Introduction
The endolymphatic sac functions to regulate endolymphatic pressure, fluid volume and ionic balance within the inner ear. It can be found on the posterior surface of the petrous temporal bone where it is in contact with the dura mater.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1 Endolymphatic sac tumour is a neuroectodermal tumour thought to emanate from the intratemporal portion of the endolymphatic sac.Reference Heffner2, Reference Doherty, Yong and Maceri3 It is extremely rare, with less than 150 cases reported. It can occur either sporadically or in association with Von Hippel–Lindau disease in 11–30 per cent of cases.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1 It was originally described as a slow-growing neoplasm that manifests as a destructive, infiltrative growth into the posteromedial aspect of the temporal bone and in some cases, into the posterior and/or middle cranial fossae.Reference Heffner2 As a result of its slow growth and initial asymptomatic phase, diagnosis is often difficult, with a reported mean interval between first symptoms and diagnosis of 97 months.Reference Timmer, Neeskens, van de Hoogen, Slootweg, Dunnebier and Pauw4 Due to the anatomical location of the tumour, a wide array of differential diagnoses exist, and difficulties can arise in distinguishing endolymphatic sac tumours both clinically and radiologically from more common lesions.Reference Heffner2, Reference Devaney, Ferlito and Rinadlo5
We present two cases of patients diagnosed with endolymphatic sac tumour and review the literature, with a focus on management.
Case reports
Patient one
A 79-year-old man presented with unilateral right-sided hearing loss, tinnitus and episodes of vertigo over a 6-month period. There were no complaints of ataxia or facial weakness. Physical examination revealed an intact right tympanic membrane with a non-pulsatile reddish-blue mass behind it. The facial nerve was intact. Audiometry confirmed a profound sensorineural hearing loss on the right, with normal thresholds on the left.
Computed tomography (CT) and magnetic resonance imaging (MRI) of the temporal bone showed a destructive lesion involving the petrous apex and extending into the posterior semicircular canal. The jugular bulb and sigmoid sinus were not involved, and no dural breach was evident. An endolymphatic sac tumour was suspected based on the radiological findings (Figure 1).
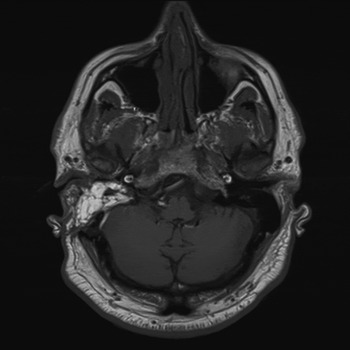
Fig. 1 Axial magnetic resonance image showing a right-sided destructive petrous apex lesion.
A right-sided transmastoid-translabyrinthine excision was performed to remove the lesion. The tumour, which extended to the common crus from the posterior semicircular canal but spared the jugular bulb, was completely excised macroscopically. Histology confirmed an endolymphatic sac tumour; sections of fibrous tissue and bone were seen to be infiltrated by a bland, papillary neoplasm.
The patient recovered well with normal facial function. The head and neck multidisciplinary team (MDT) decided on a ‘watchful waiting’ approach. The patient's first interval CT and MRI of his temporal bones showed no signs of recurrence.
Patient two
A 53-year-old man initially presented with unilateral right-sided hearing loss and pulsatile tinnitus. Examination showed a mobile tympanic membrane with a reddish-blue mass behind it. Facial function was normal. Pure tone audiometry revealed mixed moderate hearing loss on the right side and normal thresholds on the left.
Computed tomography and MRI of the temporal bones identified a highly vascular, heterogeneously enhancing mass in the right cerebellopontine angle (Figure 2). There was evidence of bony destruction of the petrous apex. Clinically and radiologically, this appeared to represent a grade B glomus jugulare (paraganglioma). The patient underwent an elective subtotal resection in order to preserve lower cranial nerve function. Histology revealed a glomus tumour.

Fig. 2 Axial magnetic resonance image showing a right-sided cerebellopontine angle lesion.
Imaging at one year post-surgery demonstrated growth of the residual tumour. Gamma knife radiosurgery was therefore undertaken. Despite this, the tumour continued to grow and a subtotal petrosectomy was performed to excise the residual tumour. Pre-operative angiography had shown the tumour to have little in the way of vascularity. Histology revealed low grade adenocarcinoma of endolymphatic sac origin.
The patient was discussed at our head and neck MDT meeting where once again it was decided that interval MRI and CT imaging would be carried out. The patient remained disease-free at his three-month interval scan.
Discussion
Endolymphatic sac tumours are rare neoplasms, almost always found in adults, typically in the third or fourth decade of life, with a slight female preponderance.Reference Doherty, Yong and Maceri3, Reference Devaney, Ferlito and Rinadlo5 Some studies have indicated that the average age (at diagnosis) of those endolymphatic sac tumour patients with Von Hippel–Lindau disease is lower (31.3 years) than those without this disease (52.5 years).Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1 The Von Hippel–Lindau disease is an autosomal dominant genetic condition caused by a mutation in the Von Hippel–Lindau tumour suppressor gene on chromosome 3p25.3. This mutation results in multiple tumours, both benign and malignant, in the central nervous system and viscera. The incidence of endolymphatic sac tumour in those with Von Hippel–Lindau disease is approximately 10 per cent; consequently, Von Hippel–Lindau disease patients are often screened for this lesion.Reference Timmer, Neeskens, van de Hoogen, Slootweg, Dunnebier and Pauw4
The typical clinical manifestations of endolymphatic sac tumours are sensorineural hearing loss with tinnitus and/or vertigo, and may also include cranial nerve paralysis, jugular foramen syndrome (glossopharyngeal neuralgia, paralysis of the hypoglossal nerve and motor deficit related to the vagus nerve) or cerebellopontine angle syndrome (hearing loss, facial paralysis and dizziness).Reference Devaney, Ferlito and Rinadlo5 Other presentations consist of serous otitis media, cerebellar signs and incidental findings.Reference Schick, Kronsbein, Kahle, Prescher and Draf6
In terms of the radiological findings for endolymphatic sac tumours, plain films reveal temporal bone destruction, with areas of mineralisation within the tumour. Computed tomography demonstrates vascular, enhancing, destructive lesions that are located in the endolymphatic sac region of the retrolabyrinth, centred in the region between the sigmoid sinus and the internal auditory canal.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1, Reference Devaney, Ferlito and Rinadlo5 Magnetic resonance imaging reveals a heterogeneous image which is enhanced with gadolinium on T1-weighted images and displays high signal intensity on T2-weighted images.Reference Devaney, Ferlito and Rinadlo5 It is particularly difficult to distinguish between glomus jugulare and endolymphatic sac tumour, as reflected in the report of patient two. This is partly due to the fact that paragangliomas are more common and are particularly vascular (like endolymphatic sac tumours). In addition, endolymphatic sac tumours can extend into the cerebellopontine angle, leading to confusion with glomus tumours.Reference Devaney, Ferlito and Rinadlo5
Early stage endolymphatic sac tumours involve the internal acoustic meatus, sigmoid sinus and medial mastoid bone. Advanced lesions can additionally affect the posterior fossa dura mater, which is the site of most frequent spread, involving the anterior cavernous sinus, the superior middle cranial fossa and the inferior jugular foramen.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1 Angiography, which is often performed pre-operatively, highlights the hypervascularity of the endolymphatic sac tumour, with branches principally arising from the external carotid artery, necessitating embolisation.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1
Macroscopically, the intra-operative appearance of an endolymphatic sac tumour has typically been described as a soft, reddish-blue mass which bleeds readily and may contain foci of mineralisation.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1, Reference Devaney, Ferlito and Rinadlo5 Histologically, an endolymphatic sac tumour has a benign appearance, displaying a haemorrhagic, highly vascularised stroma with cystic spaces and focal calcifications.Reference Doherty, Yong and Maceri3 There are two basic patterns evident on light microscopy: the follicular pattern, in which the colloid-filled follicles resembling the thyroid parenchyma are predominant; and the papillary architecture, which demonstrates a more cellular structure consisting of papillary formations, well-defined borders and bland nuclei.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1, Reference Doherty, Yong and Maceri3
The histological differential diagnosis of endolymphatic sac tumour includes middle-ear adenoma or adenocarcinoma, paraganglioma, choroid plexus papilloma, papillary meningioma, metastatic thyroid carcinoma, and renal cell carcinoma.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1 The radiological differential diagnoses for destruction of the temporal bone are divided into two categories: those which cause obliteration of the apex of the petrous bone (such as cholesteatoma, meningioma, metastatic carcinoma, chordoma and osteomyelitis), and those which cause destruction of the middle ear and/or petrous bone (such as middle-ear tumour, endolymphatic sac tumour and glomus jugulare).
The management of endolymphatic sac tumours is not standardised. This is due to the paucity of cases rigorously studied and the variance of inter-patient differences.Reference Timmer, Neeskens, van de Hoogen, Slootweg, Dunnebier and Pauw4 Surgery has been the mainstay of treatment, with various neurosurgical and skull base approaches reported, including transmastoid, retrosigmoid, transpetrosal, retrolabyrinthine and posterior fossa.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1, Reference Doherty, Yong and Maceri3 A 90 per cent cure rate has been reported for complete endolymphatic sac tumour excision without concomitant radiotherapy.Reference Doherty, Yong and Maceri3
• Endolymphatic sac tumours are rare, benign, slow-growing lesions of the petrous temporal bone that can be locally destructive
• They can be sporadic or found in association with Von Hippel–Lindau disease
• Symptoms include sensorineural hearing loss with tinnitus, vertigo, otalgia, facial weakness or other cranial neuropathies
• The tumours can be difficult to diagnose due to an initial asymptomatic phase, a wide differential diagnosis and more common lesions at this site
• No consensus regarding management and long-term follow up exist
• Surgical resection is usually favoured, but treatment with radiotherapy and gamma knife surgery has also been reported
Radiotherapy and gamma knife radiosurgery have been employed either as a primary treatment for endolymphatic sac tumours (with reports of 50 per cent efficacy with radiotherapy alone) or as an adjunct to surgery. However, these treatments remain controversial, with no clear indications apparent.Reference Doherty, Yong and Maceri3, Reference Cheng, Qin, Wang, Li, Shrestha and Wang7 Stereotactic radiosurgery has been used as a primary therapy in three cases, which led to a reduction in tumour size in one patient and no change in size for the other two patients.Reference Ferreira, Feiz-Erfan, Zabramski, Spetzler, Coons and Preul8, Reference Balasubramaniam, Deshpande and Misra9 The use of pre-operative angiography is increasing and has proved beneficial in limiting intra-operative blood loss.Reference Doherty, Yong and Maceri3
A recent case series of nine endolymphatic sac tumour patients highlighted the need to individualise treatment. Complete surgical resection was reported to offer the most optimal outcomes (with no reported recurrences), whereas subtotal resection led to persistent or progressive disease. It was recommended that follow-up investigations included annual MRI scanning.Reference Timmer, Neeskens, van de Hoogen, Slootweg, Dunnebier and Pauw4 Surgery therefore remains the cornerstone of management, but total resection of a late stage endolymphatic sac tumour is often not possible.Reference Yilmaz, Bolat, Demirhan, Aydin and Ozluoglu1
There is no consensus as to what follow up should be arranged for endolymphatic sac tumour patients, although referral for genetic evaluation may be warranted. Given the relatively benign nature of endolymphatic sac tumours (metastases have only been described in four cases in the literature), a ‘wait and scan’ protocol should be considered alongside radiotherapy.Reference Timmer, Neeskens, van de Hoogen, Slootweg, Dunnebier and Pauw4




