Introduction
Enhancement of reproduction, fertility, and population planning are critical issues in farm animal production especially in developing countries, therefore it is effective to use appropriate treatments that regulate fertility and reproduction by controlling gonadal hormone secretion (Kooti and Daraei, Reference Kooti and Daraei2017). Feed additives such as herbal plants (or their extracts) and ionophores (i.e. salinomycin) are used to ensure healthy growth and increase the productivity of farm animals. Phytoestrogens are plant-derived compounds that structurally or functionally resemble estradiol-17β. Thyme and celery are a source of phytoestrogen compounds (Zava et al., Reference Zava, Blen and Duwe1997; Modaresi et al., Reference Modaresi, Ghalamkari and Jalalizand2012) that exert oestrogenic activity via oestrogen receptors (ERs), and may also affect oestrogen synthesis and metabolism (Van-Duursen, Reference Van-Duursen2017). Thyme (or its extracts) contains substances such as phenols, thymol (68.1%), carvacrol (3.5%), monoterpene hydrocarbons, p-cymene (11.2%), and γ-terpinene (4.8%) (Grosso et al., Reference Grosso, Figueiredo, Burillo, Mainar, Urieta, Barroso, Coelho and Palavra2010) that give thyme antioxidant, antimicrobial, and aroma-regulating activity (Calsamiglia et al., Reference Calsamiglia, Busquet, Cardozo, Castillejos and Ferret2007). Celery (Apium graveolens L.) is a plant from the Apiaceae family that grows throughout Europe and in the tropical and subtropical regions of Africa and Asia, (Gauri et al., Reference Gauri, Ali and Khan2015). The chemical constituents of celery essential oil have been identified and classified into five chemical categories, monocyclic terpenes (77.10%), bicyclic terpenes (14.69%), aliphatic hydrocarbons (1.70%), ketones (0.20) and sesquiterpene (2.97%). These identified compounds account for 96.66% of the composition of celery essential oil. The remaining part, 3.34%, represents seven unknown constituents (Hassanen et al., Reference Hassanen, Eissa, Hafez and Mosa2015). Celery has been found to have potent antioxidant activity (Boğa et al., Reference Boğa, Hacıbekiroglu and Kolak2011; Stankevicius et al., Reference Stankevicius, Akuneca, Jakobsone and Maruska2011; Cao et al., Reference Cao, Chen, Song, Liu, Xie, Han, Liu, Ji and Jiang2012). It has been reported that celery decreased GSH, catalase, lipid peroxidation in the liver, and blood haemolysate (Kolarovic et al., Reference Kolarovic, Popovic, Mikov, Mitic and Gvozdenovic2009). Celery showed a significant inhibitory effect toward malonaldehyde (MDA) formation (Wei and Shibamoto, Reference Wei and Shibamoto2007). Ionophore antibiotics (i.e. salinomycin) are fermentation products of various Streptomyces spp. They function as mobile cation carriers and have the ability to complex with and transport organic amines (Butaye et al., Reference Butaye, Devriese and Haesebrouck2003; Oliveira et al., Reference Oliveira, Sousa, Queiroz, Macedo, Neves, Bianchi and Teobaldo2015). Such antimicrobials are used extensively in livestock farming as growth promoters and to improve the health and welfare of animals by controlling coccidiosis and decreasing the shedding of zoonotic pathogens (Sapkota et al., Reference Sapkota, Lefferts, McKenzie and Walker2007). During the estrous cycle and before pregnancy, large amounts of sex hormones, E2 from granulosa cells and P4 from the corpus luteum, are produced that act on the oviducts, uterus, and vagina in preparation for the breeding season (Meikle et al., Reference Meikle, Tasende, Rodriguez and Garófalo1997). Only a few studies have been conducted to investigate the effects of such herbs and ionophores on reproductive performance and sexual hormone profile during the sexual activity period in farm animals.
Therefore, this work aimed to examine the effects of thyme, celery and salinomycin as feed additives on sex hormones, reproductive traits, and antioxidant activities during the estrous cycle of native ewes.
Materials and methods
The current study was carried out at the experimental sheep farm belonging to the Nuclear Research Centre, Atomic Energy Authority, Egypt.
Medicinal plants
The authors bought dried thyme (leaves) and celery (seeds) from a spice dealer (Haraz), Bab Allouq, Cairo, Egypt. The medicinal plants were ground separately, then used in the experiment.
Animal grouping and treatments
Seventy-five mature Barki ewes, 2–3 years old, and mean body weight of 40 ± 1.5 kg were assigned randomly into five groups (15/group), as in Table 1. The concentrate feed mixture (CFM) used in the feeding trial of ewes consisted of (as a percentage): 24.0 corn, 22.5 sugar beet pulp, 20.0 wheat bran, 30.0 undecorated cotton seed cake, 1.0 sodium chloride, 0.3 mineral mix, 2.0 dicalcium phosphate, 0.1 AD3E, and 0.1 sodium bicarbonate. Ewes were fed twice daily at 07:00 and 21:00 h. Fresh drinking water was available at all times. The animals received treatments at 6 weeks before breeding and these were continued until the end of the experiment.
Table 1. Experimental design used for feeding in the study

Reproductive performance
Reproductive traits (presented in Table 3) were recorded throughout the experimental period. A teaser ram was introduced to the ewes to detect the onset of estrous. The teaser ram was joined twice daily at 7 h intervals at 9:00 h up to 16:00 h for each group for 60 min. Ewes that were receptive to the teaser and standing for mounting by the teaser were considered to be in estrous. Ewes in heat were then naturally inseminated by a fertile ram at 9:00 h and introduced to the ram again at 16:00 h to ensure insemination.
Blood sampling and hormonal analysis
Blood samples were collected from the jugular vein into evacuated nonheparinized glass tubes, and kept at room temperature for 30 to 60 min for clotting, then samples were centrifuged at 3000 rpm for 15 min to separate the serum. The serum was stored at −70°C until analysis. Samples were collected throughout different stages of the estrous cycle. Serum P4 (ng/ml) and E2-17β (pg/ml) concentrations were determined using Coat-A-count I-125 RIA kits (cat. no. IM1188, with a standard curve ranging from 0 to ~50 ng/ml and A21854, curve range 0–5000 pg/ml) obtained from Immunotech, a Beckman Coulter Company, Czech Republic.
Antioxidants determinations
GSH and GSSG activities were determined based on a chromatographic procedure with standard Sigma-Aldrich cat. no. 70-18-8, 27025-41-8 (Jayatilleke and Shaw, Reference Jayatilleke and Shaw1993). Superoxide dismutase and MDA were estimated in accordance with the manufacturer’s instructions using kits from the Biodiagnostic Company (Dokki, Giza, Egypt) (cat. nos. SD 2521 and MD 2529, respectively). Nitric oxide was determined as the sum of nitrite and nitrate using an anion-exchange column (Hamilton PRP-X100) using the parameters (150 × 4.1 mm, 10 m, mobile phase 45:55 of 0.1 M NaCl:methanol); wavelength was adjusted to 230 nm in accordance with HPLC procedure (Papadoyannis et al., Reference Papadoyannis, Samanidou and Nitsos1990).
Statistical analysis
Data were expressed as mean ± SE. To test treatment effect on E2 and P4 during the estrous cycle the differences between groups were assessed by one-way analysis of variance (ANOVA) using SAS (1998) software; two-way ANOVA was applied to the test phase of the estrous cycle (follicular and luteal) and for treatments effects on antioxidant parameters. Statistical analysis of the obtained data was performed using the general linear model (GLM). Significant differences among means were evaluated using Duncan’s Multiple Range Test.
Results
Effect of thyme, celery and salinomycin on ovarian sexual hormones during estrous stages of Barki ewes
Results in Table 2 revealed the effect of treatments on P4 and E2 concentrations during the follicular and luteal phases. During the follicular phase of the estrous cycle, groups treated with thyme, celery and T×C showed a significant (P < 0.01) increase in E2 levels compared with the control group, the highest E2 value was 14.19 pg/ml for thyme treatment. Similarly, P4 increased (P < 0.01) during the same phase due to celery and T×C supplementation compared with the control (Table 2). During the luteal phase, P4 levels were significantly higher in the celery group (2.85 ng/ml; P < 0.001) compared with others; the lowest P4 level was 1.16 ng/ml in the thyme group compared with the control. There were no significant (P < 0.05) differences in E2 concentrations between groups during the luteal phase, except for a marked decrease (6.50 pg/ml; P < 0.001) recorded in the salinomycin group compared with the others. Salinomycin treatment decreased (P < 0.01) E2 levels during both follicular and luteal phases of estrous compared with the control group (Table 2).
Table 2. Effect of thyme, celery, and salinomycin on E2 and P4 concentrations during the estrous cycle of Barki ewes
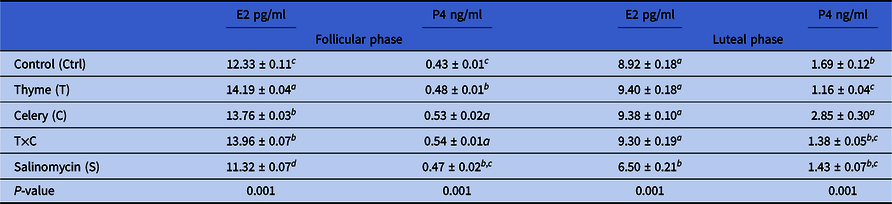
E2 estradiol-17β and P4 progesterone.
a–cColumns with different superscripts are significantly different at P < 0.05.
Table 3. Effect of thyme, celery, and salinomycin on reproductive traits of Barki ewes

Reproductive performance of Barki ewes as affected by thyme, celery and salinomycin
Data listed in Table 3 revealed that ewes supplemented with thyme and celery had a lower number of services per conception (1.3 and 1.5 service/conception, respectively). Thyme females only recorded the highest (66.67%) conception rate from the first insemination. However, ewes in the salinomycin group followed the control in the high percentage of conception rate over two inseminations. Females given both thyme and celery were lower in conception rate at 6.66% compared with other groups (Table 3).
Effect of thyme, celery and salinomycin on antioxidant activity during oestrous stages of Barki ewes
In general, the luteal phase produced higher levels (P < 0.01) of SOD, GSH, GSSG and NO activities compared with the follicular phase (Table 4). Regarding treatment effects, both thyme, celery and T×C treatments showed an increase (P < 0.01) in SOD, GSH, GSSG and NO compared with the control, whereas the salinomycin group did not show differences (P < 0.0) compared with the control except in GSH and MDA levels. With respect to the interaction between estrous phase and treatment, the thyme group showed a significant (P < 0.01) increase in SOD, GSH, GSSG and NO levels during the luteal phase of the estrous cycle compared with the follicular phase and as compared with the control (Table 4). During the follicular phase, SOD, GSH, GSSG and NO levels increased (P < 0.01) due to celery and T×C treatments, while salinomycin increased GSH (1.58 µmol/ml; P < 0.01) compared with the control. During the luteal phase T×C treatment produced higher (P < 0.01) levels of SOD, GSH and NO activities compared with the control; the same trend was observed in the celery group but not for NO. It was noticeable that MDA was elevated (P < 0.01) during the follicular phase more than in the luteal phase in all groups, including in the control group (Table 4).
Table 4. Effect of thyme, celery, and salinomycin on antioxidant activity during the estrous cycle in Barki ewes

GSH, reduced glutathione; GSSG, glutathione disulfide; MDA, malondialdehyde; NO, nitric oxide; SOD, superoxide dismutase.
A–C,a–c Columns with different superscripts are significantly different at P < 0.05.
Discussion
The current results demonstrated that phytoestrogen plants might affect serum E2 and P4 concentrations by inhibiting enzymes that metabolize steroid hormones and therefore increase their levels in serum. The current results showed an increase in E2 concentration in the thyme-treated group during the follicular phase, while P4 decreased compared with the celery-treated group (Table 2). This agreed with the study of Bushra et al. (Reference Bushra, Khudair and Alkalby2016) who demonstrated that treatment with thyme produced a decrease in P4 and an increase in estrogen in mature non-pregnant female rabbits. In contrast, Trabace et al. (Reference Trabace, Zotti, Morgese, Tucci, Colaianna, Schiavone, Avato and Cuomo2011) reported that oral administration of carvacrol (0.45 g/kg) significantly reduced E2 levels only during proestrus, while it has no effect during the diestrus phase. Moreover, oral carvacrol administration did not modify plasma P4 levels in both stages of the estrous cycle. In the same way, Zava et al. (Reference Zava, Dollbaum and Blen1998) studied estrogen and progestin bioactivity in some herbs including thyme, reporting that thyme contains relatively high ER-binding activity and may have weak or mild estrogenic effects. This sex steroid hormone mimic can bind to the respective receptors for endogenous sex hormone in the target cells of the brain and reproductive tract and induce or inhibit biological responses similar to the sex hormone (Zava et al., Reference Zava, Dollbaum and Blen1998). Progesterone, a steroid hormone synthesized from the corpus luteum (CL), and the main product in the luteal phase following ovulation, plays an essential role in the establishment of a uterine environment suitable for the development of peri-implantation conceptus (embryo and associated extraembryonic membranes) and the successful progression and maintenance of pregnancy. Apigenin is a naturally occurring flavonoid in celery, thyme, and other herbs. Its chemical structure is similar to that of estrogen, and it may act as a mimic of estrogen (Bak et al., Reference Bak, Gupta, Wahler and Suh2016), which may enhance ewe estrus signs and male acceptance for mating and fertilization. This may explain the increased percentage of conception from 1st and 2nd inseminations in thyme and celery groups, as well as the decreased number of services per conception compared with the other groups (Table 3).
There is essential evidence that reactive oxygen species (ROS) are critical factors in determining CL lifespan (Behrman et al., Reference Behrman, Kodaman, Preston and Gao2001) and that antioxidants play significant roles in CL physiology during the estrous/menstrual cycle (Sugino et al., Reference Sugino, Takiguchi, Kashida, Karube, Nakamura and Kato2000; Al-Gubory et al., Reference Al-Gubory, Ceballos-Picot, Nicole, Bolifraud, Germain, Michaud, Mayeur and Blachier2005, Reference Al-Gubory, Camous, Germain, Bolifraud, Nicole and Ceballos-Picot2006; Sugino, Reference Sugino2006). Enzyme defence systems of organisms usually neutralize ROS. It is well known that free radicals (such as superoxide, hydroxyl, and hydrogen peroxide) are generated as a result of regular metabolic activity and are eliminated by various routes. However, in the event of antioxidant deficiency and the generation of an excessive number of free radicals due to stress factors, these radicals cause both tissue damage and lipid peroxidation of fatty acids.
It is clear that serum SOD, GSH, GSSG, and NO levels of ewes treated with medical herbs, especially thyme, are higher during the luteal phase compared with the follicular phase. This may be due to oxidative stress in the luteal period when progesterone is the dominant steroid hormone; this result agrees with that of Kuru et al. (Reference Kuru, Kükürt, Oral and Öğün2018). Thyme has antioxidant (Lawrence Reference Lawrence2005), antimicrobial, and antifungal properties that help to maintain health (Basilico and Basilico, Reference Basilico and Basilico1999; Criag, Reference Criag1999). Thymol and carvacrol (the main critical bioactive contents of thyme or thyme essential oil; Aeschbach et al., Reference Aeschbach, Loliger, Scott, Muscia, Butler and Halliwell1994; Grigore et al., Reference Grigore, Paraschiv, Colceru-Mihul, Bubueanu, Draghici and Ichim2010) significantly reduce lipid peroxidation in tissues (Yanishlieva et al., Reference Yanishlieva, Marinova, Gordon and Raneva1999; Nieto et al., Reference Nieto, Bañón and Garrido2011). Gumus et al. (Reference Gumus, Ercan and Imik2017) studied thyme effects on the activity of the antioxidant enzyme SOD – which protects tissues against oxidation – and the enzyme GSH-Px, which protects intracellular lipids against peroxidation. The authors demonstrated that thyme significantly increased the serum activity of SOD and GSH-Px.
Furthermore, as the most significant indicator of lipid peroxidation, it was determined that MDA levels had significantly decreased in the serum of the treated group; these results are in line with our results. Similarly, Hashemipour et al. (Reference Hashemipour, Kermanshahi, Golian and Veldkamp2013) reported that T×C thymol and carvacrol intake caused an increase in antioxidant enzyme activity in the serum and liver of broilers. As mentioned previously, MDA had no significant effect on the thyme and celery effect, in agreement with Akbarian-Tefaghi et al. (Reference Akbarian-Tefaghi, Ghasemi and Khorvash2018), who found that blood MDA levels did not differ among treatments with herbal plants (thyme and celery), and monensin in the dairy calf. The viable antioxidant activity of thymol is due to the presence of phenolic OH groups that act as hydrogen donors to the peroxy radicals produced during the first step in lipid oxidation, therefore retarding hydroxy peroxide formation (Farag et al., Reference Farag, Badei, Hewedi and El-Baroty1989).
In conclusion, supplementation of thyme, celery and salinomycin significantly affected ovarian sexual hormones (E2 and P4) and reproductive performance, especially fertilization from 1st and 2nd inseminations for thyme and celery, as well as enhancement of antioxidant activities of Barki ewes during the estrous cycle.
Ethical Standards
This work was approved by the Committee of Egyptian Atomic Energy Authority, Cairo, Egypt.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
The authors report no conflict of interest.






