1. Introduction
The complex behaviour of subduction zone fluids is strongly controlled by the progressive metamorphism involved during subduction. Prograde metamorphism of the subducting lithosphere to mantle conditions produces a slab-derived high-pressure fluid (Peacock, Reference Peacock1993; Liebscher & Heinrich, Reference Liebscher and Heinrich2007) whose pressure, temperature and composition control dissolved solutes (Manning, Reference Manning2004). Upon upward migration, such fluids liberated from oceanic and continental crust may hydrate overlying rocks, decreasing their density, and triggering buoyancy-driven exhumation (Engvik, Austrheim & Andersen, Reference Engvik, Austrheim and Andersen2000; Scambelluri & Philippot, Reference Scambelluri and Philippot2001; Miller et al. Reference Miller, Buick, Cartwright and Branicoat2002). Fluid-mediated retrogression and/or partial melting during uplift greatly reduces the likelihood of preserving high-pressure (HP) mineral phases (Harley & Carswell, Reference Harley and Carswell1995). Systematic fluid inclusion studies allow us to investigate all these processes, specifically allowing us to understand: (1) the nature and role of the fluids during subduction and exhumation; (2) the expression of oceanic and continental slab components in eclogite-facies fluids (Scambelluri et al. Reference Scambelluri, Piccardo, Philippot, Robbiano and Negretti1997; Svensen et al. Reference Svensen, Jamtveit, Yardley, Engvik, Austrheim and Broman1999); (3) the constraints imposed by isochores and mineral equilibria on P–T conditions and the exhumation path; (4) the general magnitude of fluid flow during HP and UHP metamorphism (Philippot, Reference Philippot1993; Nadeau, Philippot & Pineau, Reference Nadeau, Philippot and Pineau1993); and (5) the involvement of fluids in the formation and exhumation of HP and UHP rocks (Erambart & Austrheim, Reference Erambart and rheim1993).
In a newly discovered UHP terrane of the Himalaya, the Tso Morari Crystalline rocks have undergone maximum crustal metamorphism (Mukherjee & Sachan, Reference Mukherjee and Sachan2001; B. K. Mukherjee, unpub. Ph.D. thesis, Univ. Srinagar, 2003; Mukherjee et al. Reference Mukherjee, Sachan, Ogaswara, Muko and Yoshika2003; Sachan et al. Reference Sachan, Mukherjee, Ogaswara, Maruyama, Ishida, Muko and Yoshika2004). Although preliminary studies on fluid inclusions have been carried out by Sachan et al. (Reference Sachan, Bodnar, Islam, Szabo and Law1999) on eclogites, the behaviour and trapping conditions of fluids at peak metamorphic conditions have received only cursory attention. Consequently, this paper focuses on ultrahigh-pressure (UHP) fluids in a Himalayan terrane, the Tso Morari Crystalline Complex. Here, fluid inclusions are shown to conserve traces of pristine metamorphic fluids, those fluids associated with prograde, peak and retrograde metamorphism are characterized, and results are interpreted within the context of the geodynamic evolution of subduction related UHP metamorphism in Himalaya.
2. Geological background
In the NW Himalaya (Fig. 1), the Tso Morari Crystalline Complex occurs within a doubly plunging dome (Fig. 2), where a UHP metamorphic core is bounded by the Indus Suture Zone to the north and by low-grade sedimentary rocks (Zanskar, Tethyan sequence) on all other sides (Thakur, Reference Thakur, Thakur and Sharma1983). The oldest rock, exposed in the core of the complex, is the Puga Gneiss, with the relatively little-deformed Polokangla and Rupshu granite bodies along its margins. These rocks lie below a metasedimentary sequence that appears to be stratigraphically equivalent to the unmetamorphosed passive margin rocks of the Tethyan sedimentary sequence. The mineralogy of the Puga Gneiss largely reflects kyanite–sillimanite-grade rocks, which surround numerous blackish patches that are, in fact, mafic eclogite boudins. These boudins are elongate parallel to the main foliation (S2; Guillot et al. Reference Guillot, De Sigoyer, Lardeaux and Mascle1997), vary from a few centimetres across to tens of metres in size, and are commonly foliated and retrogressed along the margins. The cores of the eclogite boudins retain mineralogical evidence for progressive metamorphism during subduction processes and reflect the earliest stages of burial followed by blueschist facies to UHP eclogite-facies metamorphism (Mukherjee & Sachan, Reference Mukherjee and Sachan2001; Mukherjee et al. Reference Mukherjee, Sachan, Ogaswara, Muko and Yoshika2003). These UHP bodies are often tightly wrapped by orthogneiss, paragneiss and whiteschist. The overlying Palaeozoic–Mesozoic Taglangla metasedimentary rocks are dominated by metamorphosed calcareous marl and argillaceous sedimentary rocks together with layers of metabasalt. This metabasalt horizon is thought to be the equivalent of the Panjal Traps (De Sigoyer et al. Reference De Sigoyer, Guillot, Lardeaux and Mascle1997).

Figure 1. Geological sketch maps of Himalaya. (a) India. (b) Study area is marked in the N–S cross-section of Himalaya (after Gansser, Reference Gansser1964).
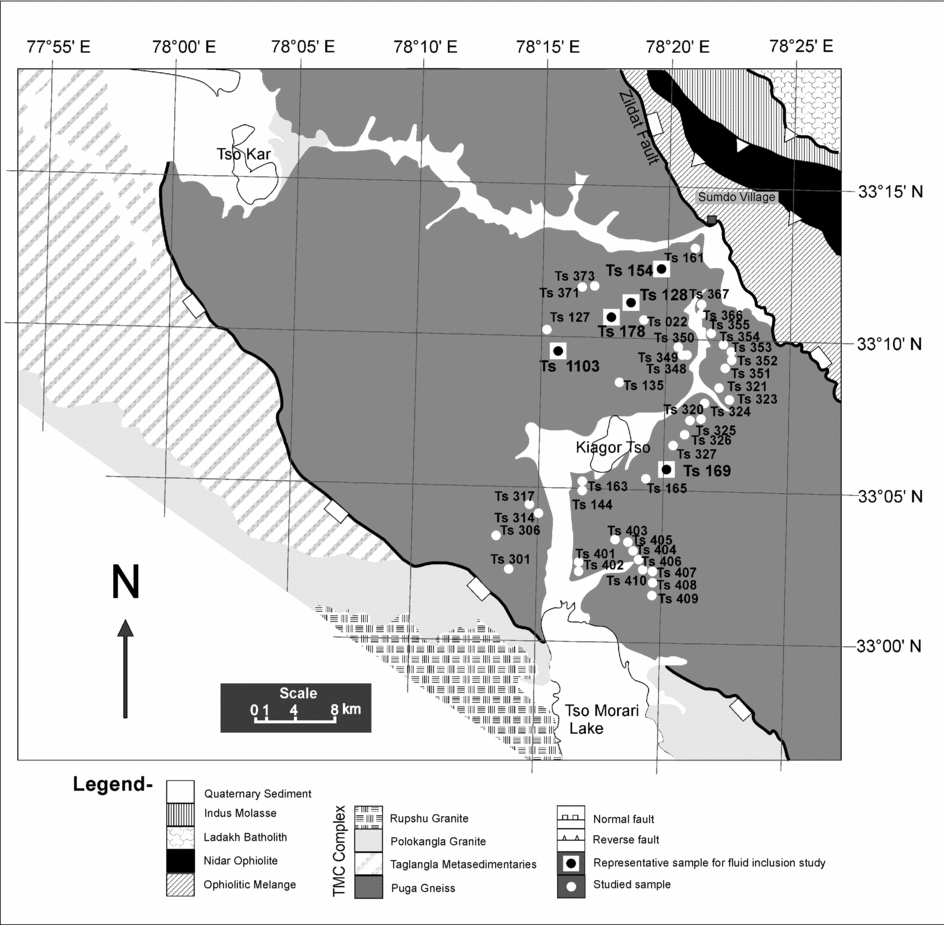
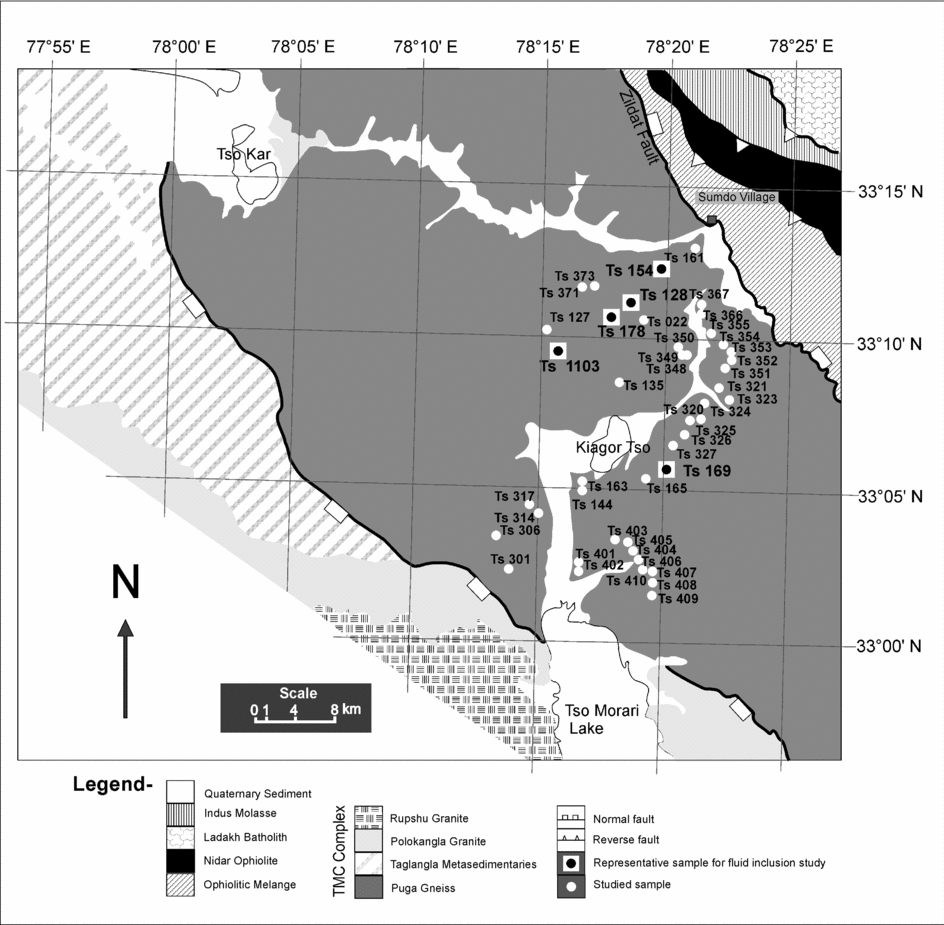
Figure 2. Part of the NW Himalaya showing Tso Morari Crystalline Complex in which samples are located in the Puga Gneiss (adapted from Mukherjee et al. Reference Mukherjee, Sachan, Ogaswara, Muko and Yoshika2003).
3. Methodology
Forty-five samples (Fig. 2) were studied for mineralogy and fluid inclusion petrography, and five representative samples (Fig. 2) were chosen for detailed mineral chemistry, fluid inclusion petrography, microthermometric measurements and Raman spectroscopy. The mineralogy and mineral chemistry for these representative samples are given in Tables 1 and 2.
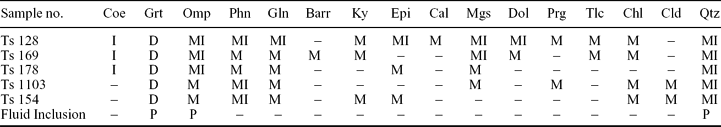
Table 1. Mineral assemblages of representative samples from Tso Morari Crystalline Complex

Abbreviations and symbols: Coe – coesite; Grt – garnet; Omp – omphacite; Gln – Glaucophane; Bar – barrosite; Ky – kyanite; Epi – epidote; Cal – calcite; Mgs – magnesite; Dol – dolomite; Prg – Paragonite; Tlc – Talc; Chl – Chlorite; Cld – chlorotoid; Qtz – quartz; I – inclusion mineral; M – matrix mineral; MI – mineral in matrix and as inclusion; D – dominant mineral, P – Present.
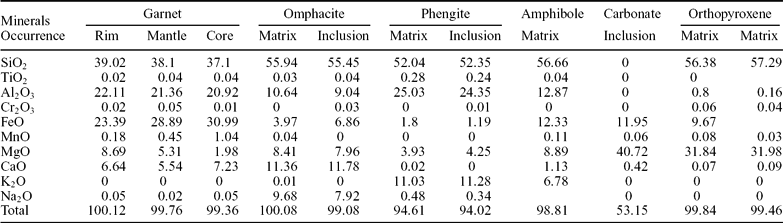
Table 2. Representative chemical composition of minerals present in UHP eclogite of Tso Morari Crystalline Complex

Mineral chemistry was measured by using a CAMECA SX-100 electron microprobe at the Wadia Institute of Himalayan Geology, Dehradun, India, and by using a JEOL JXA-733 electron superprobe at the Department of Earth Sciences, Waseda University, Tokyo, with 15 kV acceleration voltages and 15 nA beam current. The acquisition time was 20 s for each element, and the beam diameter was 3 μm.
Thermometric measurements were made on a Linkam THSM-600 heating–freezing stage attached to an Olympus BH2 microscope at the Wadia Institute of Himalayan Geology, Dehradun, India. The thin-sections were doubly polished about 120 to 180 μm thick. The minimum temperature was −195°C. The heating–freezing stage was calibrated with pure CO2 in natural fluid inclusions. Reproducibility of the melting temperature was better than ± 0.1°C, whereas for heating runs the precision is approximately 1°C.
The characterization of fluid is also supplemented by Raman spectroscopy by using the Horiba Jobin Yuvon Micro Raman Laser at Wadia Institute of Himalayan Geology, Dehradun, India, and the JASCO, NRS 2000C Raman Laser at Tokyo Institute of Technology, Japan. Spectra were excited with 785 and 514.54 nm lines of an Ar-ion Laser through a 100× objective. The laser on the inclusions surface had a spot diameter of approximately 1 μm and a power of 4–20 mW with dwell time 5–30 s. Spectral ranges for inclusions are in the order of 1000–3000 cm−1.
4. Petrography and P–T evolution
The Puga Gneiss occupies the core of the Tso Morari Crystalline Complex, and retains the best record of poly-metamorphic histories with a complex metamorphic fluid system. The mineralogy of the Puga Gneiss is dominated by quartz, feldspar, muscovite and biotite, along with some accessory phases like kyanite, chlorite, garnet, etc. The Puga Gneiss is broadly classified as orthogneiss and paragneiss with a strong kyanite–sillimanite-grade metamorphic overprint. This study mainly focused on eclogite, which is enclosed within the Puga Gneiss as irregular and isolated bodies. These eclogite bodies exhibit a wide range of mineral assemblages, namely, garnet, omphacite, phengite, kyanite, epidote and carbonates, along with secondary phases like glaucophane, chlorite, etc. Coesite inclusions in garnet porphyroblasts signal peak metamorphism at UHP conditions.
Table 1 lists the representative samples and their mineral assemblages, modes of mineral occurrences, and mineral distribution patterns. These assemblages represent the pre-peak to post-eclogitic conditions. Coesite-bearing eclogite retains peak UHP mineral assemblages. Texturally garnet is subhedral to euhedral, unevenly distributed in the matrix (Fig. 3a), and surrounded by omphacite, phengite and often by secondary chlorite and glaucophane. Garnet porphyroblasts contain various inclusion minerals, namely, omphacite, rutile, zircon, dolomite, magnesite, phengite and amphiboles (Fig. 3c). These inclusions are mostly concentrated in optically distinguishable core and mantle portions of the garnet grains. Among the important inclusions, the UHP indicator coesite is observed in the mantle part of the garnet (Fig. 3b). The coesite is typically rimmed by palisade quartz and radial cracks in the host mineral (Fig. 3b). These cracks are assumed to have developed during decompression due to volume expansion during the conversion of coesite to quartz (Ekaterina, Pascal & Jean-Pierre, Reference Ekaterina, Pascal and Jean-Pierre2006). The garnets are pyrope-rich, and MgO increases from core (1.98 wt%) to rim (8.69 wt%), consistent with preservation of prograde growth zoning (Table 2). The omphacites are jadeite-rich (Jd44 to Jd59) (Mukherjee et al. Reference Mukherjee, Sachan, Ogaswara, Muko and Yoshika2003), are bluish green in colour, and constitute 25–30% volume of the rock matrix. Some omphacite grains occur as inclusions in the coesite-bearing garnet (Fig. 3c), but the Na/Ca ratio (Table 2) does not show any marked differences between matrix grains versus inclusions.

Figure 3. (a) Photomicrograph of euhedral garnet associated with omphacite and phengite. Garnet contains various mineral inclusions. (b) Radiating fractures in host garnet are well developed around the coesite inclusion. Rim of the coesite inclusion shows quartz transformation. (c) Garnet surrounds omphacite and carbonates, which are closely associated. (d) Eclogite matrix contains bladed kyanite and epidote. (e) Clinopyroxene and orthopyroxene are closely associated. Reaction texture between clinopyroxene and orthopyroxene is developed around garnet porphyroblast. (f) Photomicrograph showing clinopyroxene breakdown reaction to secondary amphiboles; this reaction texture is well developed around garnet porphyroblasts.
Bluish-green amphiboles occur as inclusions and within the matrix. The core and mantle parts of garnet porphyroblasts are mainly occupied by inclusions of barroisite and Na-amphiboles that are assumed to have been trapped during the growth of the pre-eclogite facies at possible conditions of T~400°C and P ~ 18 kbar (Mukherjee et al. Reference Mukherjee, Sachan, Ogaswara, Muko and Yoshika2003). Omphacite is also commonly replaced by Na- and Ca-amphiboles in response to exhumation (Fig. 3f).
The occurrence of coesite with the eclogite minerals (jadeite-rich omphacite, pyrope-rich garnet and high-Si phengite) constrains peak metamorphic conditions at T ~ 750°C and P ~ 39 kbar (Fig. 8). The secondary amphiboles were formed during retrogression, whereas phengite is in textural equilibrium with omphacite and is part of UHP-eclogitic mineral assemblages. The association of orthopyroxene and clinopyroxene (Fig. 3e) in the matrix signals isothermal decompression to granulite-facies conditions of T~750°C and P ~ 12 kbar. This replacement phenomenon must have occurred soon after UHP metamorphism.
The micas are dominated by phengite, which occupies ~15% of the rock matrix (Fig. 3a). Minute flakes of phengite having high Si values (up to 3.53) are also observed as inclusions in garnet porphyroblasts. These inclusions are closely associated with coesite, implying phengite is part of the peak metamorphic mineral assemblage. Compositionally and texturally, few white micas in the matrix indicate later stage decompression reactions.
Coarse-grained magnesite, dolomite and calcite∕aragonite inclusions are restricted to the mantle and rim part of the garnet (Fig. 3c); magnesite and dolomite are commonly observed in the matrix. Epidote and kyanite are commonly regarded as eclogitic, and they occur as matrix phases (Fig. 3d). These could be of interest for inferring fluid compositions, but are not explicitly discussed here because they are not directly associated with coesite. Some eclogitic phases were strongly overprinted by amphibolite-facies metamorphism to produce chlorite + chloritoid at P ~ 5 kbar and T ~ 500°C.
We especially focused on UHP assemblages associated with and included in the garnet porphyroblasts to investigate prograde and peak metamorphic fluids, which are most reflective of subduction processes. To constrain fluids during exhumation, matrix quartz was also investigated in detail.
5. Fluid inclusion records
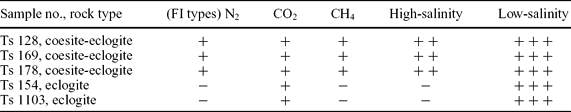
Fluid inclusions in Tso Morari eclogite show broad compositional variations from primarily aqueous-liquid to gaseous. The complex metamorphic history of eclogites generates a wide range of the fluid compositions, which in turn depend upon the pressure, temperature and reaction history. This compositional variation can be classified into five major types: high-salinity brine, N2, CH4, CO2 and low-salinity aqueous fluids (Figs 4, 5). Physically these inclusions show three different types, namely, mono-, bi- and tri-phase inclusions, which are commonly observed in quartz, garnet and omphacite.

Figure 4. (a, b) Photomicrographs showing the presence of three-phase inclusions in a quartz grain hosted by garnet. (c) Photomicrograph of N2–CH4 gaseous phase inclusion in a quartz-omphacite grain hosted by garnet. (d) Photomicrograph showing the presence of re-equilibrated fluid inclusions in matrix quartz grain.

Figure 5. (a–c) Photomicrographs showing the typical tube-shaped biphase aqueous inclusions in omphacite grains. (d–f) Photomicrographs showing high density CO2 inclusions with clear negative crystal shapes. This quartz grain also contains biphase aqueous inclusions.
Petrographically, fluid inclusions vary in their mode of occurrence, namely, the high-salinity brines are found mostly as isolated, tri-phase inclusions. They are observed in the quartz inclusions hosted by the peak metamorphic minerals (garnet and omphacite). These primary inclusions range up to 25 μm in size, and contain solid halite cubes in addition to vapour and liquid phases (Fig. 4a, b). In the same host mineral, bi-phase aqueous inclusions with liquid and vapour phases typically display decrepitation texture (Fig. 4b).
The biphase fluid inclusions in omphacite occur as tubes that are oriented parallel to the c-axes of the host grain. The bi-phase tubes average 9 μm, with a length and breadth ratio of about 4:1, and occur as non-planar clusters of parallel tubes that are populated predominantly in the cores of omphacite grains (Fig. 5a,b). Co-genetic inclusions are also observed in omphacite and quartz hosted by garnet (Fig. 5c), and we suggest they are primary. These aqueous inclusions show a wide range of salinity. Low-salinity secondary bi-phase aqueous inclusions are also found in healed fractures in the recrystallized matrix quartz (Fig. 5f).
Rarely, minute mono-phase gas-rich N2 and CH4 inclusions are present in the quartz hosted by garnet (Fig. 4c) and in omphacite grains. These inclusions are dark in colour, isolated and appear primary. N2- and CH4-type inclusions are less easily observed due to their small size, but their characteristic Raman spectra confirm their identity. Some of the CO2 inclusions that occur in the matrix quartz appear primary, displaying clear negative crystal shapes. The CO2 inclusions commonly occur along micro-shears and near the periphery of the hosting minerals (Fig. 5d, e).
Re-equilibrated inclusions in quartz grains are abundant, and show various secondary textures, such as secondary haloes, dendritic texture and shrinkage due to volume loss, etc. (Fig. 4d). According to the textural criteria of Vityk & Bodnar (Reference Vityk and Bodnar1995) and Bodnar (Reference Bodnar, Samson, Anderson and Marshall2003), we interpret secondary haloes around big inclusions as an implosion texture (Fig. 4d).
6. Fluid-chronology
Textures establish a fluid-chronology with respect to the metamorphic history in the Tso Morari eclogites. Metamorphism has been broadly classified as pre- (prograde), peak- or post- (retrograde) eclogitic conditions. In this view the metamorphic fluids may also be categorized as: (1) prograde metamorphic fluids in quartz hosted by eclogitic minerals; (2) peak metamorphic fluids in eclogitic minerals such as garnet, omphacite, etc. and (3) retrograde metamorphic fluids, which commonly occur in the matrix quartz. In this study, the high-salinity brine, N2 and CH4 gaseous phases are trapped in quartz, garnet, omphacite, etc. These minerals are closely associated with coesite and carbonates, so we interpret the inclusions to have been entrapped at or prior to ultrahigh-pressure metamorphism. That is, the high-salinity brine and gaseous phases are primary prograde and peak-metamorphic fluids. In contrast, the CO2 and low-salinity aqueous inclusions are likely secondary, so belong to the retrograde fluid, because they are found as trails along healed fractures. Some CO2 inclusions do also appear primary with respect to the host mineral.
7. Microthermometric measurements
Microthermometry reveals huge compositional variations, including high-salinity brine, N2, CH4, CO2 and low-salinity aqueous fluid. Their relative abundance is shown in Table 3. During routine microthermometry, homogenization of tri-phase brine inclusions occurred with the disappearance of halite. In most cases, the vapour bubble disappeared a few tens of degrees below the final halite dissolution temperature at 250–300°C. In many cases, the dissolution temperature could not be measured due to decrepitation.
Table 3. Types of fluid inclusion in eclogite from Tso Morari Crystalline Complex

Abbreviation and symbols: FI: fluid inclusion; –: Absent; +: present, small; + +: present average; + + +: abundant.
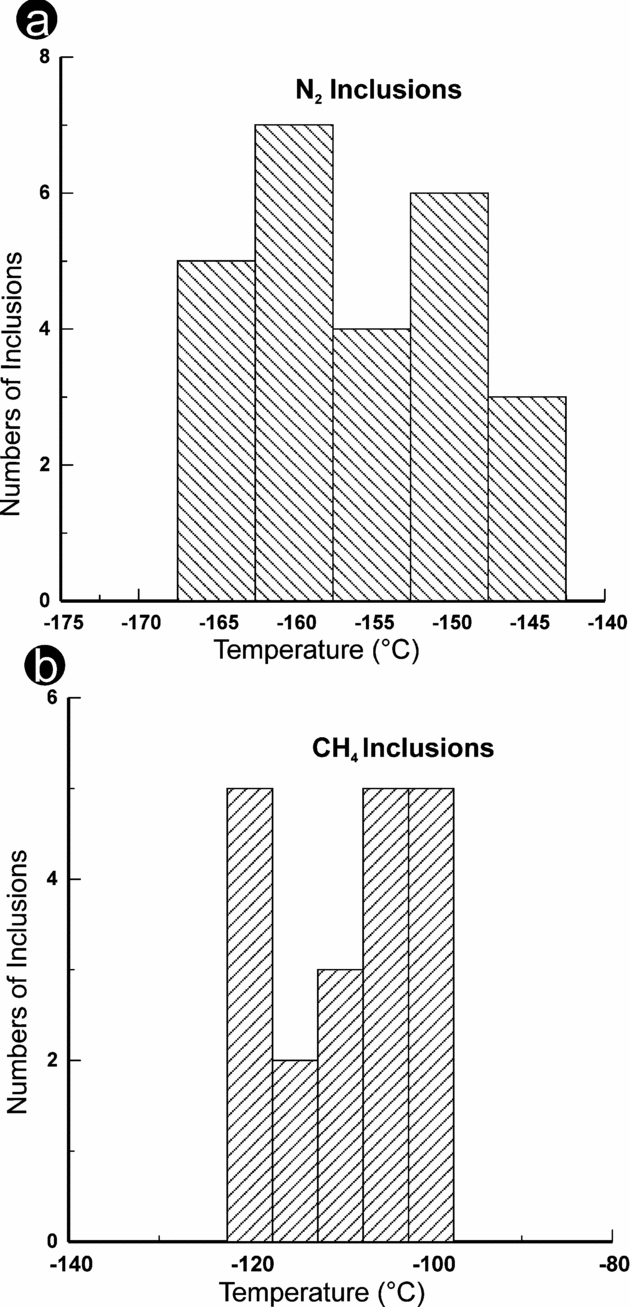
Freezing runs identify the composition of single-phase gas-rich inclusions. The temperature of homogenization for N2 falls in the range of −165 to −145°C (Fig. 6a), whereas CH4 homogenizes at −120 to −100°C (Fig. 6b). A cluster of fluid inclusions displays homogenization temperatures at −96 to −98°C, moderately below the critical homogenization temperature of N2 and CH4. This reveals the possible mixing of N2 and CH4. Nevertheless, identification of mixed fluid compositions is prone to error, so the compositions of these gaseous phases were further ascertained by Raman spectroscopy. During Raman spectroscopy, many precautions were used to avoid background noise and overlapping Raman peaks. To get the sharp Raman peak of fluids, especially for gas-rich inclusions, measurements were fixed at 20–30 s dwell time. The inclusions present in quartz and omphacite grains were identified as pure gas-rich CH4, reflecting a Raman shift at 1917.8 cm−1 (low peak), 2913.6 cm−1 (high peak) and N2 at 2334.3 cm−1. Some inclusions also indicated a pair of Raman shifts for both N2 and CH4.

Figure 6. (a) Histogram showing the homogenization temperature (Th) for N2 inclusions. (b) Histogram showing homogenization temperature (Th) for CH4 inclusion.
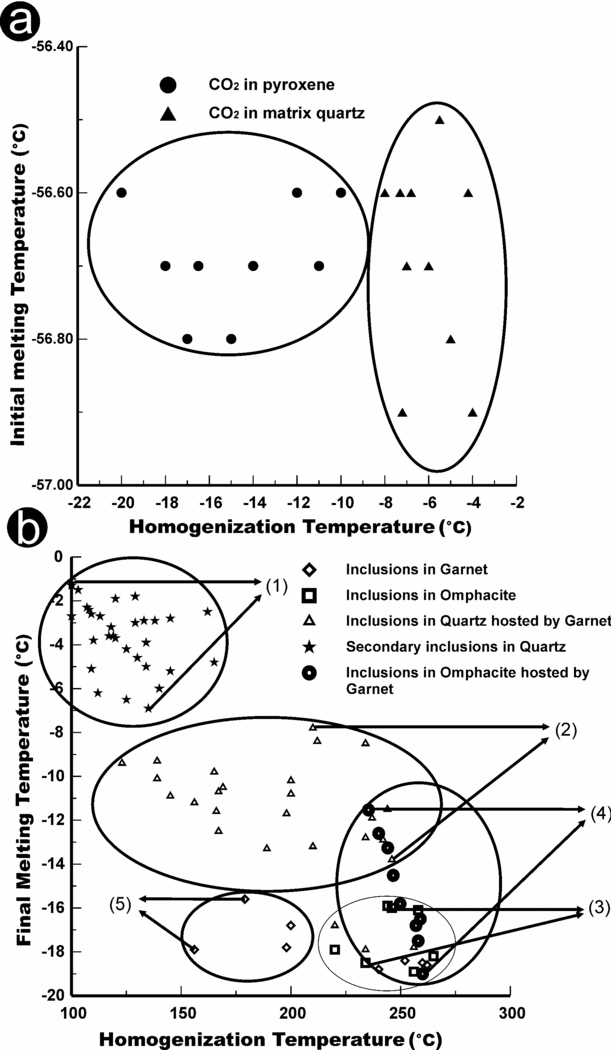
The CO2 inclusions showed an initial melting temperature (Tim) close to the pure CO2 triple point (−56.6°C). The behaviour of these inclusions during freezing/heating corresponds to the H3 type of Van den Kerkhof (Reference Van Den Kerkhof1988). Homogenization occurs between −20 and −4.2°C. The CO2 inclusions present in omphacite show a higher homogenization temperature (Fig. 7a), consistent with higher densities, compared to inclusions in the quartz grain hosted by garnet.

Figure 7. (a) Relationship between homogenization temperature (Th) and initial melting temperature (Tm) for CO2 inclusions hosted by pyroxene and matrix quartz. (b) Relationship between homogenization temperature (Th) and final melting temperature (Tfm ice) in biphase aqueous inclusions. The distribution of five zones is marked with respect to their mode of occurrences following maximum and minimum Th and Tfm.
Aqueous inclusions are by far the most abundant in all samples. They show a wide range of salinities from low- to highly saline fluid. The salinity ranges in the aqueous inclusions can be seen in Figure 7b, in which five zones have been marked according to their mode of occurrence (e.g. inclusions in garnet, in omphacite, in omphacite within garnet, in quartz, in quartz within garnet). The corresponding isochores for each zone are plotted in Figure 8 for the maximum and minimum homogenization temperatures along with the melting temperature. The data in Figure 7b show the eutectic temperature ranges from −21.2 to −25.3°C for biphase aqueous inclusions; this suggests the presence of divalent cations (K, Mg) along with Na. The final melting temperature of high-salinity inclusions ranges from −18.5 to −10°C, whereas in the low-salinity inclusions it ranges from −7 to −1.2°C (Fig. 7b). The aqueous inclusions in matrix minerals display moderately low salinity due to re-equilibration during later stage modification with an external fluid.

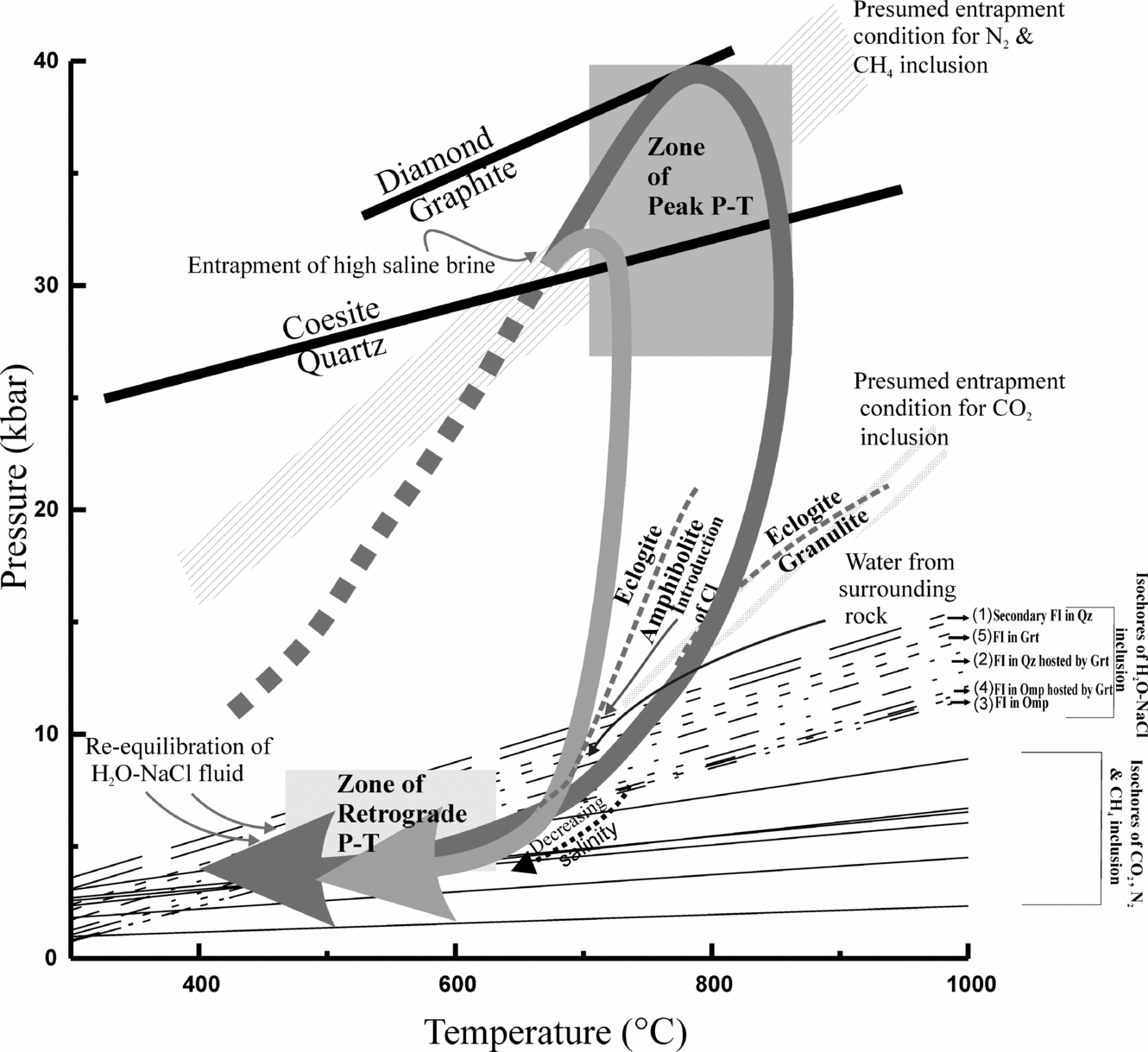
Figure 8. Schematic representation of fluid entrapment stages in the wide range of P–T conditions recorded by Tso Morari eclogite. Two grey boxes show peak and retrograde metamorphic conditions. Thick black and grey arrows illustrate P–T paths (after Mukherjee et al. Reference Mukherjee, Sachan, Ogaswara, Muko and Yoshika2003) and are constrained by peak P–T conditions and retrograde reactions (eclogite–amphibolite and eclogite–granulite); early prograde path is shown schematically by thick dashed line. Thick black lines show transitions for diamond–graphite (Kennedy & Kennedy, Reference Kennedy and Kennedy1976) and quartz–coesite (Bohlen & Boettcher, Reference Bohlen and Boettcher1982); grey dashed lines show facies boundaries (Green & Ringwood, Reference Green and Ringwood1967). Details regarding fluid entrapment conditions are given in the text. Two sets of isochores recovered from microthermometry are shown for individual fluid inclusions: isochores for H2O–NaCl fluids are shown as discontinuous lines, and isochores of gaseous phases are shown as continuous lines. Grey zone illustrates likely entrapment conditions for N2, CH4 and CO2.
8. Discussion
The fluid inclusion study provides important constraints regarding the trapping conditions (textural evidence with regard to P–T estimates), composition of metamorphic fluids and behaviour of fluids during reactive infiltration. In this regard, special attention has been paid to those inclusions that show the best textural agreement within or close to pre-, peak- and post-eclogitic conditions.
8.a. Nature of fluid during metamorphism
The Tso Morari unit records the major compositional fluid variations in metamorphic rocks, namely, high-salinity brine, N2, CH4, CO2 and low-salinity aqueous fluids. High-salinity brine, N2 and CH4 occur as primary inclusions in peak metamorphic mineral assemblages. The high-salinity brine and gaseous fluid inclusions are often seen entrapped in jadeitic omphacite, quartz in garnet, and occasionally in kyanite and epidote, presumably entrapped at or prior to ultrahigh-pressure metamorphism. Some of the brines are found within quartz and located at the core part of the garnet, which presumably grew during prograde metamorphism. The CO2 and secondary low-salinity aqueous inclusions are abundant. Nevertheless, few primary CO2 inclusions appeared to be of sufficient density (about 0.92 g cm−3) to signify granulite-facies metamorphism achieved during isothermal decompression. These inclusions were usually found within individual grains, and possibly were entrapped along microshear zones or internally modified by deformation phenomena, which may have occurred during widespread retrogression. This behaviour was also observed by Fu, Touret & Zheng (2001) in the Dabie-Sulu terrane of eastern China.
The low-salinity aqueous inclusions are dominated by H2O–NaCl and occur mostly as bi-phase types. They are usually observed in multiple generations of fluid inclusion trails (that is, in arrays) which reflect a wide range of salinity. Widespread re-equilibration partially overprinted and modified the primary signature of earlier brine and later aqueous fluid inclusions.
8.b. Relationship between isochores and mineral P–T data
In Figure 8, isochores are plotted in comparison to the inferred P–T trajectory; broadly, isochores fall into two groups: gaseous inclusions, and high-salinity (brine) to low-salinity aqueous inclusions. Isochores of gaseous inclusions (N2-, CH4- and CO2-rich) occupy the lower part of the P–T plot up to P~5 kbar. Because these inclusions are texturally primary, yet do not plot along the prograde P–T path or peak metamorphic conditions, they must have experienced considerable leakage and re-equilibration, resulting in only low-density fluid in the inclusions. Instead, we assume that N2 and CH4 gaseous phases more likely formed at or near the peak P–T conditions, ~39 kbar and ~750°C, and that CO2 formed near the eclogite–granulite transition, ~12 kbar and ~750°C. The population of aqueous inclusions (high and low salinity) is plotted on a Tm (temperature of melting; salinity)–Th (temperature of homogenization; density) diagram (Fig. 7b). The marked five zones for low-salinity aqueous inclusions trapped in the matrix quartz show homogenization temperatures of 100–160°C, although some were possibly modified through leakage. The plotted isochores for each category correspond to maximum and minimum Th/Tm (Fig. 7b), and occupy the retrograde part of the P–T trajectory between P~5 and 12 kbar (Fig. 8). However, isochores that plot at lower part of the P–T plot at ~4–7 kbar and 650 to 500°C (Fig. 8) have been greatly influenced by later stage modification during exhumation. Note that the P–T trajectory crosses multiple reactions, as indicated by textural relationships such as clinopyroxene to orthopyroxene (eclogite overprinted by granulite facies) and omphacite to glaucophane and barrosite (eclogite overprinted by amphibolite facies). Severe re-equilibration of fluid inclusions during retrogression is well documented by Sterner & Bodnar (Reference Sterner and Bodnar1989) and Kuster & Stockhert (Reference Kuster and Stockhert1997) for high-pressure and low-temperature rocks. It can be envisaged that continuous dislocation creep allowed continuous protuberation driven by small differential pressures during decompression down to the temperature of 300°C and pressure of 3–4 kbar (Kuster & Stockhert, Reference Kuster and Stockhert1997; Vityk, Bodnar & Doukhan, Reference Vityk, Bodnar and Doukhan2000). Whereas re-equilibrated inclusions represent varying density after their initial formation, there are no marked differences in isochores of high- and low-salinity aqueous inclusions.
The slope of aqueous inclusion isochores is steeper compared to the isochores of gaseous inclusions, which are nearly parallel to T-axis. The isochores of aqueous inclusions pass through retrograde P–T condition of 5 kbar at 550°C. So, pre- and peak metamorphic inclusions probably have re-equilibrated with respect to their original volume/density. In many cases, implosion textures (Fig. 4d) are also observed in the fluid inclusion, inferred as isothermal decompression (Vityk & Bodnar, Reference Vityk and Bodnar1995; Bodnar, Reference Bodnar, Samson, Anderson and Marshall2003).
8.c. Source of fluid
Compositionally primary fluids were recovered from pre-existing mineral inclusions within the garnet, omphacite, etc., although these fluids show separate P–T conditions in comparison to the hosting mineral. Externally derived fluid during retrogression and free volatile fluids at all stages (e.g. as indicated by production of secondary amphiboles from omphacite and chlorite from garnet) facilitated re-equilibration of primary fluids. Nevertheless, element mass transfer and isotopic compositions could help reconstruct the source of such fluids (brine, N2 CH4, CO2 and low-salinity aqueous fluids) in HP and UHP rocks (Andersen et al. Reference Andersen, Austrheim, Burke and Elvevold1993; Klemd, Van den Kerkhof & Horn, Reference Klemd, Van Den Kerkhof and Horn1992; Fu, Touret & Zheng, Reference Fu, Touret and Zheng2003; Vallis & Scambelluri, Reference Vallis and Scambelluri1996; Agard et al. Reference Agard, Goffe, Touret and Vidal2000; Manning, Reference Manning2004).
The high-salinity, primary brines could have formed through the dewatering of subducting carbonates and ancient meta-sedimentary rocks. This is consistent with local fluid production as well as limited fluid flow at depths of > 60 km in subduction zones, as suggested by Philippot & Selverstone (Reference Philippot and Selverstone1991), Klemd, Van den Kerkhof & Horn (Reference Klemd, Van Den Kerkhof and Horn1992) and Xiao et al. (Reference Xiao, Hoefs, Van Den Kerkhof, Fiebig and Zheng2000). The trapped N2 and CH4 gas-rich inclusions are observed in jadeitic omphacite, which formed during peak eclogitic conditions (P ~ 39 kbar and T ~ 750°C). The later-introduced CO2, probably trapped soon after peak metamorphism, underwent density resetting during rapid exhumation. This indicates that the granulite-facies overprinting on UHP eclogite followed isothermal decompression (Fig. 8). This type of high-density CO2 is also reported from ultrahigh-temperature granulites from North China (Santosh et al. Reference Santosh, Tsunogae, Ohyama, Sato Li and Liu2008). Reactive inflow during retrogression produced abundant secondary amphiboles during breakdown of omphacite, and gave rise to Cl (Markl & Bucher, Reference Markl and Bucher1998) as a major element in addition to Na. The generation of NaCl resulted in an aqueous saline environment and may indicate a crustal origin for the fluid. This aqueous saline fluid was later modified by influxes of externally derived water through microfractures. These phenomena are assumed to have taken place during the last stage of exhumation. We also interpret the large variation in salinity to result from the progressive release of alkalis during HP–UHP metamorphism, much as has been reported from the Dabie-Shan eclogite (Fu, Touret & Zheng, Reference Fu, Touret and Zheng2003).
8.c.1. Origin of N2
Several studies have been made on eclogite containing N2-rich fluids, for example, in the Caledonides (Andersen, Burke & Austrheim, Reference Andersen, Burke and Austrheim1989; Andersen, Austrheim & Burke, Reference Andersen, Austrheim and Burke1990; Andersen et al. Reference Andersen, Austrheim, Burke and Elvevold1993) and the Münchberg complex in Bavaria (Klemd, Van den Kerkhof & Horn, Reference Klemd, Van Den Kerkhof and Horn1992), although fluids from eclogites from the Alps are chiefly aqueous brines without evidence of N2 (Selverstone et al. Reference Selverstone, Spear, Franz and Morteani1984; Philippot & Selverstone, Reference Philippot and Selverstone1991; Philippot, Reference Philippot1993). Such differences in fluid regimes may be a consequence of different tectonic environments during eclogite formation (Andersen et al. Reference Andersen, Austrheim, Burke and Elvevold1993; Klemd & Bröcker, Reference Klemd and Bröcker1999). In an oceanic subduction zone, a large volume of clastic sediments is subducted together with hydrated oceanic crust, representing a considerable reservoir of nitrogen-rich material; thus eclogites formed from oceanic crust will be relatively N2-enriched.
Previous studies have shown the N2-enriched fluids belong to peak metamorphism; this is also observed in omphacite and garnet from the Tso Morari eclogite. The samples from Tso Morari show that N2-rich inclusions occur with other types of inclusions, possibly reflecting immiscible fluid formed from original metasedimentary rocks during subduction. Alternatively, nitrogen may have been present as NH4 + ions substituting for other elements (K+) in pre-peak metamorphic minerals (feldspar, amphibole and sheet silicates). This nitrogen may have been released and trapped as a fluid phase when its host minerals were consumed by metamorphic reactions to produce the eclogite mineral assemblage.
8.c.2. Origin of CH4 and CO2
Methane is a common fluid type in low-grade metamorphic rocks and is also observed in high-grade metapelites from the Kola Peninsula (Fonarev, Touret & Kotelnkova, Reference Fonarev, Touret and Kotelnikova1998) and Himalayan ophiolitic rocks (Sachan, Mukherjee & Bodnar, Reference Sachan, Mukherjee and Bodnar2007). Several processes form methane, such as inorganic synthesis, degassing of magmatic CH4, biogenic methanogenesis, etc.
In subduction zone environments, identifying primary fluid compositions is tedious and complicated. Serpentinization is commonly invoked for forming methane, but will most likely occur during obduction, which is ruled out because it will not result in prograde, primary CH4. Other abiogenic processes, such as thermogenesis (thermal decomposition) of subducted organic carbon from marine sediments are more likely in these rocks. Formation of CH4 from a mixture of organic carbon and carbon which evolved from carbonates would allow the formation of thermogenic CH4 (Fuex, Reference Fuex1979). Possibly the CH4 formed at deeper levels of the subduction zone (Sadofsky & Bebout, Reference Sadofsky and Bebout2003) as an immiscible fluid. The CH4-bearing fluid inclusions were discovered in peak eclogitic (T ~ 750°C and P~39 kbar; Fig. 8) minerals such as silica inclusions in garnet and in jadeitic pyroxene. The production of CH4 is consistent with occurrences of reduced carbon as graphite and diamond in Tso Morari eclogites (Mukherjee, Sachan & Ahmad, Reference Mukherjee, Sachan and Ahmad2005).
Carbonic fluid inclusions in Tso Morari Complex eclogites contain CO2-rich vapour and H2O liquid phases. Other phases like CH4 and/or N2 in carbonic fluid inclusions were absent; this shows fluid unmixing. The absence of CH4 and N2 phases from carbonic fluid inclusions reveals the CO2-rich fluids were not entrapped at UHP metamorphic conditions. Perhaps the CO2 (±H2O)-rich fluid inclusions were entrapped during the retrogression path, either at the eclogite–granulite transition, achieved during isothermal decompression, or by a decarbonation process. However, during granulite-facies overprinting on eclogite, the presence of brine (saline) with carbonic fluid phases cannot be ignored completely (Touret, Reference Touret, Tobi and Touret1985; Selverstone et al. Reference Selverstone, Franz, Thomas and Getty1992). Seemingly, the post-entrapment modification at a later stage of cooling and uplift governs the density resetting in carbonic fluid inclusions.
8.d. Behaviour of fluid recycling
Our data from Tso Morari eclogites show that the primary fluids were generated from mineral dehydration during prograde metamorphism (blueschist to eclogite facies). This implies that the transition of blueschist- to eclogite-facies rock might offset fluid transport from the downgoing slab at a typical temperature of 600°C. Previous to this release, highly concentrated brines might be derived from downgoing metasedimentary or mafic rocks. The production of the two gaseous phases CH4 and N2 could take place through extensive consumption of carbonate along with associated metasedimentary rocks, leading to gaseous inclusions at or near the peak metamorphic condition ~39 kbar and ~750°C.
The immiscible behaviour of these two gaseous phases could be well explained by their different formation process, such as CH4 through carbonate (or graphite) breakdown and N2 through breakdown of ammonium-bearing K minerals. Although miscibility of these two gaseous phases might be important for the formation of microdiamond (Badzian, Badzian & Lee, Reference Badzian, Badzian and Lee1993), in Tso Morari rocks (Mukherjee, Sachan & Ahmad, Reference Mukherjee, Sachan and Ahmad2005) a definitive explanation will be possible only through stable isotope analysis. The post-peak metamorphic fluids initially formed through decarbonation reactions that took place at moderately high pressure (Molina, & Poli, Reference Molina and Poli2000). The presence of CO2 ± N2 ± CH4 fluid inclusions in amphibolite facies has been studied by various workers (Touret, Reference Touret, Hollister and Crawford1981; Sisson & Hollister, Reference Sisson and Hollister1990), and in granulites from southern Norway, such a fluid could coexist with a highly saline brine (Touret, Reference Touret, Tobi and Touret1985). The formation of highly saline aqueous fluid may in part derive from K-feldspar in granulite facies (Perchuk & Gerya, Reference Perchuk and Gerya1993; Newton et al. Reference Newton, Aranovich, Hansen and Vandenheuvel1998).
Fluid recycling during exhumation produced hydrous phases mostly by clinopyroxene breakdown to amphiboles. Thus, retrograde fluids were typically water-rich. Water commonly contains a significant dissolved salt component, such as NaCl, KCl, etc. The apparent salinity of the fluid (< ~6 wt% NaCl), however, changes dramatically if CO2 is considered. CO2-rich fluid was at times a major component that was trapped soon after the Indian slab initiated exhumation. Although isothermal decompression resulted, the entrapment of high-density CO2-rich fluid was likely present during granulite-facies metamorphism. The absence of a mixed ternary (CO2–NaCl–H2O) fluid system could support separate entrapment of CO2 and NaCl–H2O fluids, but as yet no visible water (± NaCl) has been noticed with CO2-rich inclusions. In contrast, the low-salinity aqueous fluids display tremendous re-equilibration, which has been controlled by the external fluid influxes from the surrounding gneissic rock.
In summary, this paper elucidates several aspects of complex fluid behaviour in high-grade rocks. Rare fluid inclusions hosted by quartz included in garnet and by jadeitic pyroxene represent samples of primary fluids. Much of the host garnet and omphacite escaped subsequent deformation and back reaction, allowing retention of primary fluid inclusions. Thus, close attention to the selection of fluid inclusions and their compatibility with host mineral is crucial to final interpretation. Fluid mixing and unmixing at peak UHP conditions are expected to be common and create additional complexities, as does infiltration of crustally derived, compositionally diverse fluids at more normal geothermal gradients.
9. Conclusions
(1) Metamorphism of the Tso Morari Crystalline Complex produced high-salinity brines during prograde metamorphism, followed by gaseous phases enriched with methane and nitrogen at peak ultrahigh-pressure conditions. Retrograde metamorphism produced a high-density CO2 fluid, followed by low-salinity aqueous fluids. Post-peak, granulite-facies overprinting was attained through isothermal decompression to 10–12 kbar pressure.
(2) The CH4 and N2 gaseous-rich inclusions were trapped in jadeite-rich omphacite and quartz inclusions in garnet, and represent peak metamorphic fluids, with T ~ 750°C and P ~39 kbar.
(3) Pronounced inflow of secondary fluid produced low-salinity inclusions. This suggests a crustal origin at ~30 km depth. Further modification occurred by huge influx of externally derived water when the last stage of subducted crust was exhumed.
Acknowledgements
The authors (BKM and HKS) are thankful to the Director, Prof. B. R. Arora, Wadia Institute of Himalayan Geology, Dehradun, for providing lab facilities to carry out this work. Constructive and sensitive reviews by J. L. R. Touret and an anonymous referee were very much appreciated. Editorial handling by D. M. Pyle and Mrs J. M. Holland are gratefully acknowledged. We thank Profs Matthew J. Kohn and Ron Harris for help in English language exposition with a final version of the MS. This work was partially funded by the Japan Society for Promotion of Sciences, Japan, as PDF to BKM.