Introduction
The still actively forming bat guano assemblage in the Rowley mine in southwestern Arizona, USA, has proven to be a prolific source of new minerals. Including the new mineral described herein, dendoraite-(NH4), eight new minerals have now been described from this assemblage. Dendoraite-(NH4), (NH4)2NaAl(C2O4)(PO3OH)2(H2O)2, is one of only five minerals known to include both phosphate and oxalate groups, the others being davidbrownite-(NH4), (NH4,K)5(V4+O)2(C2O4)[PO2.75(OH)1.25]4⋅3H2O (Kampf et al., Reference Kampf, Cooper, Rossman, Nash, Hawthorne and Marty2019a), phoxite, (NH4)2Mg2(C2O4)(PO3OH)2(H2O)4 (Kampf et al., Reference Kampf, Celestian, Nash and Marty2019b), relianceite-(K), K4Mg(V4+O)2(C2O4)(PO3OH)4(H2O)10 (Kampf et al., Reference Kampf, Cooper, Celestian, Ma and Marty2022), and thebaite-(NH4), (NH4,K)3Al(C2O4)(PO3OH)2(H2O) (Kampf et al., Reference Kampf, Cooper, Celestian, Nash and Marty2021a); all of these, except phoxite, are known only from the Rowley mine bat guano assemblage. One of these, relianceite-(K), is intimately associated with dendoraite-(NH4) and is described in a companion paper, Kampf et al. (Reference Kampf, Cooper, Celestian, Ma and Marty2022).
The name dendoraite is for the Dendora Valley and the Dendora Ranch, which are immediately west of the Rowley mine. For naming and species definition, the total combined occupancy of the two very large cation sites (not the Na site) in the structure is employed; thereby, the ‘-(NH4)’ suffix in the name reflects the fact that NH4+ > K+. If an analogue with K+ > NH4+ were found, it would be named ‘dendoraite-(K)'.
The new mineral and name were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2020-103, Kampf et al., Reference Kampf, Cooper, Celestian, Ma and Marty2021b). The holotype specimen of dendoraite-(NH4) is deposited in the collections of the Natural History Museum of Los Angeles County, Los Angeles, California, USA, catalogue number 75275. This is also the holotype for relianceite-(K).
Occurrence
Dendoraite-(NH4) was found on the 125-foot level of the Rowley mine, ~20 km NW of Theba (small settlement and railroad depot), Maricopa County, Arizona, USA (33°2'57''N, 113°1'49.59''W). The Rowley mine is on the western slope of the Painted Rock Mountains (in the Painted Rock mining district) and overlooks the Dendora Valley, immediately to the west. It is a former Cu–Pb–Au–Ag–Mo–V–baryte–fluorspar mine that exploited veins presumed to be related to the intrusion of an andesite porphyry dyke into Tertiary volcanic rocks. Although the mine has not been operated for ore since 1923, collectors took notice of the mine as a source of fine wulfenite crystals around 1945. An up-to-date account of the history, geology, and mineralogy of the mine was recently published by Wilson (Reference Wilson2020).
The new mineral was found in a hot and humid area of the mine (see figure 26 in Wilson, Reference Wilson2020) in an unusual bat guano-related, post-mining assemblage of phases that include a variety of vanadates, phosphates, oxalates and chlorides, some containing NH4+. This secondary mineral assemblage is found growing on baryte–quartz-rich matrix and, besides dendoraite-(NH4), includes allantoin (Kampf et al., Reference Kampf, Celestian, Nash and Marty2021c), ammineite, antipinite, aphthitalite, bassanite, biphosphammite, cerussite, davidbrownite-(NH4) (Kampf et al., Reference Kampf, Cooper, Rossman, Nash, Hawthorne and Marty2019a), fluorite, halite, hydroglauberite, mimetite, mottramite, natrosulfatourea (Kampf et al., Reference Kampf, Celestian, Nash and Marty2021c), perite, phoxite (Kampf et al., Reference Kampf, Celestian, Nash and Marty2019b), relianceite-(K) (Kampf et al., Reference Kampf, Cooper, Celestian, Ma and Marty2022), rowleyite (Kampf et al., Reference Kampf, Cooper, Nash, Cerling, Marty, Hummer, Celestian, Rose and Trebisky2017), salammoniac, struvite, thebaite-(NH4) (Kampf et al., Reference Kampf, Cooper, Celestian, Nash and Marty2021a), thénardite, urea, vanadinite, weddellite, willemite, wulfenite, and several other potentially new minerals. Dendoraite-(NH4) was found in intimate association with antipinite, fluorite, mimetite, mottramite, relianceite-(K), rowleyite, salammoniac, struvite, vanadinite, willemite, wulfenite, and at least one other potentially new species.
Physical and optical properties
Crystals of dendoraite-(NH4) are colourless blades, up to ~0.1 mm in length, generally growing in sprays (Fig. 1). The blades are elongate on [010], flattened on {001}, and taper to a point; the observed crystal forms are {100}, {001}, {310} and {10⋅1⋅0} (Fig. 2). No twinning was observed. The streak is white, the lustre is vitreous, the Mohs hardness is ~2½, the tenacity is brittle and the fracture is splintery. No cleavage could be observed with certainty because of the small crystal size; however, the structure suggests two cleavages in the [010] zone, probably perfect on {001} and good on {10$\bar{1}$![]() }. The tiny crystals are virtually invisible in density liquids making the measurement of their density impossible. The calculated density is 2.122 g⋅cm–3 using the empirical formula and 2.066 g⋅cm–3 using the ideal (NH4-end-member) formula. Dendoraite-(NH4) is non-fluorescent in long- and short-wave ultraviolet light. The mineral is insoluble at room temperature in H2O, but easily soluble in dilute HCl.
}. The tiny crystals are virtually invisible in density liquids making the measurement of their density impossible. The calculated density is 2.122 g⋅cm–3 using the empirical formula and 2.066 g⋅cm–3 using the ideal (NH4-end-member) formula. Dendoraite-(NH4) is non-fluorescent in long- and short-wave ultraviolet light. The mineral is insoluble at room temperature in H2O, but easily soluble in dilute HCl.

Fig. 1. Sprays of dendoraite-(NH4) blades; FOV 0.8 mm across; holotype 75275.

Fig. 2. Crystal drawing of dendoraite-(NH4); clinographic projection in non-standard orientation, b vertical.
Dendoraite-(NH4) is optically biaxial (–) with α = 1.490(5), β = 1.540(5) and γ = 1.541(5) determined in white light. The 2V could not be measured because of the small crystal size; the calculated 2V is 15.7°. The partially determined optical orientation is X = b (length fast). The mineral is non-pleochroic.
Raman spectroscopy
Raman spectroscopy was conducted on a Horiba XploRA PLUS spectrometer using a 532 nm diode laser, 100 μm slit and 1800 gr/mm diffraction grating and a 100× (0.9 NA) objective. Full pattern peak fitting was performed using the least-squares approach using Gaussian peak shapes to minimise the difference between measured and calculated profiles, and cubic-spline was used for base-line modelling. The spectrum from 3800 to 60 cm–1 is shown in Fig. 3 including labelled mode assignments based on several references: Frost (Reference Frost2004), Frost et al. (Reference Frost, Palmer and Pogson2011), Ma and He (Reference Ma and He2012), Mohaček-Grošev et al., (Reference Mohaček-Grošev, Grdadolnik, Stare and Hadži2009), Rudolph and Irmer (Reference Rudolph and Irmer2007), Sergeeva et al. (Reference Sergeeva, Zhitova and Bocharov2019), Števko et al. (Reference Števko, Sejkora, Uher, Cámara, Škoda and Vaculovič2018) and Yakovenchuk et al. (Reference Yakovenchuk, Pakhomovsky, Konopleva, Panikorovskii, Bazai, Mikhailova, Bocharov, Ivanyuk and Krivovichev2018).

Fig. 3. Raman spectrum of dendoraite-(NH4).
Chemical analysis
Analyses (6 points) were performed at Caltech on a JEOL 8200 electron microprobe in wavelength dispersive spectroscopy mode. Analytical conditions were 15 kV accelerating voltage, 5 nA beam current and 5 μm beam diameter. During vacuum deposition of the conductive carbon coat required for the electron probe microanalysis (EPMA), dendoraite-(NH4) clearly suffered loss of much of the weakly held H2O and probably a portion of its NH4. Dendoraite-(NH4) was very sensitive to the electron beam and additional loss of these components probably occurred during the EPMA. The very large loss in H2O resulted in much higher concentrations for the remaining constituents than are to be expected for the fully hydrated phase; therefore, the other analysed constituents have been normalised to provide a total of 100% when combined with the calculated H2O content. To account for the loss of NH4, (NH4)2O was calculated so that K + NH4 = 2 atoms per formula unit (apfu) in accord with the structure. Insufficient material is available for CHN analysis; however, the fully ordered structure and detailed bond-valence analysis unambiguously established the anion (O, OH, H2O and C2O4) identities and the corresponding quantitative contents of H2O and CO2. Analytical data are given in Table 1.
Table 1. Analytical data (wt.%) for dendoraite-(NH4).

* (NH4)2O, C2O3 and H2O values in the Normalised column are based on the structure.
The empirical formula (based on P = 2 and O = 14 apfu) is [(NH4)1.48K0.52]Σ2.00Na0.96(Al0.96Fe3+0.03)Σ0.99(C2O4)[PO2.97(OH)1.03]2(H2O)2. The simplified formula is (NH4,K)2Na(Al,Fe3+)(C2O4)(PO3OH)2(H2O)2 and the ideal (NH4-end-member) formula is (NH4)2NaAl(C2O4)(PO3OH)2(H2O)2 which requires (NH4)2O 12.95, Na2O 7.71, Al2O3 12.68, P2O5 35.30, C2O3 17.91, H2O 13.44, total 100 wt.%. The Gladstone–Dale compatibility (Mandarino, Reference Mandarino2007) 1 – (K p/K c) is 0.018 in the range of superior compatibility for the empirical formula.
X-ray crystallography and structure determination
Powder X-ray studies were done using a Rigaku R-Axis Rapid II curved imaging plate microdiffractometer with monochromatised MoKα radiation. A Gandolfi-like motion on the φ and ω axes was used to randomise the sample. Observed d values and intensities were derived by profile fitting using JADE Pro software (Materials Data, Inc., USA). The powder data are presented in Table 2. Unit-cell parameters refined from the powder data using JADE Pro with whole pattern fitting are a = 10.702(7), b = 6.289(7), c = 19.236(7) Å, β = 90.961(14)°, and V = 1294.5(17) Å3.
Table 2. Powder X-ray data (d in Å) for dendoraite-(NH4).

Single-crystal X-ray studies were done using a Bruker D8 three-circle diffractometer equipped with a rotating anode generator (MoKα X-radiation), multilayer optics and an APEX-II CCD area detector. A total of 22,987 reflections were integrated from 94 s frames with a 0.3° frame width. The unit-cell dimensions were obtained by least-squares refinement of 2770 reflections with I o > 8σI. Empirical absorption corrections (SADABS; Sheldrick, Reference Sheldrick2015) were applied and equivalent reflections were merged. Systematically absent reflections are consistent with space group P21/n and the structure was successfully solved in that space group by direct methods using SHELXS-2013. The structure was refined using SHELXL-2016 (Sheldrick, Reference Sheldrick2015). Two large-cation sites were refined with joint occupancies by N and K, showing both to have N(NH4) > K. A somewhat smaller cation site was consistent with full occupancy by Na. Al was assigned to a small octahedrally coordinated cation site, but exhibited a slight excess of scattering power indicating a small Fe content; the site was ultimately refined with joint Al/Fe occupancy. Difference-Fourier syntheses located all H atom sites except those associated with the two N/K sites and with the OW2 site. Note that one possible H site associated with OW2 was indicated in the difference Fourier; however, a second H site was not apparent and, because of the strongly prolate nature of the OW2 site, we lack confidence in assigning any H sites for OW2. The H sites associated with OH1, OH5 and OW1 were refined with restraints of 0.98(1) Å on the O–H distances. Data collection and refinement details are given in Table 3, atom coordinates and displacement parameters in Table 4, selected bond distances in Table 5 and a bond-valence analysis in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Data collection and structure refinement details for dendoraite-(NH4).

*R int = Σ|F o2–F o2(mean)|/Σ[F o2]. GoF = S = {Σ[w(F o2–F c2)2]/(n–p)}½. R 1 = Σ||F o|–|F c||/Σ|F o|. wR 2 = {Σ[w(F o2–F c2)2]/Σ[w(F o2)2]}½; w = 1/[σ2(F o2) + (aP)2 + bP] where a is 0.0601, b is 2.3477 and P is [2F c2 + Max(F o2,0)]/3.
Table 4. Atom positions, occupancy and displacement parameters (Å)2 for dendoraite-(NH4).

Table 5. Selected bond lengths (Å) and angles (°) for dendoraite-(NH4).

Table 6. Bond-valence analysis for dendoraite-(NH4). Values are in valence units (vu).

Bond-valence parameters for NH4+–O are from Garcia-Rodriguez et al. (Reference García-Rodríguez, Rute-Pérez, Piñero and González-Silgo2000); all others are from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). The K/N sites were modelled using refined occupancies. Hydrogen-bond strengths are based on O–O distances according to the relation of Ferraris and Ivaldi (Reference Ferraris and Ivaldi1988).
Discussion of the structure
The N1 and N2 sites are both ten-fold coordinated, the Na site is seven-fold coordinated. The Al site is octahedrally coordinated and two P sites, P1 and P2, are both tetrahedrally coordinated by three O and one OH. One oxalate (C2O4) group includes two independent C sites, C1 and C2, and four independent O sites.
The structural unit is a double-strand chain of corner-sharing AlO6 octahedra and P1O3OH tetrahedra decorated by P2O3OH tetrahedra and C2O4 groups. This decorated [Al(C2O4)(PO3OH)2]3– chain is topologically identical to that in thebaite-(NH4), although the P2O3OH tetrahedra have distinctly different orientations in the two structures (Fig. 4). Without the decorating P2O3OH tetrahedra and C2O4 groups, this octahedral–tetrahedral double-strand chain is topologically identical to the chain in hannayite, Mg3(NH4)2(HPO4)4⋅8H2O (Catti and Franchini-Angela, Reference Catti and Franchini-Angela1976), while those in galliskiite, Ca4Al2(PO4)2F8⋅5H2O (Kampf et al., Reference Kampf, Colombo, Simmons, Falster and Nizamoff2010) and kapundaite, (Na,Ca)2Fe3+4(PO4)4(OH)3⋅5H2O (Mills et al., Reference Mills, Birch, Kampf, Christy, Pluth, Pring, Raudsepp and Chen2010) are geometrical isomers.

Fig. 4. The chains along [010] in the structures of dendoraite-(NH4) and thebaite-(NH4). [100] is vertical; [010] is canted down 20° from right to left.
The chains in the structure of dendoraite-(NH4) are linked to one another through NaO7(H2O) polyhedra. Each Na-centred polyhedron shares an edge with a P1O3OH tetrahedron, an edge with an AlO6 octahedron and a corner with a P2O3OH tetrahedron, all in the same chain. Another edge of the NaO7(H2O) polyhedron links to an adjacent chain via the bridging C2O4 group. In this manner, the chains are joined to form a [Na(H2O)Al(C2O4)(PO3OH)2]2– sheet parallel to {001} (Fig. 5). The region between the sheets contains the two (NH4)/K sites, N1 and N2, and an H2O group, OW2, that bonds to both (NH4)/K sites. The [Na(H2O)Al(C2O4)(PO3OH)2]2– sheets connect to one another through bonds to (NH4)/K and through hydrogen bonds (Fig. 6).

Fig. 5. The sheet parallel to {001} in the structure of dendoraite-(NH4).
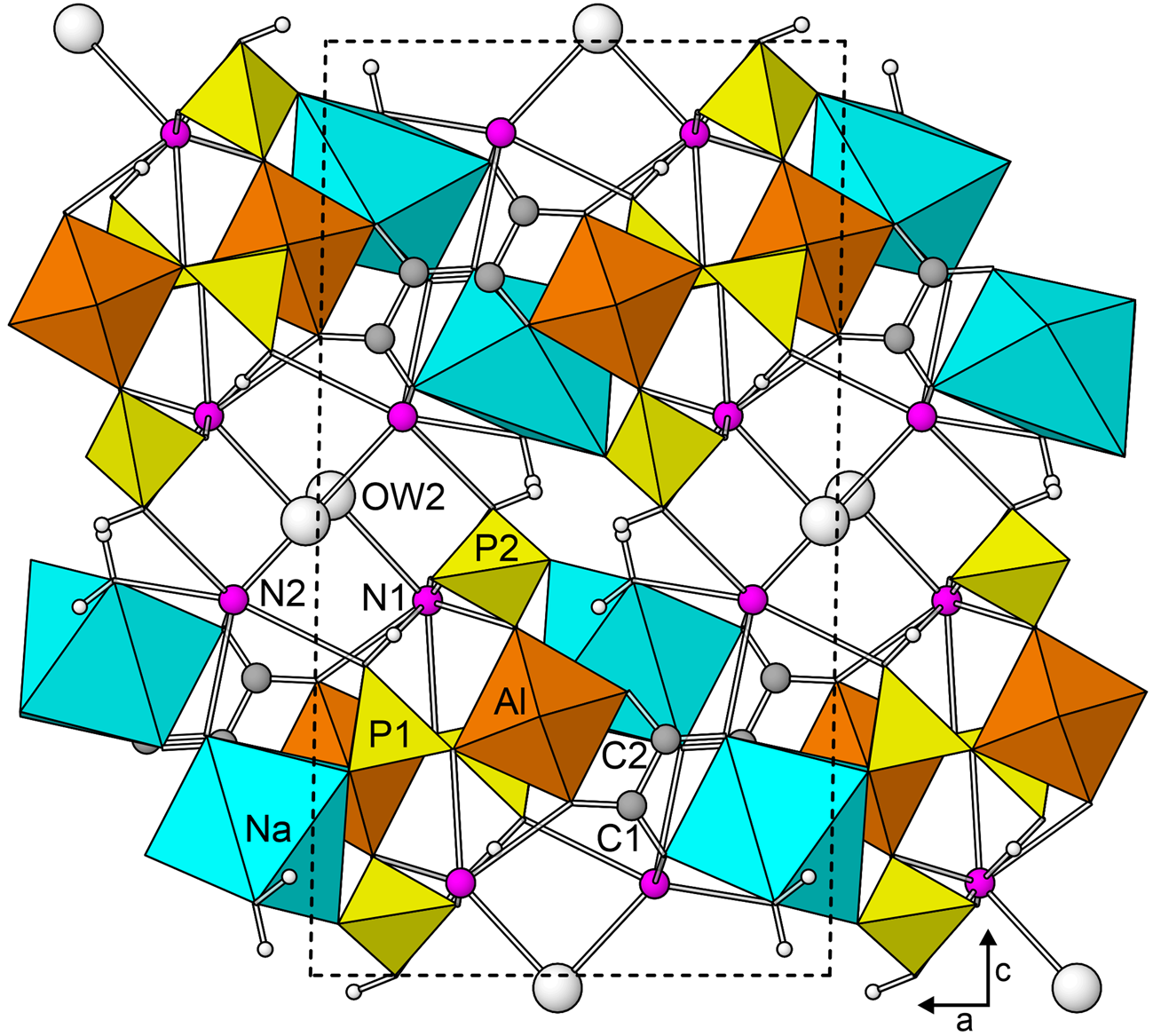
Fig. 6. The dendoraite-(NH4) structure viewed down [010]. The unit cell outline is shown with dashed lines.
As already shown, the [AlC2O4(PO3OH)2]3– chains in dendoraite-(NH4) and thebaite-(NH4) are very similar. The P1 and P2 tetrahedra are PO3(OH) groups delivering strong intra- and inter-chain H-bonds (i.e. OH⋅⋅⋅OA distances are 2.59–2.64 Å). Both structures contain similar intra-chain H-bonds directed from the OH group at OH1 of the P1 tetrahedron towards an O2– anion of the decorating P2 tetrahedron. However, the two structures differ in their inter-chain H-bond that is directed from the OH group at OH5 of the P2 tetrahedron to an O2– anion of the neighbouring chain. In dendoraite-(NH4), this inter-chain H-bond is directed towards an O2– anion of a P2 tetrahedron of the neighbouring chain, whereas in thebaite-(NH4) it is directed towards an O2– anion of the C2O4 group of the neighbouring chain. This difference in H-bond linkage between [AlC2O4(PO3OH)2]3– chains in the two structures is facilitated by a relative ~180° rotation of half of the P2 tetrahedra, and lateral offset between chains within the {010} plane (Fig. 7).

Fig. 7. Hydrogen-bond linkages between the [AlC2O4(PO3OH)2]3– chains in the structures of dendoraite-(NH4) and thebaite-(NH4) viewed down [010]. The hydrogen bonds are shown as turquoise-coloured lines pointing from the donating OH group toward the receiving O atom.
Acknowledgements
Anonymous reviewers and Pete Leverett are thanked for constructive comments, which improved the manuscript. Keith Wentz, claim holder of the Rowley mine, is thanked for allowing underground access for the study of the occurrence and the collecting of specimens, along with Frank Hawthorne for providing access to the single-crystal instrument at the University of Manitoba. This study was funded, in part, by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.98















