference Vanaverbeke, Gheskiere and Vincx2000), they are generally more abundant in fine sands (grain size <300 µm), while harpacticoid copepods become more important in coarse sediments (grain size >350 µm; McLachlan & Brown, Reference McLachlan and Brown2006). Temporal fluctuations also contribute to meiofauna variability, seasonal changes are less pronounced in tropical areas, but most meiofaunal organisms show some seasonality, with greater abundances in the warmest months (Coull, Reference Coull, Higgins and Thiel1988).
Due to the extension of its coastline and adjacent ocean area, in Brazil several islands and islets are found, with five main oceanic islands: Abrolhos, Fernando de Noronha, Rocas Atoll, St. Peter and St. Paul Rocks, and Trindade-Martim-Vaz. There are few studies on meiofaunal community and free-living marine nematode associations in the Brazilian oceanic islands (Alves & Castro, Reference Alves and Castro2006). Among them, Rocas Atoll is the most thoroughly studied island (Venekey & Santos, Reference Venekey and Santos2017), where the meiofauna community and nematode associations were characterized at different depths and habitats (see Netto et al., Reference Netto, Attrill and Warwick1999a, Reference Netto, Attrill and Warwickb, Reference Netto, Warwick and Attrillc, Reference Netto, Attrill and Warwick2003). In a recent review on nematodes of Brazilian oceanic islands, Venekey & Santos (Reference Venekey and Santos2017) observed that up to 2016, nine orders, 39 families and 143 genera had been recorded. Diversity values can be considered high when compared with overall diversity found in the coasts of Brazil (11 orders, 59 families, 294 genera and 231 species by Venekey et al., Reference Venekey, Fonseca-Genevois and Santos2010). Studies in oceanic islands are scarce, probably due to their remote locations. Thus, collecting efforts are usually concentrated in a few sampling opportunities (Venekey & Santos, Reference Venekey and Santos2017). Consequently, studies focusing on the temporal variation of meiofaunal communities in these habitats are rare.
This study aims to characterize the distribution of the meiofaunal community and free-living marine nematode associations on different volcanic sandy beaches in Trindade Island, Brazil. The following hypotheses were tested: the structure of meiofauna community and nematode associations varies (1) between different seasons; (2) among different beaches on the island; and (3) among beach zones. The results presented here provide the first ecological data for meiofauna and marine nematodes of Trindade Island which will serve as a basis for temporal studies, thus supporting the conservation and management of this remote oceanic island.
MATERIALS AND METHODS
Study area
Trindade Island (20°30′S 29°20′W) is located on the eastern end of the Vitória-Trindade submarine Ridge, 1140 km off the coast of Espírito Santo state, south-eastern Brazil (Figure 1). The island has an area of 13.5 km2 and is almost entirely comprised of volcanic and subvolcanic rocks formed between the end of the Pliocene and Holocene (Almeida, Reference Almeida and Schobbenhaus2002). The entire extension of the Vitória-Trindade Ridge is under the influence of the southward flow of the Brazilian Current (Miranda & Castro Filho, Reference Miranda and Castro Filho1982), characterized by warm (>27°C SST), relatively saline (37 psu) and oligotrophic waters (Silveira et al., Reference Silveira, Schmidt, Campos, Godoi and Ikeda2000). A tropical oceanic climate prevails in the region, ameliorated by eastern and southern trade winds, with mean surface water temperature of 27°C (DHN, 1968). Air temperature is warmer in February and colder in August (Alves, Reference Alves1998).

Fig. 1. Map showing the location of Trindade Island and the four sandy beaches studied (Tar, Tartarugas; Par, Parcel; Cab, Cabritos; Por, Portugueses).
The Trindade Island littoral zone is comprised of about 2.5 km of sandy and rocky beaches and 14 km of narrow (<1 km), steep rocky shores (Pereira-Filho et al., Reference Pereira-Filho, Amado-Filho, Guimarães, Moura, Sumida, Abrantes, Bahia, Güth, Jorge and Filho2011). The sublittoral hard bottom is comprised of boulders several hundred metres in diameter, interspersed with patches of sandy/calcareous rubble, as well as large uniform rocky expanses. Sandy substrates are characterized by volcanic sands and dominated by the presence of coarse and medium sands. The only human inhabitants at Trindade are the Brazilian Navy personnel, which have maintained a base on the island and have controlled its access since 1957, long before the beginning of the scientific research programmes on the island. Therefore, the entire island is relatively well preserved and without major human impacts.
Sampling and sample processing
Samples were collected in August (winter, rainy season) and December 2014 (summer, dry season) in four sandy beaches: Tartarugas, Parcel, Cabritos and Portugueses (Figure 1). Four replicates were collected for meiofauna in the high (HT), mid (MT) and low intertidal (LT) zones on each beach, using a cylindrical core of 3.0 cm diameter and 10 cm length. In addition, one sample was collected from each zone using the same corer for granulometric analyses (mean grain size, median and sorting). Seawater salinity was also determined in the ocean water column with a manual refractometer; sediment temperature was determined with a soil thermometer in each beach zone; and rainfall data were obtained from the National Institute of Meteorology (INMET) considering the Climatological Station of Vitória-ES.
In the laboratory, meiofauna was extracted from sediments using manual elutriation, and sieved through 0.5 and 0.045 mm meshes. Organisms retained in the 0.045 mm mesh were placed on Dollfus plates and identified to major taxonomic groups following Giere (Reference Giere2009). All nematodes were picked out, mounted on permanent slides using the methodology of de Grisse (Reference de Grisse1969) and Cobb (Reference Cobb1917), and identified to the species level when possible, following Warwick et al. (Reference Warwick, Platt and Somerfield1998) and the specialized literature provided in Nemys (Vanaverbeke et al., Reference Vanaverbeke, Bezerra, Braeckman, de Groote, de Meester, Deprez, Derycke, Gilarte, Guilini, Hauquier, Lins, Maria, Moens, Pape, Smol, Taheri, Van Campenhout, Vanreusel, Wu and Vincx2015). Nematodes were also classified into trophic groups according to Wieser (Reference Wieser1953): selective deposit feeders (1A), non-selective deposit feeders (1B), epigrowth feeders (2A) and omnivores/predators (2B).
Granulometric analysis was carried out by sieving coarse sediments and pipetting fine sediments as proposed by Suguio (Reference Suguio1973), and parameters (mean grain size, sorting, sand and gravel per cent) were calculated using the equations proposed by Folk & Ward (Reference Folk and Ward1957). Sediment grain size was determined through sediment sieving using an automatic shaker and classified following the Wentworth scale (Buchanan, Reference Buchanan, Holme and McIntyre1984).
Statistical analyses
Richness (S), abundance (N), density (ind. 10 cm−2), Shannon–Wiener diversity (H′ log2) and evenness (Pielou's J) were calculated for each sample. The homogeneity of variances was evaluated with Cochran's C tests and where necessary, data were log(x + 1) transformed. Differences between community descriptors among seasons (rainy, dry), beaches (Tartarugas, Parcel, Cabritos and Portugueses) and intertidal zones (HT, MT, LT), were tested through analysis of variance (Factorial ANOVA). The Tukey a posteriori test was performed when significant differences between factors were detected.
For each factor (Seasons, beaches and intertidal zones), the Analysis of Similarity (One Way ANOSIM) was applied to test for significant differences between meiofaunal community and nematodes associations. The effect of factors forming groups was visualized with non-Metric Multidimensional Ordinations (nMDS). The contributions of each taxon to the dissimilarity among seasons, beaches and zones were assessed using the SIMPER (SIMILARITY PERCENTAGE) routine.
Environmental data (mean sediment grain size, sorting, gravel per cent and sand per cent, temperature and water salinity) and fauna data were also analysed using multivariate methods. The abiotic parameters were log (x + 1) transformed, normalized, and analysed using Principal Components Analysis (PCA). ANOVA was also performed for environmental factors, according to the model previously described. The relationship between environmental parameters and faunal assemblage structure was assessed using the BIOENV (BIOTA-ENVIRONMENT MATCHING) procedure between seasons, beaches and zones. For all analyses the significance level of 0.05 was adopted.
RESULTS
Environmental parameters
A summary of all environmental parameters is given in Supplementary Material 1. In August, precipitation was higher and temperatures were lower than in December. Salinity ranged from 37 during rainy season (August) to 43 during dry season (December). Salinity and temperature varied between seasons (P < 0.5) but not among beaches (P > 0.5).
Mean grain size ranged from fine sand to coarse sand. Silt (4–63 µm) and very fine sand fractions (63–125 µm) were not recorded. According to the sorting coefficient, samples ranged from poorly to well sorted. Concerning beaches, Tartarugas, Parcel and Portugueses were characterized by coarse sand, moderately sorted in all seasons and zones. In Cabritos, sediment varied among zones during the rainy season; HT and MT zones were characterized by medium sand, whereas the LT zone was characterized by coarse sand. Yet, during the dry season, Cabritos beach was characterized by medium sand in all zones.
Mean grain size differed significantly between seasons, beaches and zones (P < 0.01 for all comparisons) with larger grain size on Parcel beach. Sediment sorting also differed among seasons (P < 0.01) and beaches (P < 0.01) but not among zones (P = 0.39). Sediment was moderately sorted in Tartarugas and Parcel beaches, and well sorted in Portugueses beach. Yet, in Cabritos it varied between well sorted (winter) to poorly sorted (summer) (Supplementary Material 1).
Based on sediment and abiotic parameters, the first two principal components (PC1 and PC2) explained 58% of the variance among seasons, beaches and zones (Figure 2A). All sandy beaches were characterized by an increase in sand per cent, mean grain size and temperature during dry season. During the rainy season, Parcel, Portugueses and Tartarugas were characterized by higher salinity and pluviosity, while Cabritos had an increase in gravel per cent.

Fig. 2. PCA ordination based on the environmental variables collected at Trindade Island (A – Seasons, B – Beaches). T (°C), Temperature; M.G.S, Mean Grain Size.
When grouped by beaches (Figure 2B), both PC1 and PC2 show that Parcel, Portugueses and Tartarugas were characterized by an increase in sand per cent (left side PC1 and upper half PC2), whereas Cabritos showed a higher gravel per cent, mean grain size, sorting (right side PC1 and lower half PC2). No clear pattern was detected when samples were grouped according to beach zones.
Meiofaunal community
The meiofauna was comprised of 10 major groups with a higher number of taxa during dry season (10 vs 7 taxa; see Supplementary Material 2). Across shore, the highest number of meiofaunal taxa was recorded in the MT zone of Cabritos beach (10 taxa) whereas the lowest was in the HT zone on Portugueses beach (3 taxa), both during dry season (Figure 3A). Overall, copepods (26%) and nematodes (23%) were numerically dominant in all beaches and zones, accounting for about 50% of the total meiofauna, except for Cabritos beach, where nematodes and polychaetes were numerically dominant (Figure 4A).

Fig. 3. Mean density (ind. 10 cm−2 ± standard deviations) and richness of meiofaunal major groups (A) and nematode species (B) at the sandy beaches of Trindade Island according to the sampling season.

Fig. 4. Relative abundance (%) of meiofaunal taxa (A) and most abundant nematodes species (B) at the sandy beaches of Trindade Island.
The highest density value was found during the dry season in the LT zone of Parcel beach (Figure 3A). During the rainy season, meiofaunal density ranged from 53.1 ind. 10 cm−2 in Cabritos beach to 322.5 ind. 10 cm−2 in Parcel beach. For the dry season these values fluctuated between 117.8 ind. 10 cm−2 in Tartarugas to 437.5 ind. 10 cm−2 in Parcel (Figure 3A). Overall, nematodes were the most abundant group in sandy beaches of Trindade Island (Figure 5A), except at Parcel beach (all seasons) and Portugueses beach (rainy season) where copepods dominated (Figure 5A).

Fig. 5. Mean density (ind. 10 cm−2 ± standard deviation) of the most abundant meiofaunal groups (A) and nematode species (B) at the sandy beaches of Trindade Island.
Richness (S) varied significantly among seasons, beaches and zones, whereas density varied significantly only between beaches and zones. On the other hand, evenness (J′) and diversity (H′) did not vary significantly among treatments (Tables 1 and 2). With respect to the structure of the meiofaunal communities, the ANOSIM analysis showed significant differences between seasons, beaches (except between Tartarugas and Cabritos; Tartarugas and Portugueses; and Parcel and Portugueses) and zones (except between HT and MT; LT and MT; Table 3). The MDS ordination based on meiofaunal data showed that samples grouped according to seasons (Figure 6A). However, no patterns were observed when samples were either grouped by beaches or zones (Figure 6B, C).
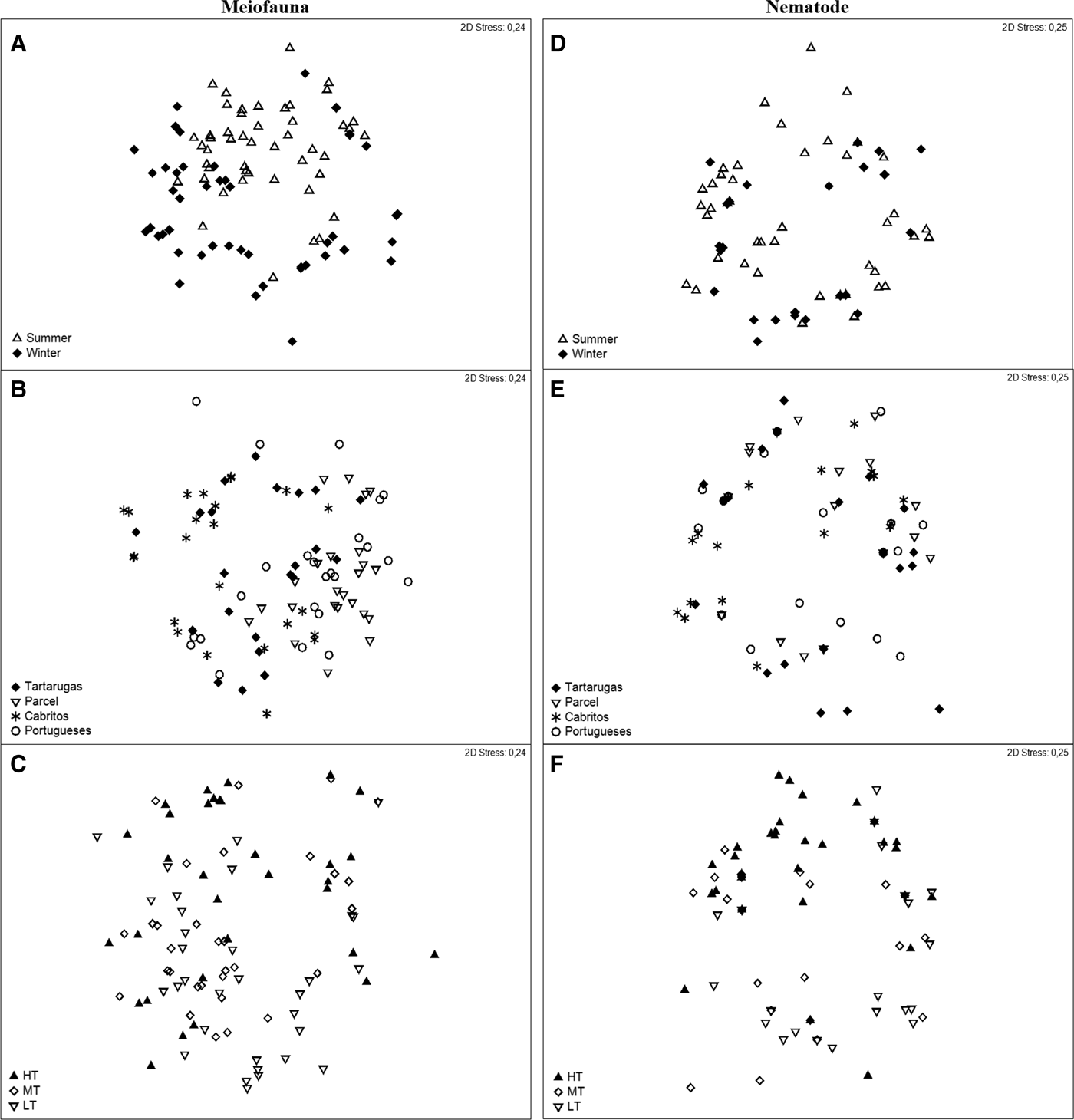
Fig. 6. nMDS for meiofaunal community [seasons (A), beaches (B) and zones (C)] and nematodes assemblages [seasons (D), beaches (E) and zones (F)] of Trindade Island.
Table 1. Results of the factorial ANOVA evaluating the significance of differences in the descriptors of meiofauna community and nematode associations of Trindade Island.

Table 2. Results of factorial ANOVA paired test for the structure of meiofauna community and nematodes associations among seasons, beaches and zones (HT – high intertidal, MT – mid intertidal, LT – low intertidal) at Trindade Island.

N.S, non-significant, P > 0.05; *P < 0.5; **P < 0.01; (–), without comparative.
Table 3. Results of ANOSIM tests for comparing the multivariate structure of meiofauna community and nematode associations among seasons, beaches and zones (HT – high intertidal, MT – mid intertidal, LT – low intertidal) at Trindade Island.

The SIMPER analysis showed that the average dissimilarity between seasons was 54.16% (Figure 6A). Oligochaeta (17.37%) and Copepoda (15.44%) were the groups that contributed most to the dissimilarity between seasons. The BIOENV analysis showed that salinity, mean grain size and sand per cent were the environmental variables that best explained the variations (r s = 0.289) in the meiofaunal community.
Nematode assemblages
A list of all recorded nematode species is shown in Appendix 1. A total of 29 species belonging to 27 genera and 12 families were found on the beaches of Trindade Island. Identification to the species level was only possible for genus Prorhynchonema. For the other genera, either the number of individuals was insufficient or their morphology was not properly preserved for measurements. Nevertheless, nematodes were separated in putative species.
Cyatholaimidae (6 genera/6 species), Xyalidae (4 genera/6 species), Oncholaimidae (3 genera/3 species) were the richest families, whereas the most abundant were Xyalidae (58.4%), Oncholaimidae (16.9%) and Cyatholaimidae (12.9%). Four species (belonging to three families) accounted for 70% of total nematode density: Theristus sp.2 (36.8%), Theristus sp.1 (13.2%), Viscosia sp. (11.2%) and Paracanthonchus sp. (10%). The most common nematode species was Theristus sp.2., present in all beaches and zones in both sampling seasons (Figure 7). Some nematode species showed strong temporal variation, appearing in high density in only one season (e.g. Metachromadora sp. (rainy season) and Innocuonema sp. (dry season)).

Fig. 7. Nematodes feeding types considering seasons, beaches, and zones at Trindade Island (1A: selective deposit feeders, 1B: non-selective deposit feeders, 2A: epistrate feeders, 2B: predators/omnivores) (HT, high intertidal; MT, mid intertidal; LT, low intertidal).
The number of nematode genera was higher in the dry season (24 genera) than in the rainy season (16 genera). With respect to intertidal zones, the highest number of nematode genera was recorded in the HT zone at Portugueses beach (13 genera) during dry season and lowest in the LT zone at Cabritos beach (2 genera) during dry season. The highest nematode density was found at Cabritos HT zone during the rainy season (Figure 3B). Mean nematode density during the rainy season ranged from 19.5 ± 3.34 ind. 10 cm−2 at Tartarugas beach to 67.8 ± 57.4 ind. 10 cm−2 at Cabritos beach; for the dry season these values varied from 34.3 ± 14 ind. 10 cm−2 at Portugueses to 57.5 ± 20.7 ind. 10 cm−2 on Cabritos. Theristus spp. and Viscosia sp. showed the highest densities in all beaches, zones and seasons, except at MT and LT zones in Cabritos beach during the dry season, where Viscosia sp. showed the highest density values (Figure 5B).
Nematode mean density varied significantly among seasons, beaches and zones, while richness varied significantly among seasons and zones but not among beaches (Tables 1 and 2). On the other hand, evenness (J′) and diversity (H′) did not vary significantly (Tables 1 and 2). The ANOSIM analysis did not detect significant differences in nematode associations among seasons, beaches and zones, except for the HT and LT zones (Table 3). Consequently, the nMDS based on nematode data did not show clear separation among seasons, beaches or zones (Figure 6D–F). SIMPER analysis revealed that the greatest assemblage dissimilarity was between high tidal zone and low tidal zone (88.34%). Theristus sp.2 contributed to the highest dissimilarities among seasons and beaches.
Nematode assemblages were predominantly comprised of non-selective deposit feeders (type 1B, 59%), followed by epigrowth feeders (type 2A, 22.5%) and omnivores/predators (type 2B, 17.4%). Selective deposit feeders (type 1A) comprised less than 2% of the total assemblage (Figure 7). Non-selective deposit feeders were the predominant group during the rainy season in all beaches and zones, except at LT zone of Tartarugas beach. During the dry season, however, there was an increase in epigrowth feeders and a decrease in non-selective deposit feeders (Figure 7). The BIOENV analysis showed that temperature and sorting coefficient were the environmental variables that best explained fauna variations (r s = 0.204) on the nematode assemblages.
DISCUSSION
Hydrodynamic and biological processes determine the structure and aggregation patterns of meiofaunal communities (Gingold et al., Reference Gingold, Ibarra-Obando and Rocha-Olivares2011). Prevalent hydrodynamic regimes are crucial for meiobenthic distribution patterns and they may vary between and within sites. However, the lack of oceanographic surveys, especially regarding currents in the area near Trindade Island, makes it difficult to have detailed knowledge on the marine circulation pattern for this region.
Spatio-temporal patterns of meiofauna community
Meiofauna diversity (i.e. major groups) encountered in this study (10) was lower compared with those in other oceanic islands and atoll from Brazil with carbonate and volcanic sediments as well as to other similar habitats located at different latitudes (Table 4). In Trindade Island, meiofauna composition did not vary among seasons, beaches and zones. Moreover, this study showed that the beaches of Trindade Island are relatively similar in terms of meiofaunal community structure, even when distant from one another (e.g. Cabritos vs Parcel, located on opposite sides of the island). This might indicate lack of spatial-temporal variation. However, such results have to be carefully interpreted, as different species within each major taxonomic group may respond differently to environmental variability (Ólafsson, Reference Ólafsson1991) by increasing or decreasing their abundances (Moens & Vincx, Reference Moens and Vincx2000). Furthermore, all beaches face north-east, and therefore, might be subjected to the same morphodynamic and oceanographic processes (i.e. waves, winds, currents).
Table 4. A summary of meiofauna community and nematode associations studies in oceanic islands with carbonate and volcanic sediments.

* Most abundant Family.
† Number of genera.
Although there are obvious difficulties in comparing locations, habitats, systems and methodological approaches, meiofauna densities recorded in this study were also lower when compared with other studies either in Brazil and/or different latitudes (see Table 4). This low density might be associated with the oligotrophic waters (i.e. low primary production) in Trindade Island. In fact, Trindade Island is under the influence of the southward flow of the Brazilian Current (Miranda & Castro Filho, Reference Miranda and Castro Filho1982), characterized by oligotrophic waters (Silveira et al., Reference Silveira, Schmidt, Campos, Godoi and Ikeda2000), thus providing low inputs of organic matter.
Total meiofaunal density in Trindade Island was higher during the summer (dry season). Temperature is known to be one of the main drivers controlling variations in the structure of meiofaunal communities in intertidal zones, particularly in temperate zones (see Giere, Reference Giere2009 for review). Although seasonal changes are less pronounced in the tropics, meiofaunal organisms still show some seasonality, with higher abundances during warmer months when temperatures are usually higher (Coull, Reference Coull, Higgins and Thiel1988; Albuquerque et al., Reference Albuquerque, Pinto, Alcântara and Gomes2007).
In tropical coarse sandy beaches with low silt content, harpacticoids are often the dominant group, although polychaetes and oligochaetes may also be present to a substantial degree (Netto et al., Reference Netto, Warwick and Attrill1999c). Similarly in Trindade Island, harpacticoids were the most abundant group in some zones of intertidal during rainy season, whereas nematodes dominated the dry season. Such variation becomes more evident when we compare beaches (e.g. Parcel vs Cabritos) where the sediment composition varied among seasons and zones.
Although nematode densities were relatively low in Trindade Island, richness of nematode genera was high. According to Heip et al. (Reference Heip, Vincx and Vranken1985), nematode density tends to be higher in finer sediments, whereas high nematode species richness is frequently recorded in coarser sediments. In fact, medium and coarse sediments are richer in micro-niches as they have relatively larger interstitial spaces and can provide areas for feeding and sheltering (Giere, Reference Giere2009). In addition, such sediment texture allows the growing of biofilms and microalgae on grain surface, which serve as food source for meiofauna.
Spatio-temporal patterns of nematodes assemblages
All species/genera found in Trindade beaches are new records for the island. Among Brazilian oceanic islands, the number of nematodes species/genera in Trindade Island (29 species in 27 genera) is relatively similar to those reported by Venekey et al. (Reference Venekey, Esteves, Fonseca-Genevois, Mohr, Castro, Costa and Alves2009) in the Archipelago of St. Peter and St. Paul, but much lower when compared with Netto et al. (Reference Netto, Attrill and Warwick1999a, Reference Netto, Attrill and Warwickb, Reference Netto, Warwick and Attrillc, Reference Netto, Attrill and Warwick2003) for Rocas Atoll. When comparing the values with oceanic islands located at different latitudes, the number of species/genera found in Trindade is usually lower or sometimes equivalent (see Table 4).
There was a remarkable variation in nematodes abundance and species richness among seasons and zones at Trindade Island, but not among beaches. This suggests that factors acting at micro-scale (e.g. such as competition or predation), have greater effect than meso-scale factors (e.g. organic matter input, transport processes) as the beaches are subject to the same environmental conditions. The increase in nematode abundance between seasons might be explained by a rise in primary production of the coral reef around the island and microphytobenthos on the beach shore. In tropical and subtropical areas, nematodes show seasonal shifts that are mainly related to food availability (Alongi, Reference Alongi1990). Another factor that may explain this seasonal variation is the difference in salinity. Unlike other organisms, nematodes are able to withstand changes in salinities, although species from the HT zone are more capable of osmoregulating than those found in the LT zone (Foster, Reference Foster1998). In fact, our results showed a higher nematode density in HT zone during dry season.
Cyatholaimidae, Xyalidae and Chromadoridae were the richest nematode families (Appendix 1) in the sandy beaches of Trindade Island. In addition, Xyalidae was also the most abundant nematode family. Along the Brazilian coast, the diversity of Xyalidae and Chromadoridae richness is well-documented in the literature, with these families occurring in several environments (Venekey et al., Reference Venekey, Fonseca-Genevois and Santos2010). Although Xyalidae has been previously reported as the most dominant family by Gourbault & Renaud-Mornant (Reference Gourbault and Renaud-Mornant1990) in calcareous shore and by Riera et al. (Reference Riera, Núñez and Brito2014) at volcanic sediments, Desmodoridae and Chromadoridae were dominant in most of the other oceanic islands studied, especially in those composed by carbonate sediments with medium-coarse sands (Boucher, Reference Boucher1997; Netto et al., Reference Netto, Attrill and Warwick1999a, Reference Netto, Attrill and Warwickb, Reference Netto, Warwick and Attrillc).
Differences in the structure of nematode associations were not detected among beaches and zones, except for the HT and LT zones where epigrowth feeders (2A) and omnivores/predators (2B) were the most abundant groups, respectively. Non-selective deposit feeders (1B) were the predominant group in all seasons and beaches at Trindade Island except for Cabritos beach during summer where nematodes of type 2B were the most abundant group. These differences in the nematode assemblage structure could be related to the oligotrophic conditions prevailing at Trindade Island. Non-selective deposit feeders ingest a variety of particles of different sizes (Moens & Vincx, Reference Moens and Vincx1997). This variety may range from individual bacteria to larger inorganic particles with attached bacteria. The presence of this trophic group, typically more dominant in the fine sediment fractions, was high in the coarse sands of Trindade due to the predominance of Theristus sp.2.
The trophic composition of the nematode associations in Trindade differed from the other Brazilian oceanic islands with carbonate or volcanic sediments. Netto et al. (Reference Netto, Warwick and Attrill1999c) found low densities of the omnivore/predator (2B) group in the entire Rocas Atoll, but the proportion of feeding types varied significantly between habitats and between size categories. Conversely, Venekey et al. (Reference Venekey, Esteves, Fonseca-Genevois, Mohr, Castro, Costa and Alves2009) found dominance of epigrowth feeders (Paracyatholaimus – 2A) and omnivores/predators (Viscosia – 2B) in the volcanic sediments of the Archipelago of St. Peter and St. Paul.
Viscosia sp., Paracanthonchus sp. and Theristus sp.2 were the most abundant species on the sandy beaches of Trindade Island. The latter two belong to the families Cyatholaimidae and Xyalidae, respectively. Cyatholaimidae can be a dominant family in exposed sandy beaches (Urban-Malinga et al., Reference Urban-Malinga, Gheskiere, Jankowska, Opaliñski and Malinga2004) and although Xyalidae is mostly a dominant family in fine to medium sands (Gourbault & Warwick, Reference Gourbault and Warwick1994; Nicholas & Hodda, Reference Nicholas and Hodda1999; Gheskiere et al., Reference Gheskiere, Hoste, Vanaverbeke, Vincx and Degraer2004), Theristus (Family Xyalidae) has already been reported as a dominant taxon in exposed and very coarse beaches (Gourbault et al., Reference Gourbault, Warwick and Helléouet1998; Urban-Malinga et al., Reference Urban-Malinga, Gheskiere, Jankowska, Opaliñski and Malinga2004).
Patterns of meiofauna and nematodes in oceanic islands with carbonate and volcanic sediments
Unlike the beaches with volcanic sands of Trindade Island, beaches mainly composed of organogenic sediments of different grain sizes (e.g. atolls, carbonate sandy beaches), generally have a more abundant and diverse fauna (Table 4). Studies in carbonate sediments have recovered at least 14 major meiofauna groups, whereas in volcanic sands this number is usually lower, 10–11, in some cases only seven. Systems with carbonate sediment that contain large coral fragments, like atolls, offer a wide variety of micro-habitats for meiofauna and may significantly contribute to the overall diversity (de Troch et al., Reference de Troch, Raes, Muthumbi, Gheerardyn and Vanreusel2008; Netto et al., Reference Netto, Attrill and Warwick1999a, Reference Netto, Warwick and Attrillc).
Granulometry also plays a role in determining the diversity and abundance of meiofauna. Often coarser sediments harbour a greater diversity, even in volcanic sands. For example, in St. Peter and St. Paul Rocks (Brazil) and Los Abrigos Del Porís Bay (Canary Islands), both with medium to very coarse sediments, 14 and 15 meiofaunal groups were found by Venekey et al. (Reference Venekey, Esteves, Fonseca-Genevois, Mohr, Castro, Costa and Alves2009) and Riera et al. (Reference Riera, Núñez and Brito2013), respectively. In the present study, however, we found both low densities and a relatively low diversity of meiofaunal groups. Coarse sands have higher permeability due to their larger pore sizes, and therefore are more oxygenated when compared with fine sands, allowing meiofauna to migrate vertically into deeper sediment layers (50 cm or even deeper according to McLachlan & Brown, Reference McLachlan and Brown2006). The lower meiofaunal density as well as sediment characteristics of Trindade Island, suggest that in this island. the meiofauna is distributed deeper than 10 cm in the sediment.
Apart from sediment granulometry, the sediment composition may also play an important role in controlling meiofaunal community structure (Semprucci et al., Reference Semprucci, Colantoni, Baldelli, Rocchi and Balsamo2010). Beaches with carbonate sediments generally offer a wide variety of micro-habitats, furthermore the high porosity of these biogenic sediments favours a greater absorption of nutrients, which gives rise to rich organic matter and large quantities of microorganisms (Wild et al., Reference Wild, Rasheed, Jantzen, Cook, Stuck, Huettel and Boetius2005). In fact, previous studies (see Table 4), have shown that such sediment features favour not only higher meiofaunal diversity, but also a larger number of genera and species, when compared with volcanic habitats. Overall, carbonate sediments have revealed rich nematode associations, usually more than 80 species, whereas volcanic sediments have been characterized by lower nematode species richness. Little is known about the characteristics of the sediments present in volcanic sandy beaches due the geographic isolation of these areas (Guo et al., Reference Guo, Gong and Amundson2003; Bockheim, Reference Bockheim2005) and the similar values for the number of genera and species found in these sandy beaches suggest that these environments, even when at different latitudes, are affected by the same environmental factors (e.g. sediment composition and organic matter input).
Comparing the dominant families and genera of nematodes there seems to be a pattern in islands with carbonate sediments. In all islands, nematodes associations are dominated by families Desmodoridae, Oncholaimidae, Epsilonematidae and Chromadoridae; the only exception is Fangataufa Atoll (very fine to fine sand) where Xyalidae is the dominant family (Gourbault & Renaud-Mornant, Reference Gourbault and Renaud-Mornant1990). However, in islands with volcanic sediments, dominance of nematode families changes drastically from locality to locality, thus reinforcing the role of endemic soils, in structuring nematode associations in volcanic sandy beaches.
Due to logistical difficulties, data of meiofaunal communities and nematode associations from oceanic islands are scarce in the South Atlantic Ocean (but see Vincx et al., Reference Vincx, Bett, Dinet, Ferrero, Gooday, Lambshead, Pfannkuche, Soltwedel and Vanreusel1994). This study provides the first ecological data for meiofaunal and nematode communities in Trindade Island. This study showed that meiofaunal community and nematode associations in the Trindade Island clearly varied along intertidal zones (HT, MT, LT) and sampling periods (rainy and dry seasons), but not among beaches. The sediment characteristics (granulometry, sand per cent and sorting) were the main drivers regulating the structure of meiofaunal community and nematode associations in Trindade Island.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315417001710.
ACKNOWLEDGEMENTS
The first author is grateful for the CAPES postgraduate research studentship (Brazil). All authors are very thankful to the Brazilian Navy (Marinha do Brasil) for all the logistical support, and to all sailors and officers of the Brazilian Navy ships APA and AMAZONAS; and to the military personnel who maintain the garrison at Trindade. A special thanks to R. F. Silva for helping with the fieldwork and to W.C. Nascimento for helping with processing the samples. Thanks also to the editor and anonymous reviewers for their comments, which helped us to improve the manuscript.
FINANCIAL SUPPORT
Sampling was conducted within the project ‘Biodiversity of meiofauna in Trindade Island with special emphasis in free-living nematodes’, funded by CNPq (Brazilian Research Council), number 405418/2012-4.
APPENDIX 1
List of nematode taxa recorded on the beaches of Trindade Island.
SYSTEMATICS
Phylum NEMATODA
Class ENOPLEA
Subclass ENOPLIA
Order ENOPLIDA
Suborder ONCHOLAIMINA
Superfamily ONCHOLAIMOIDEA
Family ONCHOLAIMIDAE
Genus Oncholaimus Dujardin, 1845
Oncholaimus sp.
Genus Pontonema Leidy, 1855
Pontonema sp.
Genus Viscosia De Man, 1890
Viscosia sp.
Family ENCHELIDIIDAE
Genus Belbolla Andrássy, 1973
Belbolla sp.
Genus Eurystomina Filipjev, 1921
Eurystomina sp.
Suborder IRONINA
Superfamily IRONOIDEA
Family IRONIDAE
Genus Trissonchulus Cobb, 1920
Trissonchulus sp.
Family OXYSTOMINIDAE
Genus Oxystomina Filipjev, 1921
Oxystomina sp.
Class CHROMADOREA
Subclass CHROMADORIA
Order CHROMADORIDA
Suborder CHROMADORINA
Superfamily CHROMADOROIDEA
Family CHROMADORIDAE
Genus Innocuonema Inglis, 1969
Innocuonema sp.
Genus Ptycholaimellus Cobb, 1920
Ptycholaimellus sp.
Genus Spilophorella Filipjev, 1917
Spilophorella sp.
Family CYATHOLAIMIDAE
Genus Longicyatholaimus Micoletzky, 1924
Longicyatholaimus sp.
Genus Metacyatholaimus Stekhoven, 1942
Metacyatholaimus sp.
Genus Paracanthonchus Micoletzky, 1924
Paracanthonchus sp.
Genus Paracyatholaimus Micoletzky, 1922
Paracyatholaimus sp.
Genus Paralongicyatholaimus Stekhoven, 1942
Paralongicyatholaimus sp.
Genus Pomponema Cobb, Reference Cobb1917
Pomponema sp.
Order DESMODORIDA
Superfamily DESMODOROIDEA
Family DESMODORIDAE
Genus Metachromadora Filipjev, 1918
Metachromadora sp.
Genus Pseudonchus Cobb, 1920
Pseudonchus sp.
Order DESMOSCOLECIDA
Suborder DESMOSCOLECINA
Superfamily DESMOSCOLECOIDEA
Family DESMOSCOLECIDAE
Genus Tricoma Cobb, 1893
Tricoma sp.
Order MONHYSTERIDA
Suborder MONHYSTERINA
Superfamily SPHAEROLAIMOIDEA
Family XYALIDAE
Genus Amphimonhystrella Timm, 1961
Amphimonhystrella sp.
Genus Prorhynchonema Gourbault, 1982
Prorhynchonema gourbaultae Nicholas & Stewart, 1995
Genus Theristus Bastian, 1865
Theristus sp1.
Theristus sp2.
Theristus sp3.
Genus Xenolaimus Cobb, 1920
Xenolaimus sp.
Order ARAEOLAIMIDA
Superfamily AXONOLAIMOIDEA
Family AXONOLAIMIDAE
Genus Ascolaimus Ditlevsen, 1919
Ascolaimus sp.
Odontophora Bütschli, 1874
Odontophora sp.
Family DIPLOPELTIDAE
Araeolaimus De Man, 1888
Araeolaimus sp.
Order PLECTIDA
Superfamily CERAMONEMATOIDEA
Family CERAMONEMATIDAE
Genus Pterygonema Gerlach, 1954
Pterygonema sp.













