Introduction
Blast explosions are the leading cause of injury to service members in the Afghanistan and Iraq wars. In a study of 2000 service members who sustained a traumatic brain injury (TBI) while deployed in Iraq between 2004 and 2008, over 80% of the injuries were mild, with 98% of those due to blast (MacGregor, Dougherty, & Galarneau, Reference MacGregor, Dougherty and Galarneau2011). A recent report confirmed that this proportion of mild TBI (mTBI) in deployment-related TBI has been stable (Helmick et al., Reference Helmick, Spells, Malik, Davies, Marion and Hinds2015). However, TBI during deployment is heterogeneous, also caused by blunt force trauma, and a small percentage of TBI is moderate or severe in severity (Helmick et al., Reference Helmick, Spells, Malik, Davies, Marion and Hinds2015).
Mild TBI in civilians is typically caused by blunt force or acceleration-deceleration injury and, while frequently without focal brain lesions, can injure white matter (WM), including diffuse axonal injury (DAI). Lack of focal lesions on clinical imaging has also been reported after TBI due to blast in veterans (Fischer et al., Reference Fischer, Parsons, Durgerian, Reece, Mourany, Lowe and Rao2014; Scheibel et al., Reference Scheibel, Newsome, Troyanskaya, Lin, Steinberg, Radaideh and Levin2012). Although decrements to WM have not been found in some diffusion tensor imaging (DTI) studies of blast-related TBI in veterans (Davenport, Lim, Armstrong, & Sponheim, Reference Davenport, Lim, Armstrong and Sponheim2012; Levin et al., Reference Levin, Wilde, Troyanskaya, Petersen, Scheibel, Newsome and Li2010; Yeh et al., Reference Yeh, Wang, Oakes, French, Pan, Graner and Riedy2014), analysis which is robust to the spatial heterogeneity of WM injuries and examination of hemispheric asymmetries have yielded evidence suggestive of DAI (Davenport et al., Reference Davenport, Lim, Armstrong and Sponheim2012; Jorge et al., Reference Jorge, Acion, White, Tordesillas-Gutierrez, Pierson, Crespo-Facorro and Magnotta2012; Yeh et al., Reference Yeh, Wang, Oakes, French, Pan, Graner and Riedy2014).
An implication of disrupted WM is altered connectivity between the gray matter regions that affected tracts connect (Greicius, Supekar, Menon, & Dougherty, Reference Greicius, Supekar, Menon and Dougherty2009). In functional connectivity magnetic resonance imaging (fcMRI), low frequency fluctuations in the blood oxygen level dependent (BOLD) signal are postulated to follow intrinsic neuronal activity (Biswal, Yetkin, Haughton, & Hyde, Reference Biswal, Yetkin, Haughton and Hyde1995; Gusnard & Raichle, Reference Gusnard and Raichle2001) and can be measured between brain areas. Functional connectivity (FC) alterations have been reported in civilians with mTBI (Mayer, Mannell, Ling, Gasparovic, & Yeo, Reference Mayer, Mannell, Ling, Gasparovic and Yeo2011; Sours et al., Reference Sours, Zhuo, Janowich, Aarabi, Shanmuganathan and Gullapalli2013; Stevens et al., Reference Stevens, Lovejoy, Kim, Oakes, Kureshi and Witt2012). In subacute civilian mTBI (i.e., 3 to 5 months post-injury), FC within the Default Mode Network (DMN) was decreased, while connectivity between the DMN and regions with which it might normally be anticorrelated (e.g., task-related network which includes the lateral prefrontal cortex) was increased (Mayer et al., Reference Mayer, Mannell, Ling, Gasparovic and Yeo2011; Sours et al., Reference Sours, Zhuo, Janowich, Aarabi, Shanmuganathan and Gullapalli2013). Disruption of electrophysiological signal within the lateral frontal lobes and the WM networks subserving them in veterans from the Iraqi and Afghanistan wars with mTBI (Sponheim et al., Reference Sponheim, McGuire, Kang, Davenport, Aviyente, Bernat and Lim2011) suggests FC of the DMN may also be disrupted in this population.
There are mixed reports regarding alteration of FC associated with blast-related TBI either during or after deployment, and little is known about the long-term consequences. In active duty service members with sub-acute mTBI, decreased connectivity has been reported between 30 and 90 days post-injury that resolved in some participants 6 to 12 months later (Han et al., Reference Han, Mac Donald, Johnson, Barnes, Wierzechowski, Zonies and Brody2014), while in another study active duty personnel with mTBI 60 to 300 days after injury demonstrated increased connectivity within posterior regions of the DMN in supplementary motor area and cerebellum, relative to active duty service members with no history of TBI (Nathan et al., Reference Nathan, Oakes, Yeh, French, Harper, Liu and Riedy2015).
In veterans, compared to healthy civilian controls, an independent components analysis showed reduced FC in six pairs of networks, in addition to alteration in the spectral power of independent components analysis (ICA) time courses and BOLD spatial maps (Vakhtin et al., Reference Vakhtin, Calhoun, Jung, Prestopnik, Taylor and Ford2013). However, Robinson et al. reported that proximity to blast, rather than the presence of concussion symptoms, was associated with disruption to the DMN in veterans an average of 2.6 years after deployment (Robinson et al., Reference Robinson, Lindemer, Fonda, Milberg, McGlinchey and Salat2015), suggesting that effects of blast may be underestimated in cases where TBI is not diagnosed.
While cortical disruptions to FC have been noted after blast, the mechanism of blast TBI suggests that subcortical structures, for example, the basal ganglia, may be impacted as well. The basal ganglia are composed of the globus pallidus, caudate and putamen (which form the striatum), thalamus, nucleus accumbens, and substantia nigra (part of the brain stem), and are involved in dopamine transmission or production (Alexander, DeLong, & Strick, Reference Alexander, DeLong and Strick1986; Cools, Reference Cools2006). In animal studies, effects of blast have been reported in the thalamus (Woods et al., Reference Woods, Colsch, Jackson, Post, Baldwin, Roux and Balaban2013), nucleus accumbens (Sajja, Galloway, Ghoddoussi, Kepsel, & VandeVord, Reference Sajja, Galloway, Ghoddoussi, Kepsel and VandeVord2013), and substantia nigra (Readnower et al., Reference Readnower, Chavko, Adeeb, Conroy, Pauly, McCarron and Sullivan2010). Disrupted catecholamine (including dopamine) expression has also been reported in rats after blast (Tumer et al., Reference Tumer, Svetlov, Whidden, Kirichenko, Prima, Erdos and Wang2013).
However, less is known in humans. Data from an in silico blast model revealed brainstem vulnerability (Taylor & Ford, Reference Taylor and Ford2009), as did a DTI study with veterans exposed to blast (Yeh et al., Reference Yeh, Wang, Oakes, French, Pan, Graner and Riedy2014). In the same DTI study, reduced structural connectivity of a fronto-striatal pathway was also implicated (Yeh et al., Reference Yeh, Wang, Oakes, French, Pan, Graner and Riedy2014). Furthermore, in a study with veterans who had experienced their most severe blast approximately 4 years earlier, altered activation in a fronto-striatal network involved in the stop signal task was found during failures to inhibit irrelevant information (Fischer et al., Reference Fischer, Parsons, Durgerian, Reece, Mourany, Lowe and Rao2014). Additionally, during a working memory task, alterations in caudate activation, accompanied by decrements in performance under the most challenging conditions, was found in veterans with similar post-blast intervals, further suggesting long-term effects on striatum (Newsome et al., Reference Newsome, Durgerian, Mourany, Scheibel, Lowe, Beall and Rao2015).
Alterations in the FC of frontal networks involved in executive function following civilian mTBI (Mayer et al., Reference Mayer, Mannell, Ling, Gasparovic and Yeo2011; Sours et al., Reference Sours, Zhuo, Janowich, Aarabi, Shanmuganathan and Gullapalli2013; Stevens et al., Reference Stevens, Lovejoy, Kim, Oakes, Kureshi and Witt2012) suggests the possibility of disruptions in task-related networks during rest in veterans who had been exposed to blast.
With the aforementioned DTI evidence for chronic effects of blast-related mTBI on structural connectivity and altered caudate activation during task-related fMRI, we hypothesized altered FC in subcortical regions in veterans exposed to TBI in comparison to veterans who without TBI who had not been exposed to blast. Given the previous studies on the DMN and the task related network, we also analyzed FC within the DMN and lateral prefrontal cortex (Smith et al., 2009).
Methods
Participants
Seventeen veterans who had sustained TBI from one or more exposures to blast (mean age=31.4 years; SD=6.2; range=21.9–45.7 years; 0 females) (Table 1) participated in this study. For comparison, 15 veterans who had been deployed, but who had not been exposed to blast and did not have a history of TBI, served as controls (mean age=31.2 years; SD=5.9; range=24.0–47.1 years; 2 females). Fifteen of the TBI participants had mild TBI, 1 had moderate TBI, and 1 had severe TBI. Radiological readings of MRI revealed scattered hyperintensities on fluid attenuated inversion recovery for the participant with moderate TBI and no findings for the participant with severe TBI. Three mild TBI participants who reported exposure to blast also experienced blunt-force mild TBI due to deployment not associated with blast, but no participants reported post-deployment TBI that required hospitalization.
Table 1. Mean (SD) Demographic Characteristics and Outcome Data for the Control and TBI Groups
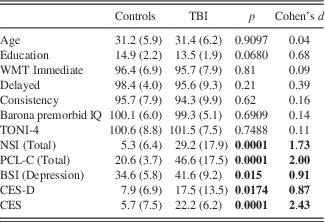
BSI=Brief Symptom Inventory; CES=Combat Exposure Scale; CES-D=Center for Epidemiologic Studies Depression Scale; NSI=Neurobehavioral Symptom Inventory; PCL-C=PTSD Checklist – Civilian; TONI-4=Test of Nonverbal Intelligence – Fourth Edition; WMT=Word Memory Test Bold values indicates p<0.05.
The TBI participants had reported exposure to an average of 4.8 primary blasts (SD=9.2; range=1–40) (Table 2), and were studied after an average interval of 5.46 years (SD=1.9; range=2.6–8.3) since their most serious blast-related TBI. According to the VA/DOD Clinical Practice Guideline, mild TBI was defined as a self-reported injury with loss of consciousness (LOC) less than 30 min and post-traumatic amnesia (PTA) duration of less than 24 hr. Moderate TBI was defined as LOC between 30 min and 24 hr, PTA between 1 and 7 days, and alteration of consciousness greater than 24 hr. Severe TBI was defined as LOC greater than 24 hr, PTA longer than 7 days.
Table 2. Number, Mean, Mode, and Median of Primary, Secondary, Tertiary, and Quaternary Blast Exposures Reported by TBI Participants
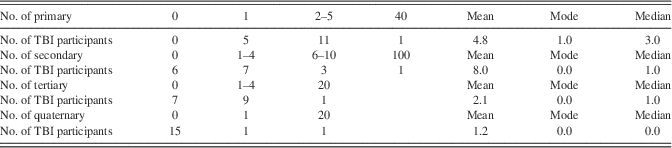
Note. As suggested by the mode, most participants had between one and four primary blasts, and zero secondary, tertiary, and quaternary blasts. The median also indicates that primary blasts were more common than the other categories. Mean responses are slightly skewed because of outlier.
All participants were recruited from the Michael E. DeBakey VA Medical Center (MEDVAMC) from two studies with an identical imaging protocol and were entered into analyses after exclusion for motion greater than 2 mm translation and 2 degrees rotation and inability to complete the scan (e.g., claustrophobia). For both groups, inclusion criteria included deployment in the Iraq or Afghanistan wars and age of 19–49 years. Inclusion criterion for the TBI group was exposure to blast, while the control group had no history of exposure to blast. Exclusion criteria for both groups were hospitalization for head injury after deployment; diagnosis of a severe psychiatric disorder (bipolar disorder, schizophrenia, psychosis not otherwise specified), epilepsy; significant alcohol and drug abuse as indicated by the Alcohol Use Disorders Identification Test (AUDIT) (Babor, Higgins-Biddle, Saunders, & Monteiro, Reference Babor, Higgins-Biddle, Saunders and Monteiro2001) score>16 and Drug Abuse Screening Test (DAST) (Skinner, Reference Skinner1982) score>2; left handed or ambidextrous; and contraindications to undergoing MRI (e.g., metal implants, pregnancy).
Because task-related fMRI was conducted in the same scanning session, failure to meet criterion on the Word Memory Test (Green, Gervais, & Allen, Reference Green, Gervais and Allen2001) was also an exclusion criterion. Groups did not significantly differ in age, sex, race, ethnicity, or estimated (pre-morbid and post-morbid) IQ (all ps>.10). A non-significant trend was observed for education (TBI=13.5 years [SD=1.9 years], Control=14.9 years [SD=2.2], p=.068). All participants were right-handed (Oldfield, 1971). All participants were interviewed by a clinician or staff member who was experienced in the evaluation of individuals with combat injury. The study was approved by the institutional review board at Baylor College of Medicine and the Research and Development Committee at MEDVAMC. Informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization were obtained for each participant.
The Polytrauma Interview was used to assess deployment and injury-related information (e.g., number of deployments and the type, number, and proximity of blasts), and associated self-reported LOC, alteration of consciousness (AOC), and PTA. This structured interview was adapted from the VA TBI secondary evaluation (Belanger, Uomoto, & Vanderploeg, Reference Belanger, Uomoto and Vanderploeg2009). In addition to deployment-related injury information, the Polytrauma Interview also collected data regarding pre- and post-deployment TBI injury history. Primary blast was described by stating “when a high explosive bomb or IED goes off, there is a blast wave of highly compressed gas that hits solid objects like a person's body and may feel almost like running into a wall”.
Secondary effects of blast were described by stating that “the blast wave is followed by a wind in which sand, debris, shrapnel, and other fragments are moving rapidly.” Tertiary effects of blast were described by stating that “the blast wave may cause a person to be thrown to the ground or into a stationary object.” Quaternary effects of blast were described as “other injuries associated with an explosive blast including burns, wounds, broken bones, amputations, breathing toxic fumes, or crush injuries from falling objects.”
Symptom and Demographic Measures
All participants completed the Neurobehavioral Symptom Inventory (NSI) (Cicerone & Kalmar, Reference Cicerone and Kalmar1995), PTSD Checklist – Civilian version (PCL-C) (Weathers, Litz, Herman, Huska, & Keane, Reference Weathers, Litz, Herman, Huska and Keane1993), Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, Reference Radloff1977), the depression scale from the Brief Symptom Inventory (BSI) (Derogatis, Reference Derogatis1993), the Combat Exposure Scale (CES) (Keane et al., Reference Keane, Fairbank, Caddell, Zimering, Taylor and Mora1989), and the Test of Nonverbal Intelligence – Fourth Edition (TONI-4) (Brown, Sherbenou, & Johnsen, Reference Brown, Sherbenou and Johnsen2010). Information was also collected to calculate the Barona IQ, an estimate of pre-morbid intelligence (Barona, Reynolds, & Chastain, Reference Barona, Reynolds and Chastain1984). Please see Table 1.
Image Data Acquisition
Whole brain imaging was performed using a 12-channel head coil on a Siemens MAGNETOM Trio 3 Tesla system. BOLD T2*-weighted echo-planar images (EPI) were acquired as 245 volumes with 40 axial slices of 3.0 millimeter (mm) thickness with a 0.3 mm gap, using a 240-mm field of view (FOV), 70×70 matrix, repetition time (TR) of 2030 ms, echo time (TE) of 28 ms, and a 90-degree flip angle. A set of three-dimensional (3D) high-resolution T1-weighted images were also acquired in 176 sagittal slices of 1.0 mm thickness (no gap) with 256 mm FOV, 256×256 matrix, TR of 2530 ms, TE of 2.6 ms, and a 7.0-degree flip angle.
Statistical Analysis
Symptom and demographic measures
Two-tailed t tests for independent groups were performed to test group differences. Effect sizes (Cohen’s d) (Cohen, Reference Cohen1988) were calculated to further characterize group differences, where d=.2 is a small effect, d=.5 is a moderate effect, and d=.8 is a large effect.
Functional connectivity image processing and analysis
The Functional Connectivity Toolbox (Conn) (Whitfield-Gabrieli & Nieto-Castanon, Reference Whitfield-Gabrieli and Nieto-Castanon2012) within SPM8 (Wellcome Department of Cognitive Neurology, University College, London, UK) implemented in Matlab (Mathworks Inc., Sherborn, MA) was used to process and analyze data. Functional images of each participant were realigned, co-registered with each participant’s high resolution anatomical image, normalized to the Montreal Neurological Institute (MNI) template, and smoothed using a 6-mm full width-half maximum Gaussian filter. Anatomical landmarks in the normalized high resolution anatomical and functional data were visually checked and compared against the MNI template for each participant. Each participant’s anatomical image was segmented into gray matter, WM, and cerebrospinal fluid (CSF) masks.
Physiological noise was addressed by using WM and CSF masks as covariates. Realignment parameters and their first-order derivatives were also covaried. The Artifact Detection Toolbox (Whitfield-Gabrieli & Nieto-Castanon, Reference Whitfield-Gabrieli and Nieto-Castanon2012) was used to repair artifact due to frame-by-frame head movement, that is, scrub (Power, Barnes, Snyder, Schlaggar, & Petersen, Reference Power, Barnes, Snyder, Schlaggar and Petersen2012), and correct global drift. Outlier time points were defined as exceeding three standard deviations from the mean image intensity of the complete resting state run. Outliers were included as regressors in the first level general linear model along with motion parameters.
Data were band-pass filtered between 0.008 and 0.09 Hz (Arnold Anteraper et al., Reference Arnold Anteraper, Triantafyllou, Sawyer, Hofmann, Gabrieli and Whitfield-Gabrieli2014; Hosseini & Kesler, Reference Hosseini and Kesler2013; Pelletier-Baldelli, Bernard, & Mittal, Reference Pelletier-Baldelli, Bernard and Mittal2015), the default frequency range in the SPM Conn toolbox. The high-pass value was selected to approximate both SPM's default value (0.0078 Hz) and a 2-min value suggested as a standard (0.0083 Hz) (Ashby, Reference Ashby2011). The low-pass value approximates the frequently reported 0.08 Hz and 0.10 Hz values and SPM’s hemodynamic response function cutoff frequency of .091 Hz. FC was measured with the following 12 seeds (1) subcortical: bilateral caudate, putamen, and globus pallidus; (2) DMN: a single seed in the medial prefrontal cortex (MPFC), a single seed in the posterior cingulate cortex (PCC), bilateral lateral parietal lobes (Fox, 2005); and (3) bilateral dorsolateral prefrontal cortex (DLPFC) (Brodmann area 9). Seeds were made available by the Conn software package and through the WFU PickAtlas (Maldjian, Laurienti, Kraft, & Burdette, Reference Maldjian, Laurienti, Kraft and Burdette2003). See the Appendix.
A general linear model was used to estimate the correlation between the seeds and the whole brain on a voxel-wise level for individual participants (first level). Pearson correlation coefficients were then transformed into Z-scores using Fisher’s method followed by group (second level) random effects analyses. For group analyses, t tests were calculated to investigate whole brain differences in FC between TBI participants and controls. For seeds in which t tests were significant, analysis of covariance (ANCOVA) was performed with age, total scores on the CES-D and the PCL-C as covariates in the model after verifying the homogeneity of slopes assumption for each seed on a voxel-wise level. One control participant was omitted in the ANCOCA for incomplete CES-D data. Furthermore, regression with time since injury was performed in the TBI group.
For all analyses, significance was defined by voxel (height) threshold p<.001 and cluster threshold p<.05 false detection rate (FDR) corrected for multiple comparisons across the whole brain. The significance criterion for the FDR-corrected cluster probability values was Bonferroni corrected for the seeds placed on both sides (left and right) and further correction was made for both tails for the three types of analyses (between-group t tests, ANCOVA, simple regression) (criterion p=.05/[12 regions×2 tails×3]=0.000694). Effect sizes (Cohen’s d) (Cohen, Reference Cohen1988) were calculated to further characterize group differences.
Results
Symptom and Demographic Measures
As shown in Table 1, groups differed on total scores of the NSI, PCL-C, CES-D, BSI, and CES, with the TBI group reporting more combat exposure and more severe post-concussion, PTSD, and depression symptoms than the control group. There were no group differences in nonverbal IQ (TONI-4; Brown, 2010) or estimated pre-morbid IQ (Barona et al., Reference Barona, Reynolds and Chastain1984).
Functional Connectivity
Time since injury
Regressions with time since injury in the TBI group were nonsignificant for all regions.
Caudate
FC from the right and left caudate body did not significantly differ between the TBI group and the controls.
Putamen
The TBI group demonstrated greater positive FC than the control group between the right putamen and a cluster (50 -54 32) that included primarily the right angular gyrus, as well as adjoining right lateral occipital cortex (Cohen’s d=−2.17). There were no significant between-group differences in the FC with left putamen. Please see Figure 1a and Table 2a. However, these results were no longer significant when age and measures of depression and PTSD were used as covariates in analyses.

Fig. 1 Between-groups t tests showing significant group differences in functional connectivity between the clusters shown and seeds in the (a) right putamen (TBI>Control); (b) right globus pallidus (Control>TBI) [b1: cluster 1, −26 −56 −10; b2: cluster 2, 26 −60 −8]; and (c) right DLPFC (Control>TBI) [c1: cluster 1, −36 −52 52; c2: cluster 2, 48 −34 54]. Mean Fisher transformed Z-scores and their standard errors associated with each cluster are also depicted. Left side of brain is on left side of image.
Globus pallidus
FC was significantly reduced in the TBI group compared to the control group between the right globus pallidus and one cluster (−26 −56 −10) that included primarily the left temporal occipital fusiform cortex, as well as adjoining occipital fusiform gyrus, lingual gyrus, and cerebellum (Cohen’s d=2.53). An additional marginally significant cluster (26 −60 −8, Cohen’s d=1.85; p=.0069) was located in the same regions on the right. In both clusters the control group demonstrated positive connectivity, while the TBI group demonstrated anticorrelation with the right globus pallidus. There were no significant between-group differences in FC with left globus pallidus. Please see Figure 1b and Table 2b. The clusters observed in the t test were significant when age and measures of depression and PTSD were entered as covariates in the analyses (Cohen’s ds=1.80 [left cluster] and 1.32 [right cluster]), and an additional cluster in the occipital pole emerged (Cohen’s d=1.45). See Figure 2 and Table 4.

Fig. 2 Results from between-groups ANCOVA demonstrating significant group differences in functional connectivity in the right globus pallidus when covarying for age, PTSD and depression symptoms (Control>TBI). (a): Depicts functional connectivity with a cluster whose most significant voxel is at -22 -58 -10. (b): Depicts functional connectivity with a cluster whose most significant voxel is at 30 -54 -18. (c): Depicts functional connectivity with a cluster whose most significant voxel is at 8 -88 28. Mean Fisher transformed Z-scores and their standard errors associated with each cluster are also depicted. Left side of brain is on left side of image.
DMN
There were no significant between-group differences in any of the four DMN seeds.
DLPFC
FC was significantly reduced in the TBI group compared to controls between the right DLPFC and bilateral superior parietal lobule, supramarginal gyrus (anterior and posterior divisions), and post-central gyrus (-36 -52 52 and 48 -34 54, Cohen’s d=2.20 and 2.25, respectively). In both clusters, the control group demonstrated positive connectivity, while the TBI group demonstrated anticorrelation. There were no significant between-group differences in FC with left DLPFC. Please see Figure 1c and Table 2c. However, when age and the PTSD and depression measures were entered as covariates in the analyses, no clusters were significant.
Discussion
The aim of this study was to investigate FC several years after blast-related TBI sustained by service members during the wars in Iraq and Afghanistan. In between-group comparisons of veterans, we found disrupted FC from the right putamen and from the right globus pallidus. However, after covarying for age, PTSD, and depression, the group difference seen for the putamen was no longer significant, while the group differences found for the globus pallidus maintained significance, and an additional cluster in the occipital pole was revealed. Thus, the right globus pallidus appears to be affected in this sample.
The globus pallidus is one of several subcortical structures that form the basal ganglia (caudate and putamen, which form the striatum, thalamus, nucleus accumbens, and substantia nigra) and is involved in memory, reward, and movement and in dopamine transmission (Eid & Parent, Reference Eid and Parent2015; Gauthier, Parent, Levesque, & Parent, Reference Gauthier, Parent, Levesque and Parent1999). Animal models of blast suggest alterations in thalamus, nucleus accumbens, and substantia nigra (Readnower et al., Reference Readnower, Chavko, Adeeb, Conroy, Pauly, McCarron and Sullivan2010; Sajja et al., Reference Sajja, Galloway, Ghoddoussi, Kepsel and VandeVord2013; Woods et al., Reference Woods, Colsch, Jackson, Post, Baldwin, Roux and Balaban2013), and a small number of DTI and fMRI studies in humans suggest that the striatum (putamen and caudate) may be impacted (Fischer et al., Reference Fischer, Parsons, Durgerian, Reece, Mourany, Lowe and Rao2014; Newsome et al., Reference Newsome, Durgerian, Mourany, Scheibel, Lowe, Beall and Rao2015; Yeh et al., Reference Yeh, Wang, Oakes, French, Pan, Graner and Riedy2014). Results of the present study revealed altered FC from the right globus pallidus, which demonstrated anticorrelation with temporo-fusiform cortex, cerebellum, lingual gyrus, and cuneus.
Why would connectivity between the right globus pallidus and temporo-occipital-cerebellar regions be altered? In this preliminary account, the precise mechanism and consequences of globus pallidus involvement are unknown. However, our results invoke a few intriguing lines of research that warrant future inquiry. First, regarding laterality, the globus pallidus has been reported to be smaller on the right than left (Kang, Herron, Ettlinger, & Woods, Reference Kang, Herron, Ettlinger and Woods2015), potentially leading to differences in functional connectivity as seen in a study of multiple sclerosis (Akbar et al., Reference Akbar, Till, Sled, Binns, Doesburg, Aubert-Broche and Banwell2015).
Second, there are known pathways between the globus pallidus and temporo-occipital regions; however, these regions are not identical to those reported here. For example, temporo-occipital cortex projects to the striatum, which projects to the globus pallidus, suggesting a route that may be impaired in deployment-related TBI. However, the area of temporo-occipital cortex that projects to the striatum is not in the temporo-occipital region specific to the fusiform area, the area reported here (Alexander et al., Reference Alexander, DeLong and Strick1986; Yeterian & Van Hoesen, Reference Yeterian and Van Hoesen1978). There is also a region in inferotemporal cortex that receives basal ganglia (which contains the globus pallidus) output (Middleton & Strick, Reference Middleton and Strick2000), but that region is anterior to the temporal region reported here.
An additional line of inquiry regarding these regions relates to structural connectivity routes described by Kwon and Jang (Reference Kwon and Jang2014), who reported strong structural connectivity between the substantia nigra and globus pallidus in healthy people, but only a limited degree of structural connectivity between the substantia nigra and temporal areas that included the fusiform cortex reported here (BA37) (Kwon & Jang, Reference Kwon and Jang2014). It is intriguing to consider that blast might have altered any similar relation in functional connectivity. A structural network that includes the globus pallidus and temporal-occipital fusiform gyrus, among other regions, has been identified in patients with fronto-temporal dementia (Ahmed et al., Reference Ahmed, Irish, Henning, Dermody, Bartley, Kiernan and Hodges2016).
A third possibility in this population is that PTSD may not have been completely accounted for, although scores representing severity of PTSD symptoms were entered as covariates in our study. The temporal-occipital fusiform cortex is associated with emotion and has been found to have altered activation in males with PTSD symptoms (Crozier, 2014). In fact, in veterans with PTSD, this region demonstrated augmented activation during the processing of combat-related scenes in a cluster with foci (-32 -50 -12) similar to one reported here (30 -54 -18) (Morey et al., Reference Morey, Dolcos, Petty, Cooper, Hayes, LaBar and McCarthy2009). Thus PTSD may influence the FC in these veterans. The cerebellum also had altered FC with the globus pallidus. Future studies may further investigate the interaction between the cerebellothalamic and pallidum pathway, which has been linked to tremor (Helmich, Janssen, Oyen, Bloem, & Toni, Reference Helmich, Janssen, Oyen, Bloem and Toni2011). Finally, the globus pallidus also showed altered activation with regions associated with lingual gyrus, cuneus, and occipital fusiform gyrus. These regions associated with visual processing had been shown to demonstrate differential activation with globus pallidus during two types of memory processing (de Rover et al., Reference de Rover, Petersson, van der Werf, Cools, Berger and Fernandez2008).
We did not find differences in the analysis of the DMN, consistent with Robinson and colleagues (Robinson et al., Reference Robinson, Lindemer, Fonda, Milberg, McGlinchey and Salat2015), who found an effect of proximity to blast but not mTBI due to blast in the posterior cingulate of the DMN in veterans approximately 30 months post-deployment. Taken together with Nathan et al. (Reference Nathan, Oakes, Yeh, French, Harper, Liu and Riedy2015), who reported increased FC within posterior DMN regions in active duty service members, effects of blast on the DMN may be more prominent in post-injury intervals closer to exposure. However, using an ICA, Vakhtin et al. (Reference Vakhtin, Calhoun, Jung, Prestopnik, Taylor and Ford2013) reported weakened FC between the DMN and two other networks in veterans with an undisclosed post-injury interval, suggesting that detection of any alterations in DMN processing may vary by analysis methods and thus be subtle, as seen in mixed reports of alterations in DTI data following blast (e.g., Davenport et al., Reference Davenport, Lim, Armstrong and Sponheim2012). However, the control participants in the study by Vakhtin et al. (Reference Vakhtin, Calhoun, Jung, Prestopnik, Taylor and Ford2013) were healthy males from the community who were not matched on military or deployment status, which may impact mental and brain health.
A pattern of disruption between the right DLPFC and superior parietal lobules was observed in the t test, but because the group difference for the DLPFC seed was no longer significant after covarying for age and PTSD and depression symptoms, the results may be accounted for by the common variance associated with PTSD, depression, and TBI, rather than variance associated with TBI alone. The fronto-parietal network is associated with flexibility in cognitive control (Cole et al., Reference Cole, Reynolds, Power, Repovs, Anticevic and Braver2013), and rumination and inability to control intrusive thoughts associated with depression and PTSD may override cognitive control.
Altered FC between frontal and parietal regions and the hippocampus has been reported in patients with depression (Rao et al., Reference Rao, Jenkins, Hymen, Feigon, Weisenbach, Zubieta and Langenecker2016); although the parietal region was the posterior cingulate rather than the superior parietal lobes, supramarginal gyrus, and postcentral gyrus found here, and in PTSD (Sakamoto et al., Reference Sakamoto, Fukuda, Okuaki, Rogers, Kasai, Machida and Kato2005), albeit in ventral rather than dorsal frontal regions. It should be noted that, because of the overlap of PTSD symptoms with symptoms of mTBI, use of the PCL-C may have captured some concussion symptoms (Brenner, Vanderploeg, & Terrio, Reference Brenner, Vanderploeg and Terrio2009). Increased FC between the right putamen and angular gyrus was found in a between-group t test, but not following ANCOVA, again suggesting any disruption may be related to PTSD or depression.
Alterations in the FC of the globus pallidus several years after blast exposure suggests the potential of a neurodegenerative process. While there is currently little support for a relationship between isolated mTBI and Parkinson’s disease (Marras et al., Reference Marras, Hincapie, Kristman, Cancelliere, Soklaridis, Li and Cassidy2014), repetitive mild and more severe TBI have been linked to Parkinson’s disease (Bower et al., Reference Bower, Maraganore, Peterson, McDonnell, Ahlskog and Rocca2003; Shahaduzzaman, Acosta, Bickford, & Borlongan, Reference Shahaduzzaman, Acosta, Bickford and Borlongan2013). It is possible that, while patients with mTBI might not develop Parkinson’s disease per se, alterations may still be detectable in subcortical structures implicated in Parkinson’s disease. Studies that directly compare active duty service members and veterans exposed to blast could elucidate any progression of changes over time.
Given previous work demonstrating changes in caudate activation during task-related fMRI, we had hypothesized that there would be alterations in the FC of the caudate. However, the caudate did not demonstrate any between-group differences in current analyses. Only the body of the caudate was investigated in this paper, and potentially changes in FC might be observed in the head or tail. It is also possible that changes in caudate activity may be sensitive to whether a task is performed and may be found only during challenging cognitive processing, as when failing to correctly inhibit irrelevant information (Fischer et al., Reference Fischer, Parsons, Durgerian, Reece, Mourany, Lowe and Rao2014) or remember high memory loads (Newsome et al., Reference Newsome, Durgerian, Mourany, Scheibel, Lowe, Beall and Rao2015). In a study investigating PTSD, veterans with mTBI of an unknown post-injury interval were reported to demonstrate a negative relation between re-experiencing (a PTSD symptom) and FC in a graph theoretic network surrounding the caudate (Spielberg, McGlinchey, Milberg, & Salat, Reference Spielberg, McGlinchey, Milberg and Salat2015), suggesting that additional disruption to subcortical structures may be revealed when specific clusters of PTSD and different network relations are investigated.
Limitations of this study include the overlap or shared variance between self-report measures of concussive, depression, and PTSD symptoms (Brenner et al., Reference Brenner, Vanderploeg and Terrio2009; Scheibel et al., Reference Scheibel, Pastorek, Troyanskaya, Kennedy, Steinberg, Newsome and Levin2015), as both depression and PTSD were more severe in the TBI group. Enrolling participants with deployment-related TBI without PTSD or depression is difficult given the high rate of co-morbidity in veterans. While it was not possible to group-match patients to controls on PTSD and depression and obtain samples that are representative of their respective populations, we matched the groups on age and IQ. Measures of PTSD and depression were also entered as covariates in follow-up analyses.
From a statistical perspective, the use of covariates must be carefully considered for any analyses in which (1) the covariates are significantly different between the groups and (2) where the difference in the covariate is believed to be inherently part of the grouping variable (i.e., disease process). Classic examples of covariates that meet both of these criteria include increased levels of anxiety in depressive disorders and decreased IQ in patients with schizophrenia (Miller & Chapman, 2001). In the present study, the potential covariate (i.e., depression) may theoretically be a direct result of the disease process (i.e., TBI) as well. Removing the variance associated with the covariate can also reduce the variance associated with the grouping variable, decreasing the sensitivity of analyses. As a conservative approach, we investigated results both without and with ANCOVA due to the controversies surrounding the use of covariates in analyses (Miller and Chapman, 2001) and focused our discussion on results that survived the more conservative analyses (globus pallidus). However, investigation of PTSD was limited to symptoms endorsed on the PCL, rather than a diagnostic test.
A further limitation includes small sample sizes, which are associated with a greater chance of false negatives, reduced likelihood of detecting true positives, and the potential of the magnitude of effects being overestimated, although the ability to correct for nuisance variables may not be impacted by sample size (Button et al., Reference Button, Ioannidis, Mokrysz, Nosek, Flint, Robinson and Munafo2013; Friston, Reference Friston2013; Lindquist, Caffo, & Crainiceanu, Reference Lindquist, Caffo and Crainiceanu2013).
The higher education of the control group than the TBI group (14.9 vs. 13.5 years) also represents a confound, but the groups did not differ in estimated pre-morbid or current IQ. Unavailability of medication information precluded the ability to investigate effects of medication. Additionally, spatial heterogeneity in WM injury location has been cited as a limitation in many studies of mTBI, and the self-report nature of injury severity variables such as duration of alteration of consciousness, LOC, and PTA, as well as TBI of mixed etiology (e.g., blast plus blunt force), are also common limitations in this population. Study strengths include exclusion of participants who reported post-deployment mTBI and ongoing alcohol or drug misuse, as well as inclusion of control participants who had been deployed, and conservative correction for multiple seeds and two tails.
Conclusions
Alterations in the FC of the globus pallidus are evident in veterans an average of 5.46 years after blast-related TBI. Future studies investigating implications of subcortical alteration may further elucidate long-term changes in the brain after blast exposure.
Table 3. Between Groups t-Tests Showing Significant Group Differences in Functional Connectivity between the Custers Shown and Seeds in the (a) Right Putamen (TBI>Control); (b) Right Globus Pallidus (Control>TBI); and (c) Right DLPFC (Control>TBI)

a Probability at the cluster level of significance after random field theory family-wise error correction over the whole brain search volume.
b Number of voxels within a cluster.
c Negative values along the x-axis are defined to be in the participant’s left hemisphere.
d Marginal, where the threshold for significance is defined as p<0.000694.
Table 4. Results from between-Groups ANCOVA Demonstrating Significant Group Differences in Functional Connectivity in the Right Globus Pallidus when Covarying for Age and Depression and PTSD Symptoms (Controls>TBI)
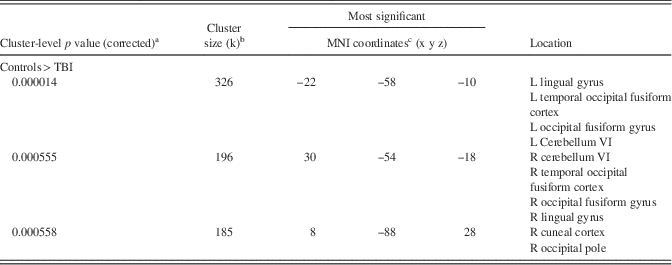
a Probability at the cluster level of significance after random field theory family-wise error correction over the whole brain search volume.
b Number of voxels within a cluster.
c Negative values along the x-axis are defined to be in the participant’s left hemisphere.
Acknowledgments
This study was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Rehabilitation Research and Development, #B6812C Traumatic Brain Injury Center of Excellence (HSL), #B45961 (HSL), #I21RX001608 (MRN), B1320-I (HSL and RSS), and O1062-I (RSS & EAW). The South Central Mental Illness Research, Education, and Clinical Center (SCMIRECC) at the Michael E. DeBakey VA Medical Center provided facilities that allowed analyses of the fMRI data. We also gratefully acknowledge the assistance and computing resources provided by the CIBR Center for Computational and Integrative Biomedical Research of Baylor College of Medicine in the completion of this work. We are grateful to Qisheng Liu for technical assistance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs. There are no potential conflicts of interest, including financial, between any of the authors and the work presented in this manuscript. We are grateful to the veterans taking part in this research.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1355617716000448








