Introduction
Grape is one of the most important fruits in the world, and it is ranked third in fruit production (FAO STAT Database at www.fao.org). The wild Vitis species are primarily distributed in three centres of diversity: East Asia (Asian species), North to Central America (American species), and Europe to Central Asia (European species). Vitis amurensis is the most important East Asian Vitis species and is widely distributed in north-eastern China. This species is able to resist extremely low temperatures ( − 40°C) and diseases, and its fruit is the most important material for winemaking in China as an important resource for hybridization with other genotypes (Alleweldt et al., Reference Alleweldt, Speigel-Roy, Reisch, More and Ballington1990; He, Reference He and He1999; Kong, Reference Kong2004).
Seed dormancy and its removal is one of the obstacles for plant breeding. Seeds of Vitis species are dormant, and many studies have been conducted on its seed dormancy release (Flemion, 1937; Singh, Reference Singh1961; Manivel and Weaver, Reference Manivel and Weaver1974; Ellis et al., Reference Ellis, Hong and Roberts1983; Spiegel-Roy et al., Reference Spiegel-Roy, Shulman, Baron and Ashbel1987; Celik, Reference Celik2001; Conner, Reference Conner2008). However, most of these studies are about European and American species, and there is little information about dormancy release and germination of V. amurensis seeds, which has impeded the use of this germplasm for grape breeding.
Inter-species variation in dormancy release and germination is a natural phenomenon found in many plant genera (Skordilis and Thanos, Reference Skordilis and Thanos1995; Araki and Washitani, Reference Araki and Washitani2000; Veasey et al., Reference Veasey, Karasawa, Santos, Rosa, Mamani and Oliveira2004; Karlsson and Milberg, Reference Karlsson and Milberg2007; Vandelook et al., Reference Vandelook, Bolle and Van Assche2007; Oliveira and Garcia, Reference Oliveira and Garcia2011). This variation can be attributed to differences in genetic characteristics as well as the environmental conditions under which the seeds mature (Grime et al., Reference Grime, Mason, Curtis, Rodman, Band, Mowforth, Neal and Shaw1981; Baskin and Baskin, Reference Baskin and Baskin1998; Allen and Meyer, Reference Allen and Meyer2002). In Vitis, this variation also appears to be clear. For example, cold stratification at 5°C for 12 weeks is required for dormancy release of V. vinifera seeds (Singh, Reference Singh1961), while in some unspecified species, seed dormancy could be released by stratification at 3–5°C for only 20 d (Ellis et al., Reference Ellis, Hong and Roberts1983). Our previous work on Vitis also indicated that stratification at 5°C for about 20 d is enough to release dormancy in seeds of V. vinifera × V. amurensis (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009). For a given species, seed dormancy and germination can also vary among populations originating from different habitats (Meyer and Monsen, Reference Meyer and Monsen1991; Andersson and Milberg, Reference Andersson and Milberg1998; Meyer and Allen, Reference Meyer and Allen1999; Schütz and Rave, Reference Schütz and Rave2003; Karlsson and Milberg, Reference Karlsson and Milberg2007; Qiu et al., Reference Qiu, Bai, Fu and Wilmshurst2010). Thus, to obtain comprehensive information about seed dormancy release and germination characteristics of V. amurensis seeds, it is important to conduct research on V. amurensis seeds not only on a species basis, but also on a population (variety) basis.
Seed dormancy is an adaptive mechanism to environmental conditions, and its removal is tightly related to the habitat at which the mother plant grows (Bewley, Reference Bewley1997; Baskin and Baskin, Reference Baskin and Baskin1998; Allen and Meyer, Reference Allen and Meyer2002). Temperature and water are two major environmental factors influencing seed dormancy. Thus, understanding dormancy release behaviour in response to various temperatures and seed moisture contents can help us discover some regeneration strategies of seeds in response to environment factors. Hitherto, research on grape seed dormancy release has focused only on chilling; chemicals like gibberellins, hydrogen peroxide and cyanamide; scarification or combinations of these treatments (Singh, Reference Singh1961; Manivel and Weaver, Reference Manivel and Weaver1974; Ellis et al., Reference Ellis, Hong and Roberts1983; Spiegel-Roy et al., Reference Spiegel-Roy, Shulman, Baron and Ashbel1987; Celik, Reference Celik2001; Conner, Reference Conner2008). The purpose of these treatments is mainly to obtain high germination percentages for agricultural purposes, but the mechanism of dormancy release in response to the natural environment is unclear. In our previous research, we used a hybrid species of V. vinifera × V. amurensis to test for dormancy release in response to various temperatures and seed moisture contents. We found that dormancy release in this hybrid was not limited to cold temperature, but could occur at a very wide range of temperatures and seed moisture conditions (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009). The regeneration strategy of grape seed in wild conditions is very complicated (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009).
In the present study, we have investigated seed dormancy and germination traits in six varieties of V. amurensis, including cultivars of wild plants and hybrid varieties from two cultivated sites. Our purpose was to determine the optimum dormancy release and germination conditions for V. amurensis seeds, and to determine the variation in dormancy and germination within this species. A series of stratification treatments was applied to release seed dormancy. Freshly harvested and dormancy-released seeds were germinated at the different temperatures. Mathematical models were applied to quantify the effects of stratification on seed dormancy release in response to a series of temperatures and seed water contents. This work will contribute to understanding the ecological adaptation of V. amurensis to its natural environment.
Materials and methods
Seed collection
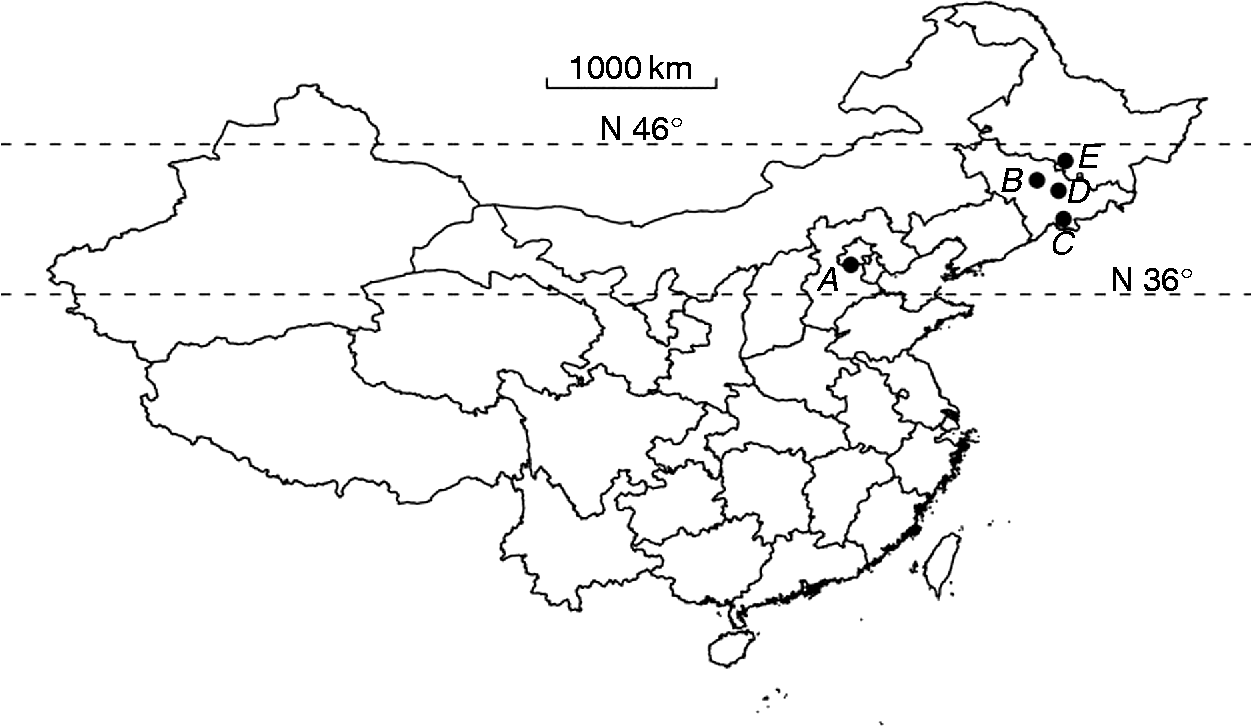
Six varieties of V. amurensis were chosen as experimental material, and seeds were collected from two cultivated sites (Table 1 and Fig. 1). The six varieties included three hybrid varieties of V. amurensis (ZS, SH and SY) and cultivars of wild V. amurensis (TH1, ZS2 and ZS1) (Table 1). The original growth sites of varieties or parent varieties were different (Table 1 and Fig. 1).
Table 1 Materials used in the present experiment

A: Germplasm repository of grapes in the Institute of Botany, the Chinese Academy of Science, Beijing in the middle of China (39.59°N, 116.13°E); B: Germplasm repository of Vitis amurensis in the Institute of Special Wild Economic Animal and Plant, the Chinese Academy of Agriculture, Zuojia in north-eastern China (44.08°N, 126.06°E); C: Changbai Mountains area, Jilin province, China (42.03°N, 127.47°E); D: Xinzhan town, Jilin province, China (43.53°N, 127.20°E); E: Shangzhi town, Heilongjiang province, China (45.12°N, 127.57°E).
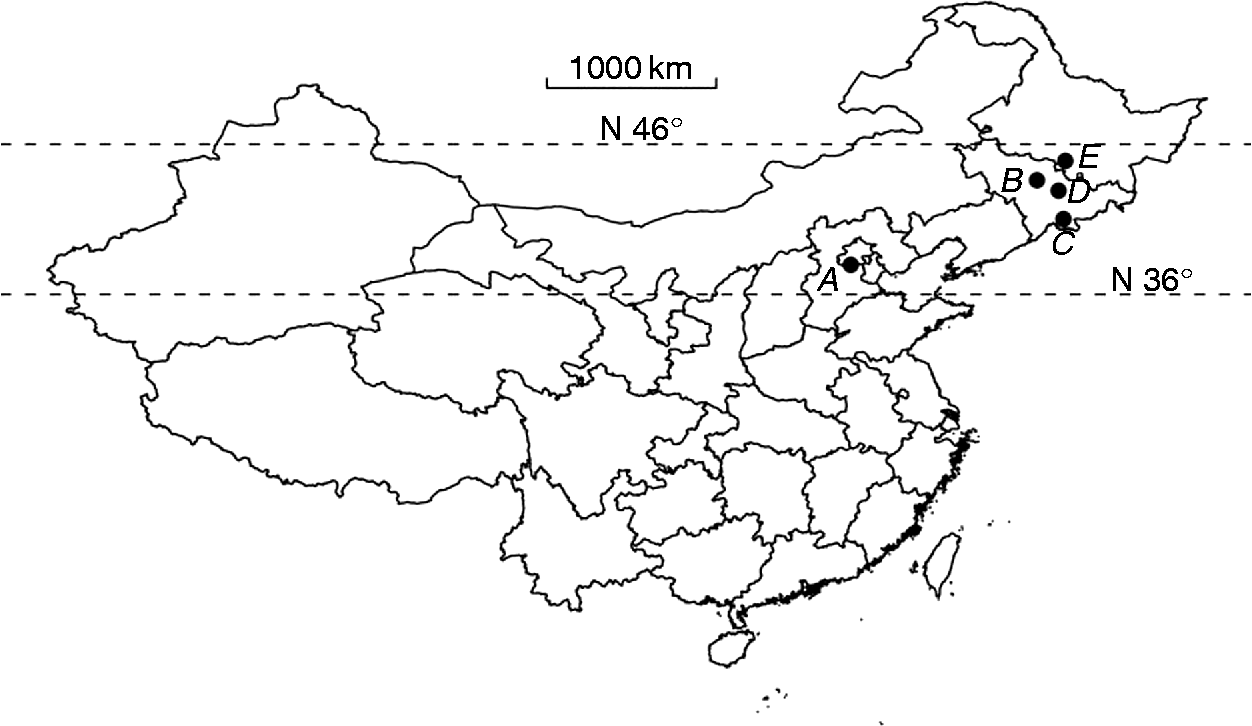
Figure 1 Original growth and cultivation sites of V. amurensis varieties.
Seeds were collected from 5–10 vines. After collection, the exocarp and mesocarp were removed, and seeds with the endocarp were washed thoroughly in water. These seeds (without drying) were called freshly harvested seeds and were used in immediate experiments.
Water content determination
Water content of seeds was determined according to the methods of the International Seed Testing Association (1999) and expressed on a dry mass basis (g H2O (g dry weight)− 1, g g− 1). Four replicates of five seeds each were used to determine water content.
Germination of freshly harvested seeds
Four replicates of 50 seeds each were incubated in darkness at four constant temperatures (30, 25, 20 and 15°C) and three fluctuating temperatures (30/20, 30/10 and 25/15°C, 12/12 h). Germination was monitored at regular intervals over a period of 30 d under daylight (light was found to have no effect on seed germination). Moist perlite (about 2.0 g g− 1) was used as the germination medium.
Stratification at different temperatures
Four replicates of more than 300 seeds each were mixed with moist perlite (2.0 g g− 1, 50 seeds per 5 g perlite) and then stratified in darkness at 5, 10, 15, 20, 25 and 15/5°C for 0, 15, 30, 60 and 120 d. After a given stratification period, 50 seeds were removed from each replicate and incubated at 30/20°C (12/12 h) in darkness for germination. Also, at the end of the stratification, 25 seeds were removed from each replicate and then dried to a water content of about 0.14 g g− 1 and stratified at 5°C for 60 d to enable further dormancy release. After stratification seeds were germinated at 30/20°C (12/12 h) in darkness for 30 d. Germination percentages of these treatments were compared to the percentages of freshly harvested seeds that had been dried and stratified to determine seed viability.
Warm stratification followed by cold stratification
Four replicates of more than 100 seeds each were stratified in moist perlite at 25°C for 30 and 60 d, after which they were transferred to 5°C for 30 d. Seeds stratified continuously at 25 or at 5°C for 30 or 60 d were used as controls. After a given stratification, 50 seeds were removed from each replicate and incubated at 30/20°C (12/12 h) in darkness for germination.
Drying treatment before or after stratification
Four replicates of more than 50 seeds each were initially dried for about 1 d in silica gel to a water content of about 0.14 g g− 1 and then stratified in moist perlite at 5°C in darkness for 60 d. In addition, four replicates of more than 50 seeds each were initially stratified in moist perlite at 5°C in darkness for 60 d and then dried to 0.14 g g− 1 before germination. After stratification or drying, seeds were tested for germination at 30/20°C (12/12 h) in darkness.
Stratification under various relative humidities
Four replicates of more than 80 seeds each were dried to about 0.14 g g− 1 and then hydrated to different levels of moisture content (MC) at controlled relative humidities (RH) over saturated salt or glycerol solutions. These seeds were stratified in darkness for 90 d at 5°C and over a range of RHs: 7.5% (saturated NaOH solution), 43% (saturated K2CO3 solution), 76% (saturated NaCl solution), 96% (20% v/v glycerol), 98% (12% v/v glycerol) and 100% RH (water). In addition, seeds were stratified in darkness for 90 d at 25°C and over a range of RHs: 33% (saturated MgCl2 solution), 43% (saturated K2CO3 solution), 75% (saturated NaCl solution), 96% (20% v/v glycerol), 98% (12% v/v glycerol) and 100% RH (water). Seeds were also embedded in moist perlite at 5 and 25°C in darkness for 90 d to serve as controls. After stratification, 50 and 5 seeds were taken out from each replicate to germinate at 30/20°C and to determine seed MC, respectively. Moreover, 25 seeds were taken from each replicate and stratified at 5°C for 60 d in moist perlite (2 g g− 1) to enable further dormancy release. Seeds after stratification were germinated at 30/20°C (12/12 h) in darkness for 30 d. Germination percentages of these treatments were compared to the percentages of the control seeds at 5°C to determine seed viability.
Germination at different temperatures following 5°C stratification
Four replicates of more than 350 seeds each were dried to about 0.14 g g− 1 and then stratified in darkness at 5°C in moist perlite for 60 d. After stratification, 50 seeds were taken from each replicate and incubated in darkness for a period of 30 d at 30, 25, 20 and 15°C and at 30/20, 30/10 and 25/15°C (12/12 h) with average germination temperatures of 25, 20 and 20°C, respectively. The ratio of maximum germination percentage at fluctuating temperature (FT) to that at constant temperature (CT) was used for quantifying the germination difference between FT and CT.
Data analysis
A mathematical model developed previously (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009) was applied to describe the effect of stratification temperature on dormancy release of V. amurensis seeds. This model is based upon the assumption that temperature affects dormancy release and dormancy induction independently and at different rates, and dormancy induction occurs simultaneously with dormancy release (Totterdell and Roberts, Reference Totterdell and Roberts1979; Kebreab and Murdoch, Reference Kebreab and Murdoch1999; Batlla et al., Reference Batlla, Grundy, Dent, Clay and Finch-Savage2009; Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009). The number of seeds expected to be germinated after stratification at a given temperature is the net effect of dormancy release and induction. If the dormancy release and induction both follow a cumulative normal distribution and the maximum numbers of seeds in which dormancy is released and induced are equal, then germination percentage (y) for a given stratification temperature and time can be predicted by:
![y = G _{\, max \,}\times \Phi ( g 1 + r 1\times t _{ sg })\quad \times \{1 - [\Phi ( g 2 + r 2\times t _{ sg }) - \Phi ( g 2)]\}](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20160921054233148-0826:S0960258511000225:S0960258511000225_eqn1.gif?pub-status=live)
where G max is the maximum percentage of seeds that can be released from or induced to dormancy in the experimental population, g1 is the initial probit percentage of non-dormant seeds in a population with 100% of seeds that can be released from dormancy, that is,
(g is the observed germination of freshly harvested seeds), r1 and r2 are the rates of dormancy release and induction, respectively, t sg is the stratification time, and g2 is the probit percentage of seeds that cannot germinate in the experimental population, that is,
Equation (1) is applicable to seeds that can germinate at harvest time. For seeds that have no germination at harvest, the predicting equation is:
![y = [\Phi ( g 2 + r 1\times t _{ sg }) - \Phi ( g 2)]\quad \times \{1 - [\Phi ( g 2 + r 2\times t _{ sg }) - \Phi ( g 2)]\}](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20160921054233148-0826:S0960258511000225:S0960258511000225_eqn4.gif?pub-status=live)
The parameters are the same as those of equation (1).
Stratification of seeds under a series of RHs at 5 and 25°C presents different patterns in dormancy release and are therefore analysed separately by two different models.
At 5°C, dormancy release is assumed to follow a cumulative normal distribution and percentage of germination (y) expected to be germinated after stratification at a given seed MC (x) is predicted according to:
where G max is the maximum percentage of seeds that can be released from dormancy; Φ is the normal probability integral, μ is the mean and σ is the standard deviation of seed MC for dormancy release.
At 25°C, dormancy release is assumed to follow two different cumulative normal distributions below and above an optimum seed MC, and the percentage of germination after stratification is:
![y = \, G _{\, max \,}\times \{\Phi [( x - \mu 1)/ \sigma 1] + \Phi [( \mu 2 - x )/ \sigma 2] - 100\%\}](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20160921054233148-0826:S0960258511000225:S0960258511000225_eqn6.gif?pub-status=live)
where μ1 and μ2 are the means, and σ1 and σ2 are the standard deviations of the normal distribution of y at sub- or supra-optimal range of seed MC, respectively. μ1 and μ2 are defined as means of the lower and upper threshold seed MC (W sl and W su) for dormancy release, respectively (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009).
Graphpad Prism 5.0 (GraphPad Software) was used for non-linear regression and parameter estimation, and a global regression with Prism 5.0 was used to make a regression with constraint parameters (Motulsky and Christopoulos, Reference Motulsky and Christopoulos2003). All data in this paper are expressed as means ± SD and after arcsine-transformation, analysed for statistical significance by analysis of variance (ANOVA). When significant effects were detected, Fisher's least significant difference (LSD) test was applied to determine the differences among treatments (P < 0.05). The one-way ANOVA was analysed by using the SPSS 17.0 package for Windows (SPSS Inc., Chicago, Illinois, USA).
Results
Germination of freshly harvested seeds
The germination percentage of freshly harvested seeds of SH, TH1, ZS2, ZS1 and SY was nil at all germination temperatures, whereas that of ZS was about 10% at 30/20°C after 30 d (data not shown).
Stratification at different temperatures
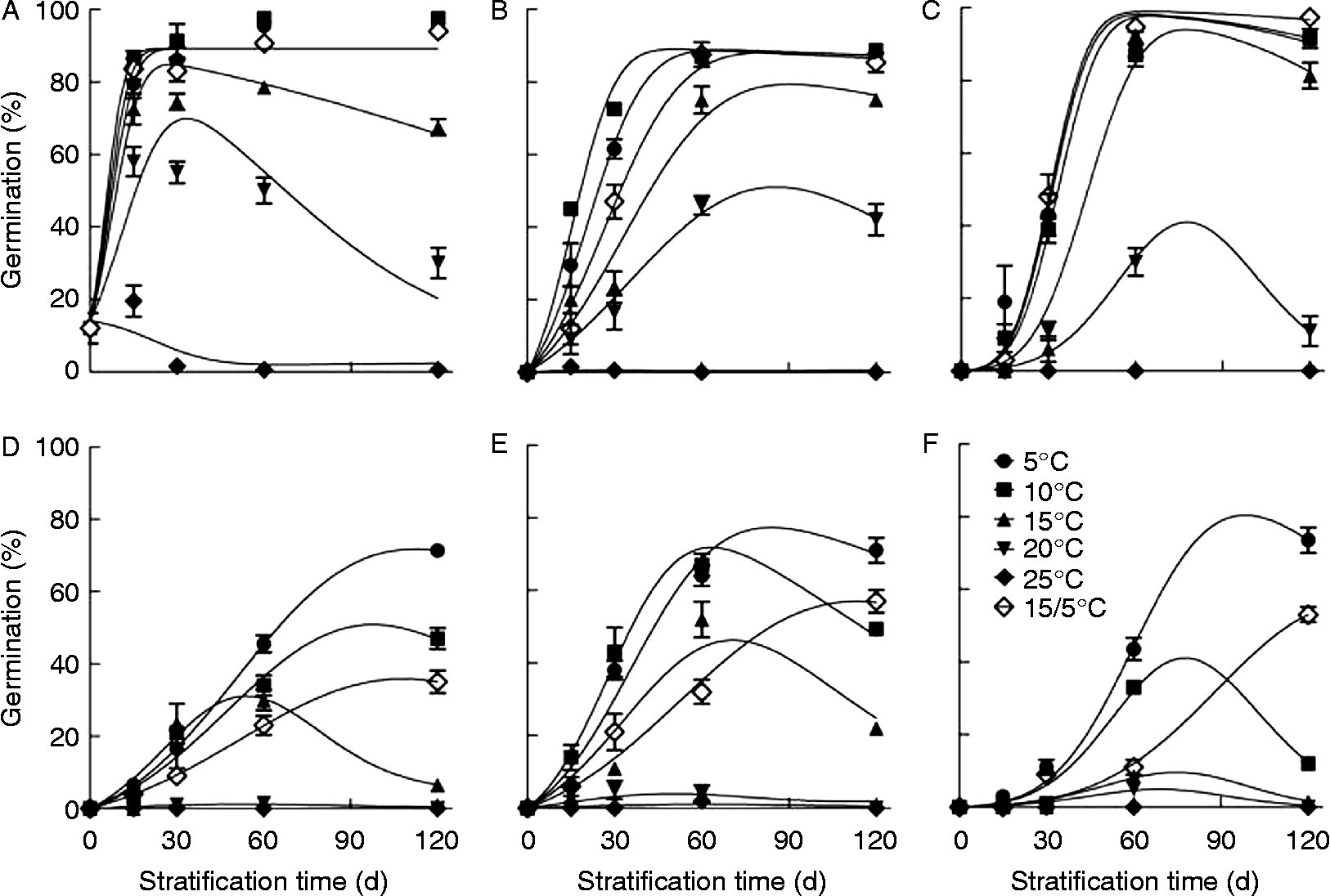
Dormancy of V. amurensis seeds was released by stratification at 5–20°C, but the effect depended on variety, stratification temperature and time (Fig. 2). For ZS, SH and TH1, ≥ 85% of the seeds were released from dormancy by stratification at 5, 10 and 15/5°C for less than 60 d, and 40–80% of the seeds were released from dormancy by stratification at 15 or 20°C (Fig. 2A, B and C). In ZS2, ZS1 and SY, less than 70% of the seeds were released from dormancy by stratification at 5, 10 and 15/5°C for 120 d.
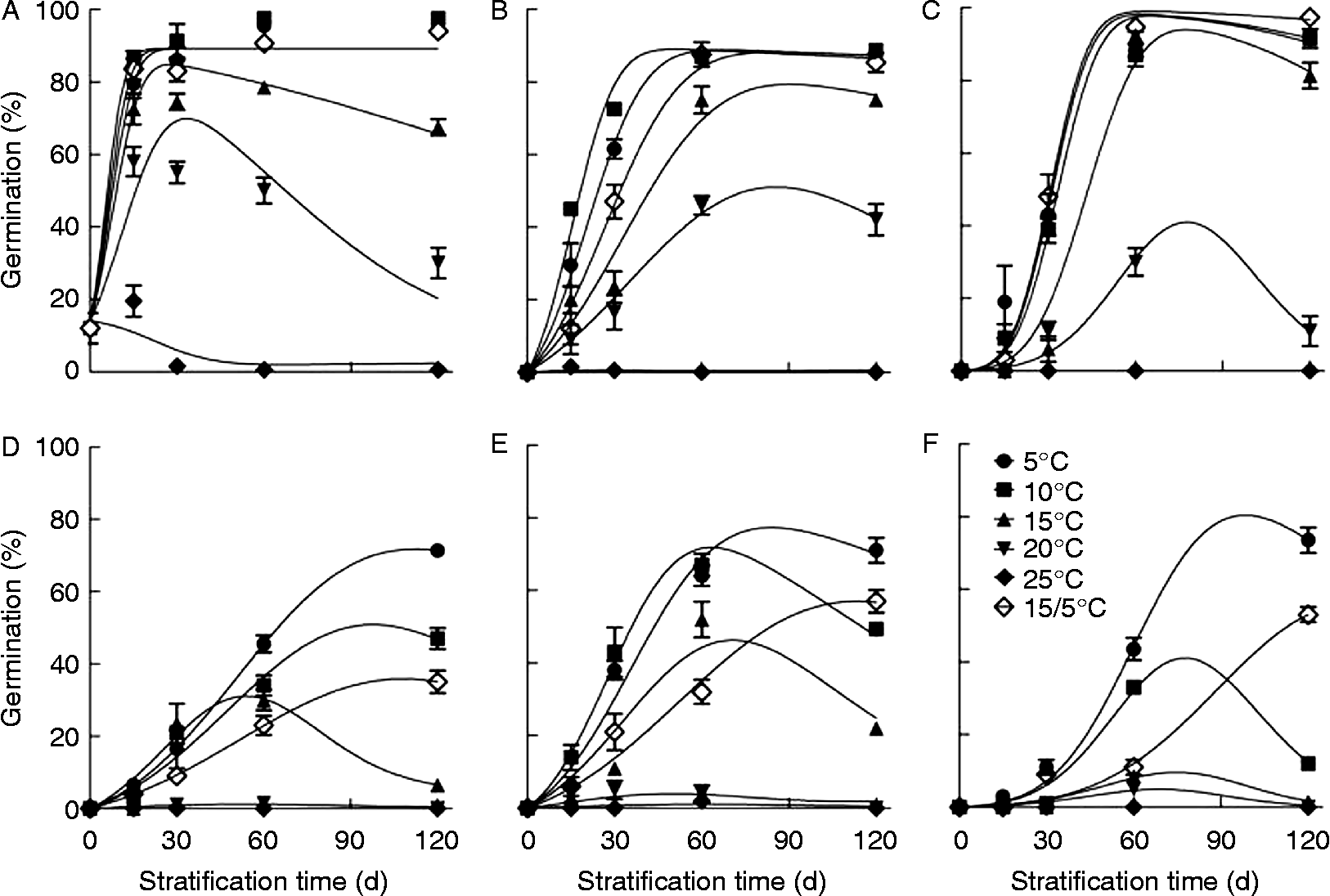
Figure 2 Seed dormancy release of V. amurensis varieties by stratification at different temperatures. Freshly collected grape seeds were mixed with moist perlite and stratified at 5, 10, 15, 20, 25 and 15/5°C (12/12 h) for 15, 30, 60 and 120 d, respectively. After stratification, seeds were germinated at 30/20°C (12/12 h) for 30 d. Data points are means ± SD (n = 4). The curves are fitted lines with equation (1) (A), and with equation (4) (B–F). (A) ZS, (B) SH, (C) TH1, (D) ZS2, (E) ZS1, (F) SY.
The percentage of germinable seeds in ZS, TH1 and ZS1 increased initially and then decreased with increasing stratification time at 15 to 25°C (Fig. 2A, C and E), and in ZS2 and SY, at 10 to 25°C (Fig. 2D and F). The decrease in germination percentage during stratification could result from dormancy induction and/or loss of seed viability. Further treatments for dormancy release indicated that seed stratified at 5 to 25°C for 120 d could germinate as well as freshly harvested seeds after full dormancy release (data not shown). Thus, seeds did not lose their viability, and thus secondary dormancy must have been induced during the stratification process.
To model the effect of stratification on seed dormancy, we applied a model that integrates processes of dormancy release and dormancy induction (equations 1 and 4). This model described well the change of germinable seeds with stratification time after stratification at 5–25°C (Fig. 2). Based upon this model, the rates of dormancy release and induction were obtained. The rates of dormancy release and induction were temperature and variety dependent. The rate of dormancy release in ZS1 seeds increased with temperature rising from 5 to 15°C; in ZS, SH and ZS2 seeds it increased with temperature rising from 5 to 10°C; and in TH1 and SY seeds it kept constant with temperature rising from 5 to 10°C, and then decreased with a further temperature increase (Fig. 3A). Among the six varieties, dormancy release was fastest in ZS seeds, with a maximum rate of 0.19 d− 1 at 10°C, and slowest in ZS1 seeds, with a maximum rate of 0.037 d− 1 at 15°C (Fig. 3A). Dormancy release almost stopped at 25°C for all varieties (Fig. 3A). The rate of dormancy induction continuously increased with rising temperature, but it varied greatly among varieties (Fig. 3B).
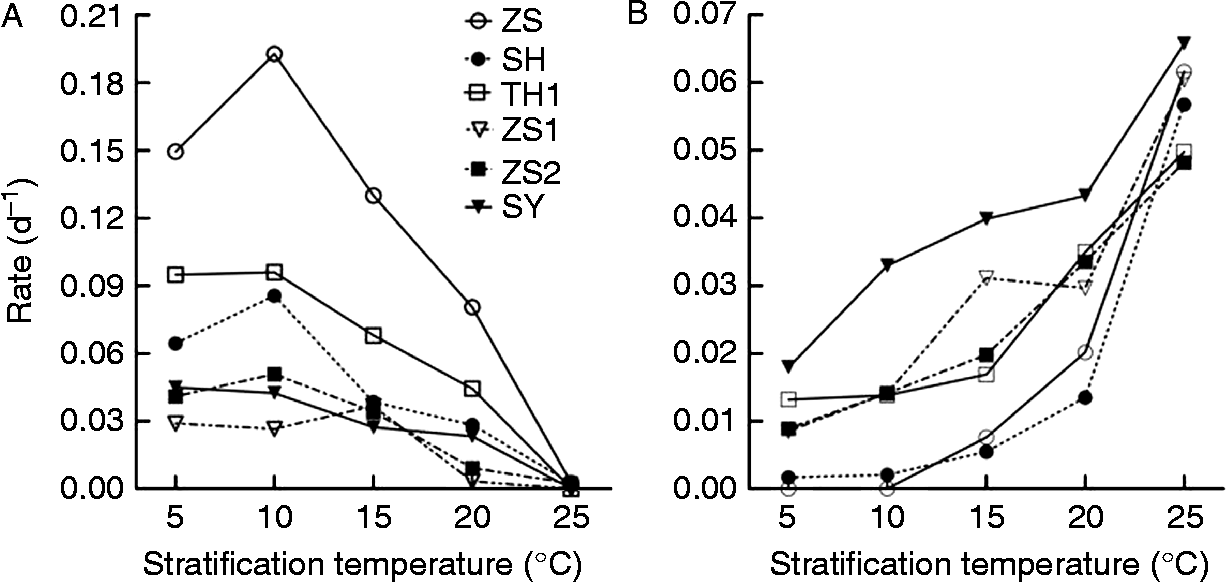
Figure 3 Rates of dormancy release and induction in different V. amurensis varieties. Based on the regression between stratification time and germination percentage of seeds after stratification, the rates of release (A) and induction (B) of seed dormancy in different varieties were estimated and plotted against stratification temperature.
Warm stratification followed by cold stratification
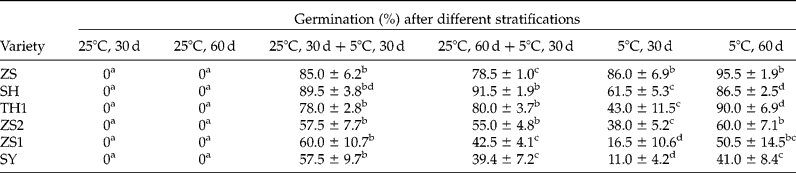
Warm stratification at 25°C rarely resulted in dormancy release (Table 2). However, stratification at 25°C for 30 d plus 5°C for 30 d resulted in higher germination than a single 5°C stratification for 30 d in all varieties collected from site B (Table 2) and even higher than 5°C stratification for 60 d in SY (Table 2, P < 0.05). However, when the time of 25°C stratification was extended to 60 d in the combined stratification, it did not increase the effect of 5°C stratification on dormancy release, and in contrast, depressed the effect in some varieties, such as ZS1 and SY (Table 2).
Table 2 Effect of warm stratification (25°C) prior to 5°C stratification on seed dormancy release
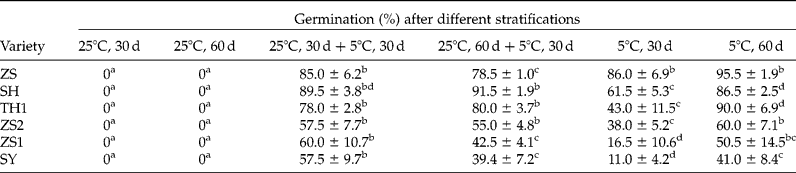
Seeds were stratified at indicated temperatures for different periods of time, and then germinated at 30/20°C (12/12 h) for 30 d. The letters a–d indicate significant differences at P < 0.05 among different treatments.
Drying before or after seed stratification
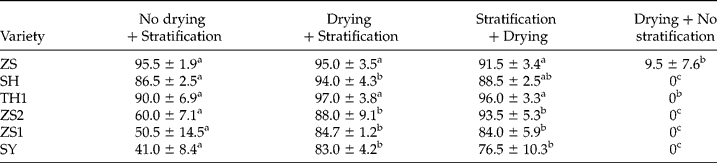
Seed drying before or after stratification markedly promoted the effect of 5°C stratification on dormancy release in ZS1, ZS2 and SY (Table 3). In ZS1 and SY, drying before or after stratification enhanced germination by 40% of seeds that were stratified at 5°C (Table 3).
Table 3 Effect of drying before or after stratification on seed dormancy release
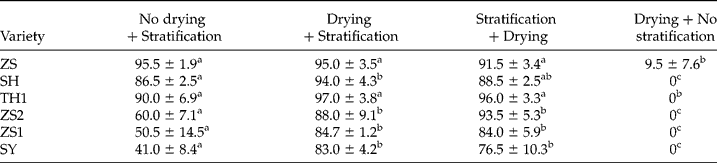
For seed stratification, seeds were mixed with moist perlite (about 2 g g− 1) and then stratified at 5°C for 60 d; for seed drying, seeds were dried to a water content of 0.14 g g− 1 by silica gel. Seeds were germinated at 30/20°C (12/12 h, in darkness) for 30 d. The letters a–d indicate significant differences at P < 0.05 among different treatments.
Stratification under various relative humidities
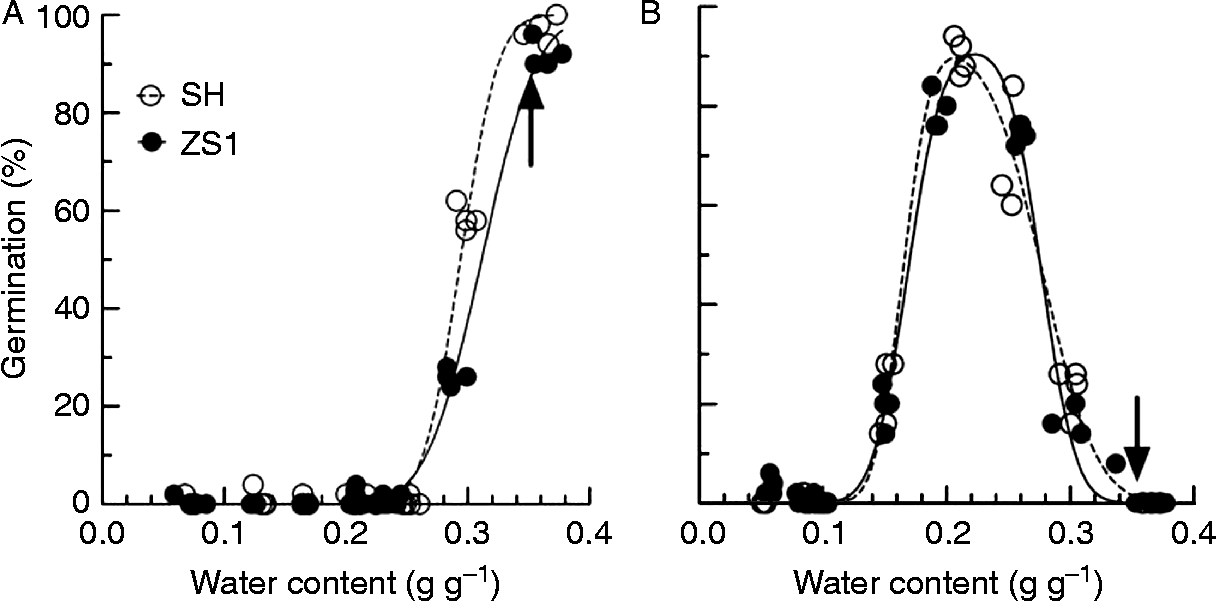
The MC at which seeds were stratified significantly influenced dormancy release, and the effect of MC was closely related to stratification temperature (Fig. 4). At 5°C, release of seed dormancy required moist conditions, and only seeds stratified in moist perlite could be fully released from dormancy (Fig. 4A). At 25°C, low seed MC (about 0.22 g g− 1) was optimal for dormancy release (Fig. 4B). The effect of seed MC on dormancy release appeared to vary little between SH and ZS1 (Fig. 4). When seeds were stratified at 5°C, the estimated mean seed water contents for dormancy release (equation 5) were 0.29 and 0.31 g g− 1 for SH and ZS1, respectively. For seeds stratified at 25°C, the estimated means of lower and upper threshold MC (W sl and W su; equation 6) for dormancy release were 0.16 and 0.28 g g− 1, respectively, for SH and 0.17 and 0.28 g g− 1, respectively, for ZS1, while the estimated optimal MC for dormancy release was 0.21 g g− 1 for SH and 0.22 g g− 1 for ZS1.
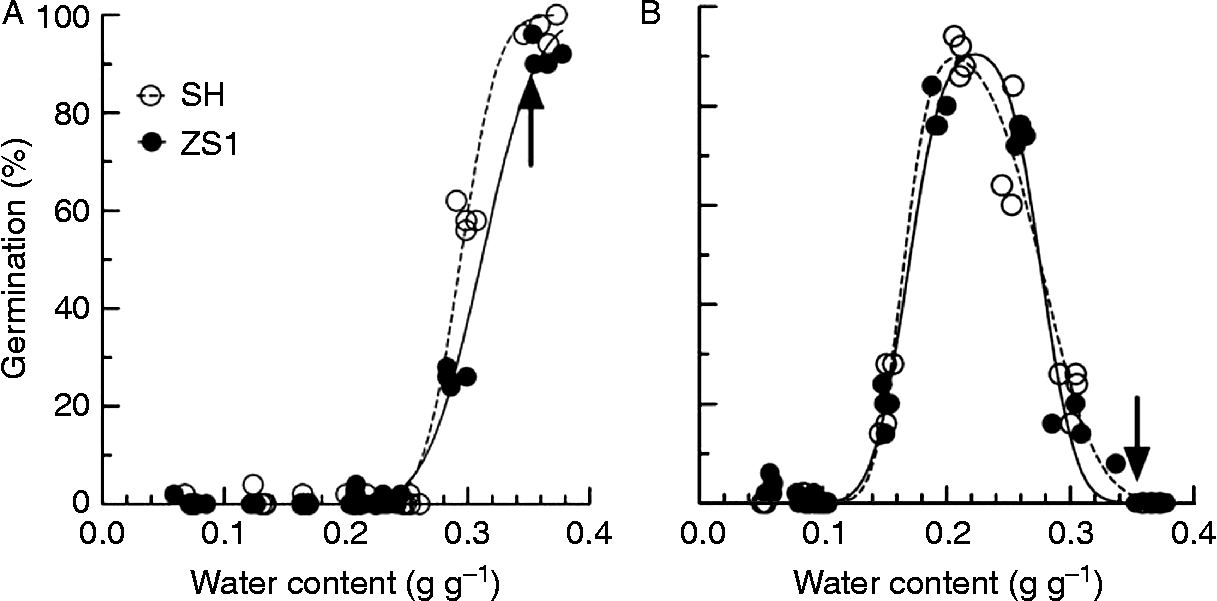
Figure 4 Dormancy release of seeds from V. amurensis varieties with a series of seed moisture contents during stratification at 5 and 25°C. After equilibrium at different relative humidities, seeds were stratified at 5°C (A) and 25°C (B) for 90 d, and then germinated at 30/20°C for 30 d. The germination of seeds stratified at 5°C related to water content with equation (1) and at 25°C with equation (2). The arrows indicate the germination of seeds stratified within moist perlite (water content of 2 g g− 1 dry weight).
Germination at different temperatures after 5°C stratification
Germination percentage of seeds stratified at 5°C for 60 d was higher at fluctuating temperatures (FT) than at constant temperatures (CT) of the same average temperature (P < 0.05, Fig. 5). In general, the optimal germination temperature (OGT) for all V. amurensis varieties was 30/20°C of FT and 30°C of CT. The germination difference between FT and CT, indicated by the ratio of F/C, was lowest in ZS (F/C ≈ 1.00, Fig. 5A), followed by SH (F/C = 1.19, Fig. 5B), ZS2 (F/C = 1.61, Fig. 5D), and then TH1 (F/C = 2.79, Fig. 5C) and greatest in ZS1 and SY (F/C>3.00, Fig. 5E and F). The OGT ranges were different among the varieties. In ZS, the seeds could germinate over the widest OGT range (30°C, 30/20°C, 30/10°C and 25/15°C, Fig. 5A), followed by SH (30/20°C, 25/15°C, Fig. 5B). TH1, ZS2, ZS1 and SY could only germinate best at 30/20°C (Fig. 5C–F).

Figure 5 Seed germination of V. amurensis varieties after 5°C stratification at different temperatures. F/C is the ratio of germination percentage at optimum fluctuating temperature (30/20°C) to that at optimum constant temperature (30°C). Data columns are means ± SD (n = 4). The letters a–f indicate significant differences (P < 0.05) among germination at different temperatures. (A) ZS, (B) SH, (C) TH1, (D) ZS2, (E) ZS1, (F) SY.
Discussion
In the present study, we conducted a series of stratification and germination treatments on V. amurensis seeds to investigate the conditions for dormancy release and germination in this species. In V. amurensis, (1) seed dormancy release could take place below 25°C (Fig. 2), but was best at cold temperature, 5°C for most varieties (Fig. 2); (2) dormancy induction occurred simultaneously with dormancy release during stratification (Fig. 2); (3) rates of dormancy release and induction depended on stratification temperature (Fig. 3); (4) seed MC affected dormancy release with a temperature-dependent pattern, that is, dormancy release required moist conditions (moist perlite) at cold temperature (Fig. 4A) and dry conditions at warm temperature (Fig. 4B); (5) seeds of all varieties germinated best at a fluctuating temperature of 30/20°C (Fig. 5). In addition, according to Baskin and Baskin (Reference Baskin and Baskin2004), seed dormancy of this species should be categorized as physiological dormancy (PD), because embryos of V. amurensis seeds do not grow after dormancy release (Gan et al., Reference Gan, Li, Song, Wang and Cheng2008), excluding it from morphological dormancy (MD); excised embryos can produce normal seedlings (Gan et al., Reference Gan, Li, Song, Wang and Cheng2008); and stratification for 1–2 months can fully remove seed dormancy.
As has been suggested for other Vitis species (Singh, Reference Singh1961; Manivel and Weaver, Reference Manivel and Weaver1974; Ellis et al., Reference Ellis, Hong and Roberts1983; Celik, Reference Celik2001), stratification at cold temperature (3–5°C) is the most effective pathway for dormancy release of V. amurensis seeds. However, in V. amurensis, we also found that dormancy release could occur at temperatures below 25°C (Fig. 3A), but the numbers of seeds expected to germinate depended on the net effect of stratification on dormancy release and induction. The effect of temperature on dormancy status as a combination of dormancy release and induction was first proposed by Totterdell and Roberts (Reference Totterdell and Roberts1979) in Rumex and subsequently this was observed in other species (Kebreab and Murdoch, Reference Kebreab and Murdoch1999; Batlla et al., Reference Batlla, Grundy, Dent, Clay and Finch-Savage2009; Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009). In these species, however, dormancy release and induction in response to temperature appears different. Totterdell and Roberts (Reference Totterdell and Roberts1979) hypothesized that in Rumex species the rate of dormancy release was independent of temperature and that of dormancy induction increased with temperature. Kebreab and Murdoch (Reference Kebreab and Murdoch1999) found that in Orobanche species the rate of dormancy release increased and that of dormancy induction decreased with increasing temperature. In Polygonum species the rate of dormancy release decreased, while that of dormancy induction increased with increasing temperature (Batlla et al., Reference Batlla, Grundy, Dent, Clay and Finch-Savage2009). The change of the rate of dormancy release and induction with temperature in V. amurensis varieties ZS, SH, ZS2 and ZS1 (Fig. 3) agrees with our recent study on a hybrid Vitis species. In this species, the rate of dormancy release increased first and then decreased, and that of dormancy induction increased with temperature (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009). In the TH1 and SY varieties, the change of rate of dormancy release with temperature appears to be different from the other varieties. In these two varieties, the rate was the same at 5 and 10°C, and decreased with increasing temperature (Fig. 3A). However, the behaviour of dormancy release below 5°C and between 5 and 10°C is unclear from the present study and, thus, it is not possible to assess the accurate trend in change of rates of dormancy release with temperature. Releasing primary dormancy followed by re-induction of secondary dormancy is a very important mechanism for species to regenerate in their natural environment. These two processes regulate the timing of germination proceeding to the most favourable season (Baskin and Baskin, Reference Baskin and Baskin1998; Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2007, Reference Batlla and Benech-Arnold2010).
In V. amurensis, dormancy release is not only regulated by stratification temperature, but also by the seed MC. At cold temperature (5°C) dormancy was only fully released in moist perlite (Fig. 4A). At warm temperature (25°C), seed dormancy could not be released in moist perlite, but could be fully released under dry conditions with an optimum at a seed MC of approximately 0.21 g g− 1 (Fig. 4B). These results have also been found in a cross-breed of Vitis species (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009). In general, seed dormancy of summer species can be released by incubating seeds under fully hydrated conditions, a process commonly named ‘stratification’, and that of winter species can be released through storing seeds under dry conditions, a process commonly termed ‘afterripening’ (Baskin and Baskin, Reference Baskin and Baskin1998). Although V. amurensis is a summer species, their seeds release dormancy both through stratification and afterripening.
Although we observed that afterripening was an alternative pathway to release dormancy of V. amurensis seeds besides stratification, the regeneration strategy of this dormancy-releasing behaviour deserves to be fully considered. Seeds of V. amurensis mature and are dormant in autumn, and subsequently they will go through the cold winter and release their dormancy in this season. Therefore, it is obvious to find that dormancy release is effective through cold stratification. However, the afterripening behaviour of V. amurensis seeds indicates that this species is also able to release seed dormancy in the summer, provided the seed MC is within an optimal range. If dormancy is released during this season, the seeds may germinate in late summer or autumn, after which the seedling must survive the cold temperature in winter. In their original distribution area, winters are extremely cold and the temperature can be as low as − 40°C. Thus, it appears that dormancy release of V. amurensis seeds through afterripening is not an advantage for plant regeneration in their natural environment. Thus, the present results cannot give a reasonable explanation for dormancy release through afterripening. Further investigations on dormancy release, germination and seedling formation and survival under original growth conditions may address this problem.
Dry afterripening is dependent on seed MC and various studies have shown that seed dry afterripening is effective at MCs from c. 0.05 to 0.2 g g− 1 (Leopold et al., Reference Leopold, Glenister and Cohn1988; Foley, Reference Foley1994; Probert, Reference Probert and Fenner2000; Steadman et al., Reference Steadman, Crawford and Gallagher2003; Bair et al., Reference Bair, Meyer and Allen2006; Bazin et al., Reference Bazin, Batlla, Dussert, El-Maarouf-Bouteau and Bailly2011). In V. amurensis, seed MC for afterripening appears to be higher than this range. For SH and ZS1 afterripening occurred within a range of seed MC from 0.14 g g− 1 to 0.34 g g− 1, and the optimum MC to release dormancy was at 0.22 and 0.21 g g− 1, respectively. In a cross-hybrid Vitis species, the optimum MC for afterripening at 20 and 30°C even reached 0.26 and 0.23 g g− 1, respectively (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009). The high MC for afterripening of V. amurensis seeds may relate to the original habitat of grapevine, the forest. In a forest, air relative humidity and soil moisture are higher than in open areas.
Although seed dormancy and germination have intra-species similarities, the variation is clear among varieties of V. amurensis, as indicated by the optimum temperature for dormancy release (Figs 2 and 3A) and germination (Fig. 5), rate of dormancy release and induction (Fig. 3), the requirement for warm stratification (Table 2) and drying (Table 3) to promote germination percentage. The differences among varieties in response to stratification treatments can be regarded as the result of different dormancy strengths (Karlsson and Milberg, Reference Karlsson and Milberg2007). Stratification could nearly completely release the dormancy in ZS seeds over a wider temperature range (Fig. 2A), the rate of dormancy release was highest among Vitis varieties (Fig. 3A) and seeds did not need warm stratification and drying treatment to promote germination (Tables 2 and 3). Thus, ZS seeds appear to possess non-deep dormancy. In comparison, dormancy of ZS2, ZS1 and SY seeds were only effectively released by 5°C stratification (Fig. 2D, E and F), rate to release dormancy was lower (Fig. 3A) and the seeds relied very strongly on warm stratification and drying to promote germination (Tables 2 and 3). Therefore, ZS2, ZS1 and SY seeds seem to have relatively deep dormancy. The dormancy strengths of SH and TH1 seeds were intermediate to those of the above varieties.
TH1, ZS2 and ZS1 seeds were collected from the same site (Table 1). Thus, the weather conditions during plant growth and seed maturation, which have been shown to be a factor affecting seed dormancy (Andersson and Milberg, Reference Andersson and Milberg1998; Meyer and Allen, Reference Meyer and Allen1999; Schütz and Rave, Reference Schütz and Rave2003) were similar. However, these varieties are cultivars from wild V. amurensis with different original distributions (Fig. 1, Table 1). Apparently, the seeds ‘remember’ their wild ancestor's adaptation to the original habitat, and thus keep their dormancy strength and release pattern.
In addition to the original growth conditions, the cultivated environment may be another factor influencing seed dormancy and germination of V. amurensis. ZS and SY have the same hybrid father plants, and thus, the major difference in dormancy strength must result from the hybrid mother plants, i.e. ZS1 versus TH1. TH1 has stronger dormancy strength than SY, while the dormancy strength in ZS1 is weaker than that in TH1 and similar to that in SY. Thus, it is expected that the dormancy strength in ZS is weaker than that in its mother plant ZS1 and in SY, but that was not the case. ZS was cultivated at site A (Fig. 1) where the average annual temperature is about 12°C and where there are 4 months in which the temperature is below 0°C. SY was cultivated at site B (Fig. 1), where the average annual temperature is about 5°C, and where there are 5 months in which the temperature is below 0°C. Thus, the difference in climatic conditions at the cultivated sites may be causal to the difference in dormancy strength between ZS and in SY. Furthermore, the significant difference in the optimum temperature for seed germination (Fig. 3A) and the requirement for warm stratification to promote cold stratification effect (Table 2) between ZS and the varieties from site B may also reflect the influence of the environment at the cultivated sites on seed dormancy.
A requirement for fluctuating temperature for germination is one of the regeneration strategies for forest species (Thompson and Grime, Reference Thompson and Grime1983; Benech-Arnold et al., Reference Benech-Arnold, Ghersa, Sánchez and García Fernandez1988; Schütz and Rave, Reference Schütz and Rave1999). In the wild, grapevines mainly grow in forest environments. The requirement for fluctuating temperature to germinate in V. amurensis and in other Vitis species, such as V. vinifera (Ellis et al., Reference Ellis, Hong and Roberts1983), V. labrusca (Celik, Reference Celik2001), V. rotundifolia (Conner, Reference Conner2008) and V. vinifera × V. amurensis (Wang et al., Reference Wang, Song, Li, Gan, Wu and Cheng2009) may reflect a conservative regeneration strategy of Vitis seeds in the forest.
Acknowledgements
We thank the Institute of Special Wild Economic Animals and Plants, the Chinese Academy of Agriculture for supplying seeds of V. amurensis in site B; the Chinese National Natural Science Foundation Program (30470183) and the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-Z-058) for financial support; and Professor Carol C. Baskin (School of Biological Sciences, University of Kentucky, USA) for helping with the English in our previous manuscript, Professor Ian M. Møller (visiting professor of the Chinese Academy of Sciences) for helping with the English and Dr Zhangwu Dai for his suggestions on data analysis and discussion.