Introduction
The two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), is a worldwide pest of many plant species including several economically important agricultural crops (Jeppson et al., Reference Jeppson, Keifer and Baker1975; Van de Vrie et al., Reference Van de Vrie, McMurthy, Huffaker, Helle and Sabelis1985). T. urticae is often difficult to manage because of its ability to quickly develop resistance to acaricides. The high reproductive potential, extreme short lifecycle and arrhenotokous parthenogenesis, combined with frequent acaricide applications, facilitates establishment of resistance. Genetically fixed mechanisms of pesticide resistance in spider mites were similar to those found in pest insects and include enhanced metabolic detoxification of acaricides through cytochrome P450-dependent monooxygenases, esterases or glutathione-S-transferases, and/or an altered target site conferring target site resistance (Knowles, Reference Knowles and Sjut1997).
Depending on the chemistry and number of applications, a high level of resistance to acaricides can develop and is often associated with cross-resistance. Failures in the chemical control of spider mites caused by resistance have been reported for various compounds, such as organophosphates (Herron et al., Reference Herron and Rophail1998; Stumpf et al., Reference Stumpf, Zebitz, Kraus, Moores and Nauen2001), carbamates (Cranham & Helle, Reference Cranham, Helle, Helle and Sabelis1985), dicofol (Unwin, Reference Unwin1971; Van Leeuwen et al., Reference Van Leeuwen, Van Pottelberge and Tirry2005), organotins (Goodwin et al., Reference Goodwin, Herron, Gough, Wellham, Rophail and Parker1995), hexythiazox, clofentezine (Herron et al., Reference Herron, Edge and Rophail1993), abamectin (Campos et al., Reference Campos, Dybas and Krupa1995; Stumpf & Nauen, Reference Stumpf and Nauen2002), bifenthrin (Herron et al., Reference Herron, Rophail and Wilson2001; Van Leeuwen & Tirry, Reference Van Leeuwen and Tirry2007) and chlorfenapyr (Herron & Rophail, Reference Herron and Rophail2003).
Since 1994, several cases of resistance have been described against the METI (mitochondrial electron transport inhibitors)-acaricides in strains of Tetranychus spp. from Japan, Korea, Belgium, Australia and England (Ozawa, Reference Ozawa1994; Cho et al., Reference Cho, Kim, Ahn, Yoo and Lee1995; Bylemans & Meurrens, Reference Bylemans and Meurrens1997; Herron & Rophail, Reference Herron and Rophail1998; Devine et al., Reference Devine, Barber and Denholm2001; Nauen et al., Reference Nauen, Stumpf, Elbert, Zebitz and Kraus2001). The METIs fenazaquin, fenpyroximate, pyridaben and tebufenpyrad, which are now in widespread use globally, were developed in the 1990s and inhibit complex I (NADH: ubiquinone oxidoreductase) of the mitochondrial respiratory pathway, probably by binding to a subunit of the associated electron transport particles (Hollingworth & Ahammadsahib, Reference Hollingworth and Ahammadsahib1995). However, the underlying resistance mechanism(s), the patterns of cross-resistance and the inheritance of the resistance trait in T. urticae have only been investigated in a few strains (Devine et al., Reference Devine, Barber and Denholm2001; Stumpf & Nauen, Reference Stumpf and Nauen2001).
In the present study, a field-collected resistant strain of T. urticae from Belgium was investigated. A preliminary screening with several commercially important acaricides revealed, amongst others, a striking resistance to several METI acaricides. Therefore, this strain was put under continuous pressure of tebufenpyrad in order to reach homogeneity and was named MR-VP. Resistance to tebufenpyrad, fenpyroximate and pyridaben was studied in detail. The effect of synergists, known to inhibit important detoxification routes, was investigated to gain insight in METI-detoxification in the resistant strain. Piperonyl butoxide (PBO), S,S,S-tributyl-phosphorotrithioate (DEF) and diethylmaleate (DEM) were used to inhibit cytochrome P450 monooxygenases, esterases and glutathione-S-transferases, respectively (Van Leeuwen et al., Reference Van Leeuwen, Stillatus and Tirry2004). These data were backed up by analysis of enzyme activities in crude homogenates. Finally, the number of genes involved, their dominance and a possible maternal effect in resistance were investigated by crossing susceptible and resistant mites.
Materials and methods
Acaricides and chemicals
The acaricides were commercial formulations (Fyto Vanhulle, Belgium) of fenazaquin (200 g a.i. l−1 Suspension Concentrate [SC]), fenpyroximate (50 g a.i. l−1 SC), pyridaben (150 g a.i. l−1 SC) and tebufenpyrad (200 g a.i. l−1 SC).
All other chemicals were of analytical grade and purchased from Sigma-Aldrich (Belgium).
Strains
Two strains of T. urticae were used in this study. The German susceptible strain (GSS) has been maintained in laboratory culture without acaricide treatment since 1965 (Nauen et al., Reference Nauen, Stumpf, Elbert, Zebitz and Kraus2001). The METI-resistant strain (MR-VP) was originally collected from different cultivars of bean plants in a greenhouse at the national botanical garden (Brussels, Belgium) in September 2005. The spray history revealed that spider mites were controlled in the last ten years by applying mainly Pyranica (tebufenpyrad 200 g a.i. l−1 SC) and Sanmite (pyridaben 150 g a.i. l−1 SC) and occasionally Apollo (clofentezine 500 g a.i. l−1 SC), Nissorun (hexythiazox 100 g a.i. kg−1 WP), Talstar (bifenthrin 8 g a.i. l−1 SC), Torque (fenbutatin oxide 550 g a.i. l−1 SC), Vertimec (abamectin 18 g a.i. l−1 SC) and Vydate (oxamyl 250 g a.i. l−1 SC). Since 2004 the application of these products appeared to be unsatisfactory for complete control of mite outbreaks. The strain was further pressurised in the laboratory with 1000 mg l−1 tebufenpyrad to reach homogeneity.
Mites of the GSS and MR-VP strain were reared on potted kidney bean (Phaseolus vulgaris L. cv. Prelude) plants in a climatically controlled room at 26 (±0.5)°C, 60% relative humidity (RH) and 16/8 h light/dark photoperiod. The strains were changed weekly. MR-VP was maintained on bean plants sprayed with a hand-held spraying device (Birchmeier, Switzerland) until runoff with 1000 mg l−1 tebufenpyrad to avoid contamination. Six months after the start of the selection with 1000 mg l−1 tebufenpyrad, mites were used in the experiments.
Toxicity bioassays
Adulticidal bioassays were conducted using a standard method described recently (Van Leeuwen et al., Reference Van Leeuwen, Stillatus and Tirry2004). Briefly, 20–30 young adult female mites were transferred to the upper side of 9 cm2 square-cut kidney bean leaf discs on wet cotton wool, which had been sprayed with 0.75 ml of spray fluid at 1 bar pressure in a Cornelis spray tower (1.58±0.06 mg aqueous acaricide deposit cm−2) (Van Laecke & Degheele, Reference Van Laecke and Degheele1993). The plates were then placed in a climatically controlled room at 26±0.5°C, 60% RH and 16/8 h (L/D) photoperiod. Four replicates of six concentrations of each acaricide plus a control (de-ionised water) were tested. Mortality was assessed after one day, except for fenazaquin, where mortality was assessed after three days. Mites were scored as being alive if they could walk normally after they were prodded with a camel's hair brush. All control mortalities were lower than 10%. LC50-values, slopes and 95% confidence limits were calculated by probit analysis (POLO, LeOra Software, Berkeley, USA) (Robertson & Preisler, Reference Robertson, Preisler, Robertson and Preisler1992a).
Synergism studies
In order to check for metabolic resistance through synergistic ratios, female mites were placed onto leaf discs which had been sprayed with PBO, DEF or DEM. Twenty-four hours later, living mites were collected and used in toxicity experiments with tebufenpyrad, fenpyroximate or pyridaben. Based on preliminary tests, the concentrations of PBO, DEF and DEM were chosen as the concentration that caused maximum 5–10% mortality (GSS: 1000 mg l−1 PBO, 500 mg l−1 DEF, 2000 mg l−1 DEM; MR-VP: 5000 mg l−1 PBO, 2000 mg l−1 DEF, 2000 mg l−1 DEM). Before use, PBO (~90% purity), DEF (98% purity) and DEM (97% purity) were dissolved in a mixture of N,N-dimethylformamide and emulsifier W (3:1 by weight) and subsequently diluted with de-ionised water (100-fold). Synergism ratios and their confidence limits were calculated using the formula and statistics of dose ratios (Robertson & Preisler, Reference Robertson, Preisler, Robertson and Preisler1992b). If the 95% confidence interval includes 1, then the LC50 of the acaricide alone is not significantly different from the LC50 of the acaricide+synergist.
Crossing experiments
In an attempt to estimate the dominance of the resistance, individuals of the susceptible (GSS; genotype SS) and resistant (MR-VP; genotype RR) strain were reciprocally crossed to produce hybrid F1 females (SR, RS). This was achieved by placing 50 female teleiochrysalids of one strain and 100 adult males of the other strain on the upper side of a primary bean leaf on wet cotton wool in a Petri dish (four replicates) as described previously (Van Leeuwen et al., Reference Van Leeuwen, Stillatus and Tirry2004). Directly after moulting, the diploid females were fertilised by the haploid males. After three days, fertilised females were collected and placed on fresh bean leaves and were allowed to lay eggs for 14 days. Every day, the egg laying females were collected and placed on a fresh leaf. The resulting F1 females were collected ten days after hatching and were used after maturation (1–3 days) in a bioassay with the appropriate concentrations of tebufenpyrad, fenpyroximate or pyridaben with at least four replicates per concentration. The degree of dominance (D) was estimated for the F1 females using the formula (Stone, Reference Stone1968):
in which X 1 is the log of the LC50 of the R strain, X 2 is the log of the LC50 of the F1 females and X 3 is the log of the LC50 of the S strain. This formula will result in a value of –1 if resistance is fully recessive, a value of 0 if there is no dominance and a value of +1 if resistance is fully dominant.
To obtain F2 females, F1 females and males were allowed to mate in a similar manner as described above. Because males are haploid and inherit their genes only from the mother, the F2 progeny obtained from the crosses were genetically equivalent to backcross progeny. The resulting F2 females were treated with several concentrations (20–30 mites per concentration) in four replicates, covering the range of 0–100% mortality. Analysis of the concentration-mortality data for the F2 females was done to determine whether the responses fit the model of single major gene inheritance for an arrhenotokous (haplo-diploid) species. The expected responses of the F2 generation were calculated as described by Georghiou (Reference Georghiou1969) :
where c is the expected mortality at a given concentration and W is the observed mortality of the parental types at a given concentration. Observed and calculated dose-response lines for F2 females were compared using a χ2 goodness-of-fit test.
Enzymatic assays
The O-deethylation of 7-ethoxy-4-trifluoromethylcoumarin (7-EFC) by P450 monooxygenases was measured according to the method of DeLuca et al. (Reference DeLuca, Dysart, Rasnick and Bradley1988) and Buters et al. (Reference Buters, Schiller and Chou1993), but the assay was rescaled and adapted to the 96-well plate format, as previously described (Van Leeuwen et al., Reference Van Leeuwen, Van Pottelberge and Tirry2005).
For the determination of esterase activity towards the substrates 4-nitrophenyl-acetate (4-NPA), 4-nitrophenyl propionate (4-NPP), 4-nitrophenylbutyrate (4-NPB), 1-naphthyl acetate (1-NA) and 2-naphthyl-acetate (2-NA), procedures were previously described (Van Leeuwen et al., Reference Van Leeuwen, Van Pottelberge and Tirry2005).
Glutathione-S-transferase (GST) activity was assayed using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate, based on the method of Habig & Jakoby (Reference Habig, Jakoby and Jakoby1981) with slight modifications as recently described (Van Leeuwen et al., Reference Van Leeuwen, Van Pottelberge and Tirry2005). GST activity was also determined using the non-fluorescent monochlorobimane (MCB) as substrate, according to Nauen & Stumpf (Reference Nauen and Stumpf2002). Thirty fresh adult females were homogenized in 500 μl Tris-HCl buffer (0.05 M, pH 7.5). The homogenate was centrifuged for 10 min at 10,000 g and 4°C. The total reaction volume per well in a 96-well microtiter plate was 300 μl, consisting of 50 μl supernatant, 50 μl buffer, 100 μl MCB (9 mM, dissolved in buffer with a volume fraction of 0.01 ethanol) and 100 μl reduced glutathione (9 mM, dissolved in buffer). The plate was incubated for 20 min at 22°C and fluorescence was measured with a SPECTRAmax™ GEMINI XS dual-scanning microtitre plate spectrofluorometer at 465 nm while exciting at 390 nm. The nonenzymatic reaction of MCB without homogenate served as control.
All protein concentrations were measured with a Coomassie protein assay (Perbio Science, Belgium). All enzymatic assays were repeated independently at least three times.
Results
Toxicity bioassays
The toxicity bioassay data determined on adults of T. urticae by foliar spray application indicated high resistance ratios to tebufenpyrad (RR=184), fenpyroximate (RR≈1500) and pyridaben (RR≈6000) (table 1). Doses of 20,000 mg l−1 pyridaben or fenpyroximate did not cause full mortality, but at these concentrations phytotoxic effects were already visible. Resistance to fenazaquin was considerable, but rather low compared to the other METI-acaricides (LC50=188 mg l−1, RR=35), and therefore it was not included in further experiments.
Table 1. Toxicity of tebufenpyrad, fenpyroximate and pyridaben with and without synergists to female adults of strains GSS and MR-VP.

a SR, LC50 of acaricide alone/LC50 of acaricide+synergist; RR, resistance ratio; SR, synergism ratio; CL, confidence limits.
Even though the MR-VP strain was under continuous selection pressure of 1000 mg l−1 tebufenpyrad during more than half a year, the LC50 value (≈1200 mg l−1) was just slightly higher than the selection concentration. In addition, the response of MR-VP to tebufenpyrad appeared to be more homogeneous (slope of probit line=3.5) than to fenpyroximate, pyridaben or fenazaquin (slope=1.5, 1.2 and 1.9, respectively).
The GSS strain was fully susceptible, and LC50 values were far below the field concentrations of tebufenpyrad (100 mg a.i. l−1), fenpyroximate (50 mg a.i. l−1), pyridaben (180 mg a.i. l−1) and fenazaquin (40 mg a.i. l−1).
Synergism studies
Pre-treatment of the MR-VP strain and the GSS strain with PBO, DEF or DEM revealed different effects on the toxicity of the METI-acaricides (table 1).
PBO caused a 9.6-fold increase in toxicity of tebufenpyrad to MR-VP and reduced the resistance ratio from 184 to 21. DEF and DEM had no synergistic effect on the toxicity of tebufenpyrad.
When the effect of PBO, DEF or DEM was tested on fenpyroximate resistance of MR-VP, the synergism ratios were very low. However, a high synergistic effect could be detected in the GSS strain after pre-treatment with PBO (SR≅19), resulting in a tenfold higher resistance ratio.
PBO and DEF enhanced the toxicity of pyridaben to MR-VP by 96- and 66-fold, respectively, and decreased the resistance ratio to 381 and 389 compared with GSS. Synergism ratios observed after treatment with DEM were rather low.
Inheritance of resistance
In all cases, reciprocal crosses between GSS and MR-VP were successful and yielded female progeny. Results of the crosses are given in table 2.
Table 2. Probit statistics for the crosses (F1) tested against tebufenpyrad, fenpyroximate and pyridaben.

n, number of mites; D, degree of dominance.
With all tested METIs, the concentration-mortality data for F1 females resulting from GSS♀×MR-VP♂ and MR-VP♀×GSS♂ reciprocal crosses revealed no maternal inheritance.
For tebufenpyrad, the estimated degree of dominance (D) for the F1 females from GSS♀×MR-VP♂ crosses and from MR-VP♀×GSS♂ crosses were 0.59 and 0.60, respectively, illustrating an incompletely dominant inheritance of resistance.
Both the concentration-mortality data for fenpyroximate in the reciprocal F1 females were close to that of the MR-VP strain. The D values were 1.12 for cross GSS♀×MR-VP♂ and 0.93 for cross MR-VP♀×GSS♂, indicating a completely dominant inheritance of resistance to fenpyroximate.
A dominant inheritance of resistance was also observed for pyridaben (D=0.70 in cross GSS♀×MR-VP♂ and 0.83 in the reciprocal cross).
Figures 1–3 show the expected (on the basis of monogenic control) and observed concentration-mortality lines of the three acaricides for backcross F2 females. Since inheritance of tebufenpyrad, fenpyroximate and pyridaben resistance was shown to be (incompletely) dominant, only the offspring of cross (GSS×MR-VP) F1♀×GSS♂ was investigated. The Chi-square (χ2) goodness-of-fit analysis indicated that the observed mortalities of the F2 females for fenpyroximate and pyridaben were not significantly different (χ2=15.88, df=13 and χ2=7.31, df=13, respectively, p>0.05) from the expected values for single gene control. These results suggest that the resistance to fenpyroximate and pyridaben is under monogenic control (figs 2 and 3). The pronounced plateau (at about 50% mortality) in response to the backcross emphasizes that a single factor confers a high level of resistance. Only the observed mortality for tebufenpyrad was significantly different (χ2-test, p=0.05) from that expected on the basis of monogenic inheritance, leading to the conclusion that more than one gene is involved in resistance.

Fig. 1. Concentration-mortality curves for tebufenpyrad in MR-VP, GSS, the reciprocal crosses, the (GSS×MR-VP) F1♀×GSS♂ backcross (■) and the theoretical backcross on the basis of monogenic inheritance (□).

Fig. 2. Concentration-mortality curves for fenpyroximate in MR-VP, GSS, the reciprocal crosses, the (GSS×MR-VP) F1♀×GSS♂ backcross (■) and the theoretical backcross on the basis of monogenic inheritance (□).

Fig. 3. Concentration-mortality curves for pyridaben in MR-VP, GSS, the reciprocal crosses, the (GSS×MR-VP) F1♀×GSS♂ backcross (■) and the theoretical backcross on the basis of monogenic inheritance (□).
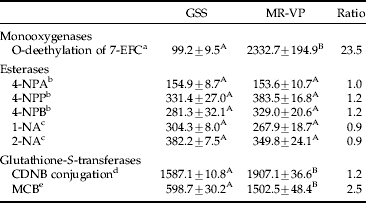
Detoxification enzymes
A significant difference in monooxygenase-mediated 7-EFC deethylation activity was found between GSS and MR-VP. The activity was increased 23.5-fold in the METI-resistant strain (table 3). Monooxygenase activity in the GSS♀×MR-VP♂ and MR-VP♀×GSS♂ reciprocal crosses was 8.8- and 10.5-fold enhanced, respectively, i.e. the activity dropped by 2.7- and 2.2-fold compared with MR-VP (fig. 4). Pre-treatment of the MR-VP strain with PBO could only reduce the monooxygenase activity ex-vivo by 1.6-fold.

Fig. 4. 7-EFC O-deethylation activity of the GSS and MR-VP strain, their reciprocal crosses and MR-VP after 24 h pre-treatment with PBO (5000 mg l−1). Data are mean values±SEM (n=3). Different letters indicate significant differences (Tukey-test, p<0.05).
Table 3. Detoxifying enzyme activities in the GSS and MR-VP strain
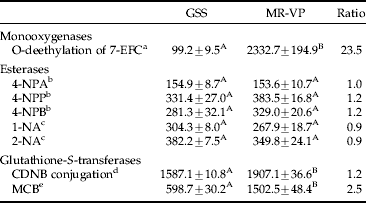
Means (±SEM) within a row followed by the same capital letter are not significantly different (t-test, P>0.05).
a pmol 7-hydroxy-4-(trifluoromethyl)-coumarin (30 min)−1 mg−1 protein (±SEM, n=3).
b nmol 4-nitrophenol min−1 mg−1 protein (±SEM, n=3).
c nmol 1- or 2-naphthol min−1 mg−1 protein (±SEM, n=3).
d nmol glutathione conjugated min−1 mg−1 protein (±SEM, n=3).
e relative fluorescence units (RFU) μg−1 protein (±SEM, n=4).
Quantitative analysis of general esterase activity measured by the production of the metabolites 4-nitrophenol, 1- and 2-naphthol by various substrates, revealed no significant differences between strains GSS and MR-VP (table 3).
The conjugation of glutathione with CDNB was significantly different between the two strains (t-test, p<0.05), though the increase in activity compared to GSS was small (1.2-fold). The GST activity was measured additionally in a fluorometric assay with MCB. Compared to strain GSS, MR-VP exhibited a 2.5-fold increased GST activity.
Discussion
The original field population of T. urticae, collected from a greenhouse near Brussels, Belgium, showed a considerable cross-resistance between the METI-acaricides tebufenpyrad, fenpyroximate, pyridaben and fenazaquin. None of these METIs were 100% effective at the recommended field dose rate, suggesting structural similarities selecting for similar mechanisms of resistance. After pressurising with tebufenpyrad in the laboratory, the strain proved to be resistant to the tested METIs in the order pyridaben>fenpyroximate>tebufenpyrad>fenazaquin. The extremely high resistance ratios of pyridaben and fenpyroximate are not unusual and were previously reported (Goka, Reference Goka1998; Nauen et al., Reference Nauen, Stumpf, Elbert, Zebitz and Kraus2001; Sato et al., Reference Sato, Miyata, Da Silva, Raga and De Souza Filho2004).
METI-acaricides attack a target-site in complex I (NADH:ubiquinone oxidoreductase) of the mitochondrial respiratory pathway (Hollingworth & Ahammadsahib, Reference Hollingworth and Ahammadsahib1995; Lümmen, Reference Lümmen1998). But clearly, a considerable degree of uncertainty exists regarding the exact relationship of inhibitor binding sites on Complex I (Hollingworth & Ahammadsahib, Reference Hollingworth and Ahammadsahib1995; Schuler & Casida, Reference Schuler and Casida2001).
All METIs are compounds containing heterocyclic rings with two nitrogen atoms associated with long hydrophobic tail structures with at least one tertiary butyl group. Despite their similar chemical structure and identical mode of action, a variation in susceptibility of METI-acaricide-resistant T. urticae strains to members of this chemical class of acaricides exists. The TUK4-strain, collected in 1999 from hops in England with a short history of tebufenpyrad use, exhibited resistance to tebufenpyrad, pyridaben, fenazaquin and fenpyroximate, with resistance factors of 46, 346, 168 and 77, respectively (Devine et al., Reference Devine, Barber and Denholm2001). The Japanese METI resistant strain AKITA, collected in 1996, also exhibited 1100-, 870- and 33-fold cross-resistance to pyridaben, fenpyroximate and tebufenpyrad, respectively (Stumpf & Nauen, Reference Stumpf and Nauen2001). In Western Australia, a strain of T. urticae collected from an apple orchard, where it had been exposed to five tebufenpyrad applications over four seasons, exhibited 63-, 210- and 25-fold resistance to tebufenpyrad, pyridaben and fenpyroximate, respectively, when tested with adult females (Herron & Rophail, Reference Herron and Rophail1998). Cross-resistance between METIs was also found in laboratory selected strains (Kim et al., Reference Kim, Lee, Lee and Ahn2004, Reference Kim, Park, Cho and Ahn2006).
These observations do illustrate the complexity of mechanisms of resistance to METI acaricides. Based on their chemical structure, hydroxylation is thought to be one common mechanism of oxidative detoxification for all METIs in T. urticae (Stumpf & Nauen, Reference Stumpf and Nauen2001). Stumpf & Nauen (Reference Stumpf and Nauen2001) reported that the highly METI-resistant strain AKITA showed a 2.4-fold increase in O-deethylation activity of the artificial substrate 7-ethoxycoumarin (7-EC). In this study, oxidative detoxification was characterized by the highly sensitive fluorometric microplate assay using 7-ethoxy-4-trifluoromethylcoumarin, a substrate similar to the more common 7-EC. MR-VP showed a highly elevated O-deethylation activity (23.5-fold) compared with the susceptible strain GSS. This supports that metabolic detoxification via cytochrome P450 monooxygenases is likely to play a major role in METI resistance in the MR-VP strain, whereas enhanced esterase and GST activity appears to be a minor resistance contributing factor, since only minor differences were found in enzymatic activity between strains.
In the current study, none of the synergists caused METI resistance to drop to full susceptibility. These results suggest either the existence of an additional resistance mechanism toward the METIs, the inability of the synergists to fully suppress the enzymatic detoxification mechanisms or that the amount of synergist may have been too low to fully block detoxification. In case of oxidative METI-degradation, this could also be confirmed with the observation that the O-deethylation activity of the MR-VP after 24-h pre-treatment with PBO was only 1.6-fold lower than that of the untreated MR-VP and still 15 times higher than GSS. It has also been reported that the enhanced 7-EC O-deethylation activities of the AKITA and UK-99 strains were only partially suppressed by PBO, possibly caused by a restricted binding to the active site of the cytochrome P450-dependent monooxygenases involved (Stumpf & Nauen, Reference Stumpf and Nauen2001).
However, our synergism studies revealed some interesting results in terms of possible differences in resistance mechanisms towards tebufenpyrad, fenpyroximate and pyridaben. Pretreatment with the monooxygenase inhibitor PBO in vivo resulted in a significant decrease of tebufenpyrad and pyridaben resistance in the MR-VP strain and reduced the resistance ratio from 184 to 21 for tebufenpyrad and from 5971 to 381 for pyridaben, which confirms the role of oxidative detoxification for these two compounds. While PBO has also been reported to inhibit resistance-related esterases in some insect species (Gunning et al., Reference Gunning, Moores, Devonshire and Jones1998, Reference Gunning, Moores and Devonshire1999; Young et al., Reference Young, Gunning and Moores2005), there is no evidence that PBO inhibits mite esterases (Van Pottelberge et al., unpublished data). Resistance to pyridaben could be significantly reduced by DEF as well, possibly reflecting the role of both monooxygenases and esterases in pyridaben resistance. However, it has been proposed that DEF is not a completely specific inhibitor of esterases and that it can also inhibit microsomal oxidases at high concentration (Scott, Reference Scott, Roush and Tabashnik1990; Valles et al., Reference Valles, Koehler and Brenner1997). It can be noted that DEM halved the resistance ratio to pyridaben. Taking into consideration the significant, but limited, increase in vivo of glutathione-S-transferase activities, resistance to pyridaben can be partly caused by these enzymes, although the effect is negligible compared to esterases and monooxygenases.
Surprisingly, the level of resistance to fenpyroximate in MR-VP could not be suppressed by PBO or DEF, suggesting either the involvement of monooxygenases insensitive to PBO inhibition or that the high level of resistance to fenpyroximate is not caused by detoxification. If the lack of synergism on fenpyroximate toxicity finds its origin in an insensitive P450 monooxygenase pathway, this would infer a completely different oxidative detoxification route for fenpyroximate in comparison to tebufenpyrad and pyridaben, since resistance to the latter two could be significantly synergised by PBO. This would also imply that cross-resistance between METIs in this strain is not caused by P450 monooxygenase cross-activity. Up to date, all METI resistance in T. urticae could be synergised, at least partially, by PBO (Stumpf & Nauen, Reference Stumpf and Nauen2001; Kim et al., Reference Kim, Lee, Lee and Ahn2004). This lack of synergism, together with the clear monogenic inheritance of fenpyroximate resistance, could well point to a target-site-based resistance mechanism. These findings challenge the general thought that METI resistance is solely caused by enhanced oxidative detoxification. This conclusion is mainly based on synergist experiments, which can draw the attention from other possible effects. A detoxification mechanism that is the same in a sensitive and a resistant strain can have a much greater impact on degradation if an altered site of action in the resistant strain retards the intoxication. The effect of a synergist that blocks the detoxification will then be much larger, which could lead to the false conclusion that the detoxification is the major resistance mechanism (Oppenoorth, Reference Oppenoorth1984, Reference Oppenoorth, Kerkut and Gilbert1985).
Regardless of the underlying mechanisms, determination of genetics of the resistance is necessary for resistance risk assessments (McKenzie & Batterham, Reference McKenzie and Batterham1998) since it reveals important information on the susceptibility of heterozygotes and, thus, the stability and the potential to spread on the field. Resistance to all METIs in the MR-VP strain was inherited as a dominant trait as was also found by Devine et al. (Reference Devine, Barber and Denholm2001) and Sato et al. (Reference Sato, Miyata, Da Silva, Raga and De Souza Filho2004). However, Goka (Reference Goka1998) examined resistance patterns in a Japanese METI-resistant strain of T. kanzawai and reported that the inheritance was intermediately dominant for tebufenpyrad and fenpyroximate, but completely recessive for pyridaben, suggesting that the mechanism conferring pyridaben resistance was almost certainly different from that conferring tebufenpyrad resistance and that the resistance gene loci for the three acaricides are not identical.
Furthermore, the responses of reciprocal crosses between strains revealed no maternal effect for all three METI-acaricides. METI-acaricides attack a target site in complex I of the respiratory pathway. Only a minority of the subunits of complex I are products of mitochondrial genes, while the remainder originate from nuclear DNA (Walker, Reference Walker1992). Recently, it was suggested that a few subunits of complex I (TYKY, PSST, ND1, ND5 and 49-kDa) form the catalytic core of the enzyme and are possible inhibitor binding sites (Schuler & Casida, Reference Schuler and Casida2001). Hence, a mutation in ND1 or ND5, which are encoded by the mitochondrial genome, could result in maternal inherited METI resistance. This was clearly not the case. Autosomally inherited resistance was reported by most authors (Goka, Reference Goka1998; Devine et al., Reference Devine, Barber and Denholm2001; Sato et al., Reference Sato, Miyata, Da Silva, Raga and De Souza Filho2004), but Stumpf & Nauen (Reference Stumpf and Nauen2001) observed a slight maternal effect in the inheritance of pyridaben and fenpyroximate resistance.
The responses of F2 females from the reciprocal crosses suggest that the resistance to fenpyroximate and pyridaben was under monogenic control, while resistance to tebufenpyrad was under control of more than one gene (polygenic). It is possible that resistance to tebufenpyrad in MR-VP was originally a monogenic resistance; but, due to selection, additional (minor) resistance mechanisms appeared. Such a hypothesis is supported by earlier findings on Lucilia cuprina, the sheep blow fly, where four populations of L. cuprina, already nearly fixed for a major allele, were brought into the laboratory for eight further generations of selection, resulting in a polygenic resistance (McKenzie et al., Reference McKenzie, Dearn and Whitten1980, cited in Roush & McKenzie, Reference Roush and McKenzie1987). Monogenic resistance, which is considered more likely to spread within populations than polygenic resistance, tends to be more stable and is less easily managed (Roush & McKenzie, Reference Roush and McKenzie1987).
After considering all experimental evidence obtained throughout the study, it can be concluded that a high level of resistance to all METI-acaricides can occur in the field and that different genetically established resistance mechanisms can play a role in resistance to each of these METIs.