Healthcare-associated infections (HAIs) cause serious health consequences for patients and result in prolonged hospitalizations and increased healthcare expenditures, particularly when the causative microorganisms are antibiotic resistant (AR).Reference Mauldin, Salgado, Hansen, Durup and Bosso 1 – Reference Haeusler, Mechinaud and Daley 7 Pediatric hospital patients are especially vulnerable to adverse outcomes from AR infections due to factors such as immature immune systems, acquired or congenital immunodeficiencies, need for chronic parenteral nutrition, and congenital anomalies.Reference Siegel 8 The unique impact HAIs have on pediatric patients is underscored by the fact that rates of device-associated infections are higher in some pediatric unit types than in corresponding adult units, despite a lower device utilization ratio.Reference Dudeck, Edwards and Allen-Bridson 9 A recent analysis of device-associated infection data has demonstrated a decline in the incidence of central line-associated bloodstream infections (CLABSIs) and ventilator-associated pneumonia infections (VAPs) in pediatric units between 2007 and 2012.Reference Patrick, Kawai and Kleinman 6 However, data specifically describing antibiotic resistance among pathogens associated with pediatric device-associated infections and surgical site infections (SSIs) are lacking.Reference Milstone, Bryant, Huskins and Zerr 10
Providing data to inform HAI and antibiotic resistance prevention efforts is an essential function of the Centers for Disease Control and Prevention’s (CDC’s) National Healthcare Safety Network (NHSN). Although NHSN reports describing HAI and antibiotic resistance data in the United States have been published,Reference Hidron, Edwards and Patel 11 – Reference Weiner, Webb and Limbago 13 these reports did not provide separate results for adult and pediatric inpatient locations. We used methods similar to those of prior NHSN reports to describe the prevalence of antimicrobial resistance among HAIs reported from pediatric locations.
METHODS
HAI Reporting
We used data from CLABSIs, catheter-associated urinary tract infections (CAUTIs), VAPs, and SSIs that (1) occurred from 2011 to 2014 in pediatric units, (2) met NHSN HAI surveillance definitions in place at that time, and (3) were reported to NHSN by December 16, 2015. Analyses of datasets from later months in this period may yield different results because NHSN users are able to edit their data as needed. NHSN surveillance methodology has been reported previously.Reference Hidron, Edwards and Patel 11 , 14 – 18 Pediatric HAIs can be reported to NHSN from acute-care hospitals, long-term acute-care hospitals (LTACHs), and inpatient rehabilitation facilities (IRFs); facility type is self-identified by facilities during initial enrollment into the NHSN. Neonatal intensive care units (NICUs) included in this report are those classified by NHSN CDC location codes as level II/III, a combined nursery housing both level II and III newborns and infants, or level III, a NICU with personnel and equipment to provide continuous life support and comprehensive care for extremely high-risk newborn infants and those with complex and critical illnesses.
NHSN HAI surveillance protocols provide procedures for attributing device-associated infections (CLABSIs, CAUTIs, and VAPs) to CDC location types and SSIs to CDC operative procedure categories. 14 – 17 We included device-associated infection data reported from pediatric locations in long-term acute care (LTAC) and inpatient rehabilitation facilities (IRFs). Because HAIs were not included in the Centers for Medicaid & Medicare Services Quality Reporting Programs for LTAC hospitals and IRFs until October 2012, 19 , 20 data from these facility types might not have been reported for the entire 4-year period. Also, VAP data reporting by NICUs ended in December 2013 16 ; this report includes NICU VAP data from 2011 to 2013 and VAP data from pediatric critical care locations for all 4 years. CAUTIs are not reported by NICUs.
Laboratory Reporting
For each HAI, data contributors were able to report up to 3 causative pathogens. For selected pathogens, the NHSN also required users to report antimicrobial susceptibility information. Clinical laboratories in facilities reporting data to NHSN were expected to use Clinical and Laboratory Standards Institute standards for antimicrobial susceptibility testing in place at the time. Bacterial susceptibility results were reported categorically to NHSN as “susceptible” (S), “intermediate” (I), “resistant” (R), or “not tested” (N).
We grouped pathogens and defined antimicrobial resistance according to methods described previously.Reference Weiner, Webb and Limbago 13 Staphylococcus aureus was defined as methicillin-resistant (MRSA) if an isolate was reported to be R to oxacillin, methicillin, and/or cefoxitin. Enterococcal species were defined as ampicillin resistant if an isolate was reported to be I or R to ampicillin, and vancomycin resistant if reported to be R to vancomycin. Pseudomonas aeruginosa was defined as resistant to extended-spectrum cephalosporins (ESCs) if an isolate was reported as I or R to ceftazidime or cefepime; fluoroquinolone resistant if an isolate was reported as I or R to ciprofloxacin or levofloxacin; and aminoglycoside resistant if an isolate was reported as I or R to gentamicin, amikacin, or tobramycin. Escherichia coli was defined as fluoroquinolone resistant if an isolate was reported to be I or R to ciprofloxacin, levofloxacin, or moxifloxacin. Enterobacteriaceae were defined as ESC resistant if an isolate was reported as I or R to ceftazidime, cefepime, ceftriaxone, or cefotaxime and as aminoglycoside resistant if an isolate was reported as I or R to gentamicin, amikacin, or tobramycin. Selected gram-negative pathogens were defined as carbapenem-resistant if an isolate was reported to be I or R to imipenem, meropenem, or doripenem, as these were the surveillance definitions for NHSN in 2011–2014.Reference Weiner, Webb and Limbago 13 Because the classification “susceptible-dose dependent” (S-DD) is used in place of I for azole antifungals (eg, fluconazole), Candida spp were defined as fluconazole resistant if an isolate was reported to be S-DD or R to fluconazole.
Criteria for defining multidrug resistance were similar to published interim standard definitions.Reference Weiner, Webb and Limbago 13 , Reference Magiorakos, Srinivasan and Carey 21 To be defined as multidrug-resistant (MDR), a gram-negative pathogen must have been reported to be I or R to at least 1 agent in 3 or more antimicrobial categories. MDR categories included ESCs, fluoroquinolones, aminoglycosides, and carbapenems (all organisms); piperacillin or piperacillin/tazobactam (Enterobacteriaceae and P. aeruginosa); and ampicillin/sulbactam (Acinetobacter spp).
Statistical Analysis
Data were analyzed with SAS software, version 9.3 (SAS Institute, Cary, NC). For analyses of device-associated infections, pediatric or neonatal NHSN inpatient location types were grouped into 4 mutually exclusive categories: NICUs, pediatric intensive care units (PICUs), pediatric oncology wards, and pediatric wards (eg, medical, surgical, and stepdown units). Absolute frequencies and distributions of reported HAIs or pathogens were calculated by hospital type, hospital size, HAI, surgery, and location type where applicable.
For device-associated infections, the most common 15 pathogens for each infection type–location combination were identified and ranked. Similarly, for SSIs the 15 most common pathogens were ranked overall and by type of surgical procedure.
The percentage of pathogens tested for susceptibility (sum of pathogens tested for susceptibility, divided by the sum of total pathogens isolated, multiplied by 100) was calculated for each pathogen–antimicrobial class combination. Pooled mean percent resistance was calculated for each pathogen–antimicrobial combination (sum of pathogens that tested resistant, divided by the sum of pathogens tested for susceptibility, multiplied by 100), for each HAI or type of surgical procedure, and for device-associated infections, stratified by pediatric location type. Pooled mean percent resistance was not calculated for any resistance phenotype where fewer than 20 pathogens were tested.Reference Weiner, Webb and Limbago 13
Statistical comparisons of antimicrobial resistance differences between locations or procedure types are beyond the scope of this report. Only the absolute differences in resistance percentages are reported and discussed, so this report does not provide definitive conclusions regarding resistance differences between locations.
RESULTS
Distribution of Pediatric Healthcare-Associated Infections by Hospital, Surgical Procedure, and Location Types
From 2011 to 2014, 1,003 hospitals reported 20,390 HAIs to NHSN from pediatric units. Of these, the most frequent hospital type was general acute care, which comprised 88% of facilities that reported 62% of HAIs. Children’s hospitals comprised only 7% of facilities but reported 33% of HAIs. Hospitals with > 200 beds represented 74% of reporting facilities and reported 91% of HAIs (Table 1). Most HAIs reported (69%) were CLABSIs. A description of the number of events and pathogens reported by HAI and surgery type can be found in Tables 2 and 3. Device-associated infection pathogen distribution by inpatient location type and SSI pathogens by surgical type are located in Tables S1–S4 and Table S5 of the online supplement, respectively.
TABLE 1 Characteristics of Hospitals Reporting Pediatric Healthcare-Associated Infections (HAIs) to the National Healthcare Safety Network (NHSN), 2011–2014

a Reported at least 1 HAI between 2011 and 2014.
b Includes free-standing rehabilitation facilities only. No inpatient rehabilitation facilities within acute-care hospitals reported HAIs to NHSN.
TABLE 2 Types of Pediatric Healthcare-Associated Infections (HAIs) and Surgical Site Infections (SSIs) Reported to the National Healthcare Safety Network, 2011–2014

NOTE. CLABSI, central line-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; VAP, ventilator-associated pneumonia; SSI, surgical site infection; Ob/Gyn, obstetrical and gynecological.
a Appendectomy, bile duct, liver, or pancreatic surgery, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, abdominal surgery, and rectal surgery.
b Breast surgery only.
c Cardiac surgery, coronary artery bypass graft with chest incision with or without donor incision, pacemaker surgery, and thoracic surgery.
d Kidney surgery only.
e Craniotomy and ventricular shunt.
f Cesarean section, abdominal hysterectomy, ovarian surgery, and vaginal hysterectomy.
g Open reduction of fracture, hip prosthesis, knee prosthesis, limb amputation, spinal fusion, refusion of spine, and laminectomy.
h Heart transplant, kidney transplant, and liver transplant.
i Abdominal aortic aneurysm repair, shunt for dialysis, carotid endarterectomy, and peripheral vascular bypass surgery.
TABLE 3 Pediatric Surgical Site Infections (SSIs) Reported to the National Healthcare Safety Network, by Surgery Type, 2011–2014Footnote a ,Footnote b

a Surgeries with fewer than 15 SSIs reported are not shown, with the exception of herniorrhaphy.
b Beginning in 2014, only surgeries with primary closure are included.
c Other includes hip prosthesis (n=14, 0.5%), knee prosthesis (n=7, 0.2%), ovarian surgery (n=6, 0.2%), thoracic surgery (n=6, 0.2%), abdominal aortic aneurysm repair (n=5, 0.2%), kidney transplant (n=5, 0.2%), rectal surgery (n=5, 0.2%), coronary artery bypass graft with both chest and donor site incision (n=3, 0.1%), breast surgery (n=2, 0.1%), limb amputation (n=2, 0.1%), spleen surgery (n=2, 0.1%), heart transplant (n=1, 0.03%), kidney surgery (n=1, 0.03%).
Pathogen Distribution
Across HAI types, 22,323 pathogens were reported. Overall, the most common pathogens were S. aureus (17%) and coagulase-negative staphylococci (17%), followed by E. coli (11%) and K. pneumoniae/oxytoca (9%). Pathogen rankings varied between HAI types. Staphylococcal species were the most frequent for CLABSI (coagulase-negative staphylococci), SSI (S. aureus), and VAP (S. aureus), but E. coli was the most frequent CAUTI pathogen and ranked second among SSI pathogens. P. aeruginosa was the second most frequent pathogen reported for both CAUTI and VAP (Table 4).
TABLE 4 Distribution and Rank Order of Selected Pediatric Healthcare-Associated Infection (HAI) Pathogens Reported to the National Healthcare Safety Network, Overall and by HAI Type, 2011–2014

NOTE. CLABSI, central line-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; SSI, surgical site infection; VAP, ventilator-associated pneumonia.
a VAP data from neonatal critical care locations from 2011 to 2013.
b The 15 most common pathogens are listed in this table and are ranked according to reporting frequency of all pathogens reported to NHSN.
c For CAUTI, SSI, and VAP, the top 15 pathogens did not correspond to the top 15 pathogens overall. The complete listing of the top 15 pathogens for each device associated infection can be found in Tables S2 (CAUTI), S3 (SSI), and S4 (VAP) of the online supplement.
Among 15,538 CLABSI pathogens, 51% were reported from NICUs, 23% from PICUs, 15% from oncology units, and 11% from pediatric wards. Staphylococcus aureus and coagulase-negative staphylococci were the most frequently reported CLABSI pathogens in critical care locations. In oncology wards, viridans group streptococci (15%) and K. pneumoniae/oxytoca (12%) were the 2 most common pathogens reported; K. pneumoniae/oxytoca was the most common CLABSI pathogen in pediatric ward locations (15%) (Table S2, online supplement).
Among 2,366 CAUTI pathogens, 83% were reported by PICUs and 15% were reported by pediatric wards. Pathogen distribution was similar between these 2 locations: E. coli and P. aeruginosa were the first and second most common pathogens for both locations, respectively, and K. pneumoniae/oxytoca, Enterobacter spp, and C. albicans were among the 5 most common pathogens in both locations (Table S3, online supplement).
Among 1,366 VAP pathogens, 63% were reported from NICUs and 37% from PICUs. Staphylococcus aureus, P. aeruginosa, K. pneumoniae/oxytoca, and Enterobacter spp were the 4 most common pathogens in both location types. Streptococcus pneumoniae ranked fifth in PICUs, and E. coli ranked fifth in NICUs (Table S4, online supplement).
Of the 3,053 SSI pathogens reported, S. aureus was the most common pathogen overall (22%) and for orthopedic surgery SSIs (39%) and cardiac surgery SSIs (55%), and S. aureus was the second most common for neurological surgery SSIs (28%). Coagulase-negative staphylococci were the most common pathogen for neurological surgery SSIs (31%). Escherichia coli was the most common pathogen for abdominal surgery SSIs (28%) and the second most common overall (18%) (Table S5, online supplement).
Percent Resistance by HAI Type
For almost all pathogen-antibiotic combinations reported for CLABSIs, resistance was generally lower in NICUs than in other location types. Conversely, resistance was highest in oncology locations for multiple pathogen–antibiotic combinations, including ampicillin and vancomycin resistance for Enterococcus faecium; ESC and multidrug resistance for E. coli and K. pneumoniae/oxytoca; and fluoroquinolone resistance for E. coli. Resistance to carbapenems was infrequent (<4%) among Enterobacteriaceae in all locations. Fluconazole resistance was infrequent (<4%) for Candida albicans and C. parapsilosis, but it did reach 41% for other Candida spp in oncology wards. However, no more than 50% of Candida spp isolates were tested in any location. For P. aeruginosa, resistance was highest for all pathogen–antibiotic combinations in pediatric wards. In addition, resistance was higher in pediatric wards than PICUs for S. aureus, E. faecalis, and E. coli, for all pathogen–antibiotic combinations evaluated (Table 5).
TABLE 5 Percent of Pathogens Reported from Pediatric Central-Line–Associated Bloodstream Infections (CLABSIs) that Tested Resistant to Selected Antimicrobial Agents, by Reporting Location, 2011–2014
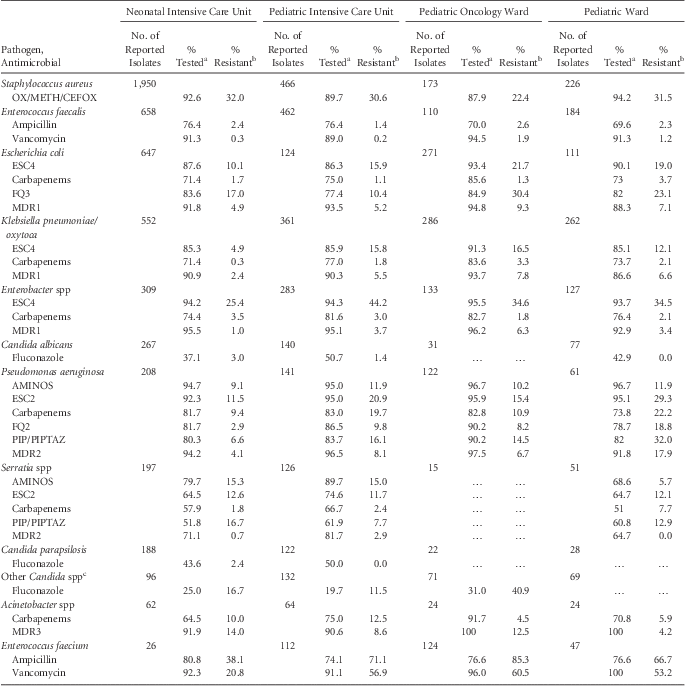
NOTE. AMINOS, aminoglycosides (amikacin, gentamicin, tobramycin); carbapenems (imipenem, meropenem, doripenem); ESC2, extended-spectrum cephalosporin (cefepime, ceftazidime); ESC4, extended-spectrum cephalosporin (cefepime, cefotaxime, ceftazidime, ceftriaxone); FQ2, fluoroquinolones (ciprofloxacin, levofloxacin); FQ3, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin); MDR1, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the classes (ESC4, FQ3, AMINOS, carbapenems, and PIP/PIPTAZ); OX/METH/CEFOX, oxacillin/methicillin/cefoxitin; MDR2, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 5 classes (ESC2, FQ2, AMINOS, carbapenems, and PIP/PIPTAZ); MDR3, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 6 classes (ESC4, FQ2, AMINOS, carbapenems, PIP/PIPTAZ and ampicillin/sulbactam); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam.
a If the percentage of isolates tested is less than 70%, caution should be used when interpreting the percent resistance.
b Percent resistance is only calculated when at least 20 isolates have been tested. Ellipses (…) in percent tested and percent resistance column indicates that fewer than 20 isolates were tested.
c Non-albicans, non-parapsilosis.
For CAUTIs, resistance was higher in pediatric wards than PICUs for most pathogen–antibiotic combinations. The percentage of E. coli and P. aeruginosa resistant to fluoroquinolones and of K. pneumoniae/oxytoca resistant to ESCs was approximately two-fold higher in pediatric wards than PICUs. Overall, carbapenem resistance was infrequent (<4%), but on pediatric wards, 13% of P. aeruginosa isolates were carbapenem resistant. The proportion of E. faecalis resistant to vancomycin was 15% in pediatric wards compared to 1% in PICUs (Table 6).
TABLE 6 Percent of Pathogens Reported from Pediatric Catheter-Associated Urinary Tract Infections (CAUTIs) that Tested Resistant to Selected Antimicrobial Agents, by Reporting Location, 2011–2014
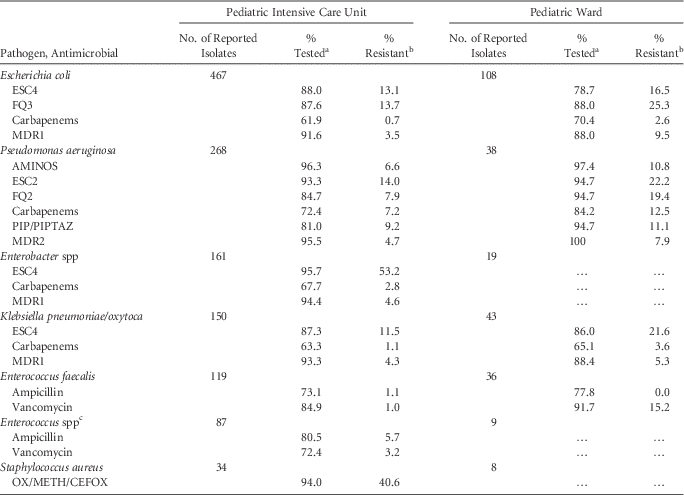
NOTE. AMINOS, aminoglycosides (amikacin, gentamicin, tobramycin); Carbapenems (imipenem, meropenem, doripenem); ESC2, extended-spectrum cephalosporin (cefepime, ceftazidime); ESC4, extended-spectrum cephalosporin (cefepime, cefotaxime, ceftazidime, ceftriaxone); FQ2, fluoroquinolones (ciprofloxacin, levofloxacin); FQ3, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin); MDR1, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 5 classes (ESC4, FQ3, AMINOS, carbapenems, and PIP/PIPTAZ); OX/METH/CEFOX, oxacillin/methicillin/cefoxitin; MDR2, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 5 classes (ESC2, FQ2, AMINOS, carbapenems, and PIP/PIPTAZ).
a If the percentage of isolates tested is <70%, caution should be used when interpreting the percent resistance.
b Percent resistance is only calculated when at least 20 isolates have been tested. Ellipses (…) in percent tested and percent resistance column indicates that fewer than 20 isolates were tested.
c Non-faecalis, non-faecium.
For VAPs, among K. pneumoniae/oxytoca and P. aeruginosa, resistance was higher overall in PICUs than in NICUs. In PICUs, >10% of K. pneumoniae/oxytoca and P. aeruginosa were resistant to carbapenems (Table 7).
TABLE 7 Percent of Pathogens Reported from Ventilator-Associated Pneumonias (VAPs) that Tested Resistant to Selected Antimicrobial Agents, by Reporting Location, 2011–2014
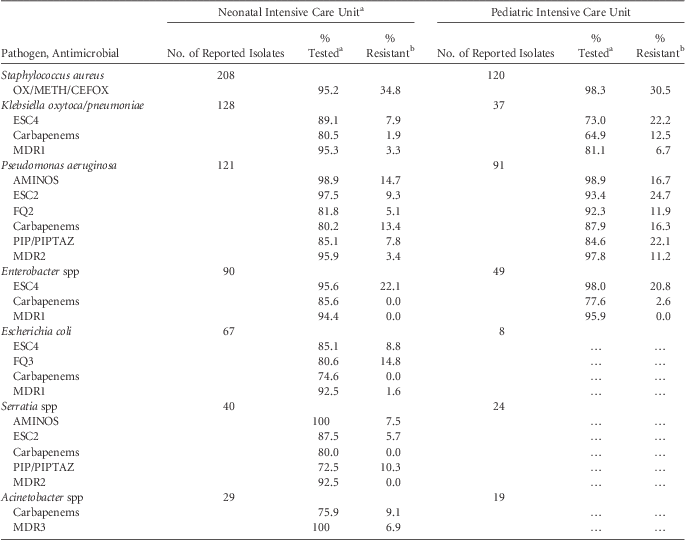
NOTE. AMINOS, aminoglycosides (amikacin, gentamicin, tobramycin); Carbapenems (imipenem, meropenem, doripenem); ESC2, extended-spectrum cephalosporin (cefepime, ceftazidime); ESC4, extended-spectrum cephalosporin (cefepime, cefotaxime, ceftazidime, ceftriaxone); FQ2, fluoroquinolones (ciprofloxacin, levofloxacin); FQ3, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin); MDR1, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 5 classes (ESC4, FQ3, AMINOS, carbapenems, and PIP/PIPTAZ); OX/METH/CEFOX, oxacillin/methicillin/cefoxitin; MDR2, multidrug resistance, [must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 5 classes (ESC2, FQ2, AMINOS, carbapenems, and PIP/PIPTAZ)]; MDR3, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 6 classes (ESC4, FQ2, AMINOS, carbapenems, PIP/PIPTAZ and ampicillin/sulbactam); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam.
a VAP data from neonatal critical care locations from 2011 to 2013.
b If the percentage of isolates tested is <70%, caution should be used when interpreting the percent resistance.
c Percent resistance is only calculated when at least 20 isolates have been tested. Ellipses (…) in percent tested and percent resistance column indicates that fewer than 20 isolates were tested.
For SSIs, percent of pathogens resistant to ESCs was lower for E. coli, P. aeruginosa, and K. pneumoniae/oxytoca (range, 4%–16%) and higher for Enterobacter spp (range, 22%–35%) across types of surgical procedures. Carbapenem resistance was highest among P. aeruginosa isolates causing SSIs due to abdominal surgery (7%). The proportion of methicillin-resistant S. aureus was similar among infections due to abdominal, orthopedic, and neurological surgery types, ranging from 26% in neurological procedures to 31% in abdominal procedures (Table 8).
TABLE 8 Percent of Pathogens Reported from Pediatric Surgical Site Infections (SSIs) that Tested Resistant to Selected Antimicrobial Agents, by Type of Surgery, 2011–2014Footnote a
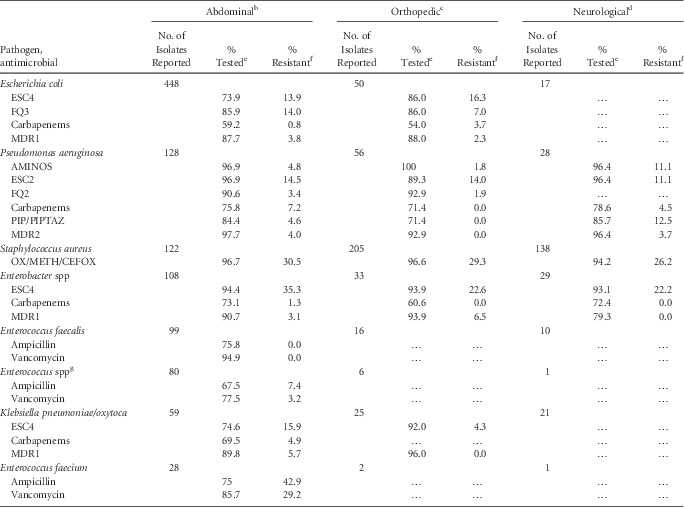
NOTE. AMINOS, aminoglycosides (amikacin, gentamicin, tobramycin); carbapenems (imipenem, meropenem, doripenem); ESC2, extended-spectrum cephalosporin (cefepime, ceftazidime); ESC4, extended-spectrum cephalosporin (cefepime, cefotaxime, ceftazidime, ceftriaxone); FQ2, fluoroquinolones (ciprofloxacin, levofloxacin); FQ3, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin); MDR1, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 5 classes (ESC4, FQ3, AMINOS, carbapenems, and PIP/PIPTAZ); OX/METH/CEFOX, oxacillin/methicillin/cefoxitin; MDR2, multidrug resistance, must test either ‘I’ or ‘R’ to at least 1 drug in 3 of the 5 classes (ESC2, FQ2, AMINOS, carbapenems, and PIP/PIPTAZ); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam.
a Breast, kidney and vascular surgeries had insufficient numbers for reporting, while cardiac and ob/gyn surgeries had sufficient isolates tested for Staphylococcus aureus only: cardiac (n=173, 95.4% tested, 21.2% resistant), ob/gyn (n=31, 93.5% tested, 44.8% resistant).
b Appendectomy, bile duct, liver, or pancreatic surgery, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, abdominal surgery, and rectal surgery.
c Open reduction of fracture, hip prosthesis, knee prosthesis, limb amputation, spinal fusion, refusion of spine, and laminectomy.
d Craniotomy and ventricular shunt.
e If the percentage of isolates tested is <70%, caution should be used when interpreting the percent resistance.
f Percent resistance is only calculated when at least 20 isolates have been tested. A (…) in percent tested and percent resistance column indicates that fewer than 20 isolates were tested.
g Non-faecalis, non-faecium.
DISCUSSION
This report is the first pediatric-specific description of antimicrobial resistance data reported to the NHSN, and it addresses a critical need for the pediatric infectious disease and infection control communities.Reference Milstone, Bryant, Huskins and Zerr 10 , Reference Balkhy and Zingg 22 – Reference Sandora 26 Most previous studies describing pathogens and antimicrobial resistance among pediatric HAIs have come from single institutions, whereas the data presented here represent approximately 1,000 healthcare facilities across the United States.Reference Patel and Saiman 27 Furthermore, this report complements previous publications of pediatric NHSN dataReference Patrick, Kawai and Kleinman 6 , Reference Dudeck, Edwards and Allen-Bridson 9 , Reference Weiner, Webb and Limbago 13 , Reference Hocevar, Edwards, Horan, Morrell, Iwamoto and Lessa 28 by including both pathogen distribution and resistance data from pediatric critical care, oncology and pediatric ward locations to inform infection prevention and antimicrobial stewardship activities.
The pathogen distribution among NICU device-associated infections reported to NHSN between 2006 and 2008 was reported previouslyReference Hocevar, Edwards, Horan, Morrell, Iwamoto and Lessa 28 ; since that report, NHSN data have shown changes in the NICU CLABSI pathogen distribution. Coagulase-negative staphylococci (28.0% of 2,378 reported pathogens) and S. aureus (28.0%) have remained the 2 most common pathogens (28.1% and 24.9% of 7,842 reported pathogens, respectively). Previously Candida spp were the third most common CLABSI pathogens at 13.0%, but when data were pooled across reported species in this report, the proportion decreased to 7.0% (1,192 reported Candida spp pathogens). For VAPs, in 2006–2008, the most common pathogen was P. aeruginosa (16.1% of 830 reported pathogens) followed closely by S. aureus (15.8%). In the current report, S. aureus was the most common (24.2% of 860 reported pathogens).
The most recent NHSN antimicrobial resistance reportReference Weiner, Webb and Limbago 13 represents data from all patient locations, and most of those data are from adult patients, who have often accumulated numerous healthcare and antibiotic exposures over many years and, therefore not surprisingly, tend to have HAIs caused by more resistant pathogens. Our report demonstrates that resistance was lower among pathogens causing pediatric HAIs than in the combined data. For most pathogens and device-associated infection types, carbapenem resistance was lower in NICUs than in PICUs, oncology wards, and pediatric wards, perhaps reflecting a combination of patient age and the relative lack of cumulative antibiotic exposure among NICU patients compared to pediatric patients in other locations. Infections due to carbapenem-resistant organisms primarily affect patients with healthcare exposures, are associated with high mortality, and have been identified as emerging public health threats. 29 – Reference Perez, Hujer, Hujer, Decker, Rather and Bonomo 32 Fortunately, our data show that prevalence overall remains low among pediatric patients, although others have shown increases in recent years, with children who are critically ill disproportionately affected.Reference McGrath and Asmar 31 , Reference Logan 33
An unexpected result of this analysis was the higher rates of resistance for select pathogen-antibiotic combinations, including P. aeruginosa (CLABSIs and CAUTIs) and E. coli (CAUTIs), reported from pediatric wards compared with from PICUs (and even oncology in some instances). Potential explanations include the possibility that patient characteristics and treatments in some pediatric ward locations pose increased risks for infections with resistant pathogens. Although device utilization typically is lower in pediatric wards than in critical care units, children in some ward locations may be treated for complex medical conditions that call for high indwelling device usageReference Dudeck, Edwards and Allen-Bridson 9 or frequent antibiotic usage, placing those children at particular risk for device-associated infections with resistant pathogens. For example, pediatric patients with short gut syndrome, who are dependent upon parenteral nutrition, are at high risk for recurrent central line infections and thus may have higher cumulative antibiotic exposure than even some critical care and oncology patients.Reference Drews, Sanghavi, Siegel, Metcalf and Mittal 34 – Reference Terra, Plopper and Waitzberg 37 For such patients, pathogens causing CLABSIs may be more likely to be antibiotic resistant. Other potential explanations include differences in infection control practices or opportunities for transmission in pediatric ward locations compared to critical care or oncology locations. In addition, it is possible that facilities reporting data to NHSN from pediatric wards may have higher levels of overall antibiotic resistance than facilities only reporting data to NHSN from critical care or oncology locations. Testing this hypothesis is beyond the scope of this paper.
Sparse data for some pathogen–location combinations are another limitation. When the number of reported pathogens is comparatively small for specific locations, between-location comparisons are challenging. Sparse data also limit or preclude meaningful comparisons over time. Increased reporting of HAIs to NHSN from pediatric locations would improve the value of these data. Also, facilities select the locations and HAIs to report to NHSN, so differences in the number of events by HAI or location type may not reflect true differences in the actual frequency of events. We hope that reporting will increase over time, enhancing the representativeness and utility of these data.
Variations in laboratory reporting and testing practices are another study limitation. Antimicrobial susceptibility data are reported to NHSN categorically according to interpretation (i.e., without information on minimum inhibitory concentrations); therefore, any variability in reporting that exists between facilities as well as any changes in testing and reporting practices over time cannot be assessed. Finally, data reported for most isolates indicated resistance, but when less than 70% of reported isolates are tested for resistance to a particular antibiotic, caution should be used when interpreting resistance data for that pathogen–antibiotic combination.Reference Weiner, Webb and Limbago 13
This report presents pediatric antimicrobial resistance data that can be used as a baseline for comparison with future reports. Pathogens associated with HAIs vary in their mode and risk of transmission to patients as well as the mechanisms through which resistance is acquired. The differences in antimicrobial resistance seen in this report may indicate priority areas for prevention work. Overall, lower antimicrobial resistance rates for most pediatric HAIs compared to previously published data on adult HAIs highlight the opportunity for the pediatric healthcare community to pursue novel policies and practices to protect their patients from the acquisition and transmission of highly resistant organisms while these events remain uncommon. NHSN data have the potential to play an important role in monitoring and evaluation of these endeavors.
ACKNOWLEDGMENTS
We thank the NHSN participants and the infection control community for their ongoing efforts to monitor infections and improve patient safety, and we acknowledge our colleagues in the Division of Healthcare Quality Promotion, who work to support this unique and growing public health network. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the Agency for Toxic Substances and Diseases Registry.
Financial support: The NHSN surveillance system is supported by the Division of Healthcare Quality Promotion, CDC.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.236










