INTRODUCTION
Many studies, including some of those presented in this Special Issue, demonstrate the power of parasites in stock assessment – the process of distinguishing populations of species of animals. Such use of parasites as ‘biological tags’ can be both powerful and highly cost-effective. However, the approach must be used with care (Lester and MacKenzie, Reference Lester and MacKenzie2009). MacKenzie and Abaunza (Reference MacKenzie and Abaunza1998) discussed six criteria for the inclusion of parasite species in such studies. The fifth of these was: ‘The parasite should be easily detected and identified. Examination of the host should involve the minimum of dissection, otherwise time can become a limiting factor.’ An important component of the ‘easily detected and identified’ aspect of this criterion is that these studies depend significantly on the accuracy of the identification of the parasites concerned; if identity is mistaken then meaning may be hidden or misrepresented, or resolution lost. Recently it has become increasingly apparent that cryptic species may represent a significant part of parasite biodiversity and that we ignore the possibility of their presence at our peril. As Pérez-Ponce de León and Nadler (Reference Pérez-Ponce de León and Nadler2010) put it in the title of their review of the problem: ‘What we don't recognize can hurt us.’ The extent to which parasite identification may impinge on the effectiveness of stock assessment, and specifically the issue of cryptic species, is the subject of this review. The scope of our analysis encompasses the various helminth parasite groups found in aquatic animals, with special reference to trematodes.
What is the potential problem?
Biological tagging studies seek to determine whether samples of an animal species (usually fishes) from different areas can be considered part of the same population. In essence the tagging process seeks to identify the parasites present, identify those most likely to be useful relative to published criteria for informativeness (MacKenzie and Abaunza, Reference MacKenzie and Abaunza1998; MacKenzie, Reference MacKenzie2002; Lester and MacKenzie, Reference Lester and MacKenzie2009; Palm, Reference Palm and Mehlhorn2011), and then to explore the level of difference between the putative populations. In the simplest terms, three outcomes are possible when the distribution of a parasite is considered for samples from two localities:
-
(a) The parasite may be present at one site and absent at the other.
-
(b) The parasite may be present at both sites, but far more prevalent or abundant at one than at the other.
-
(c) The parasite may occur in equivalent numbers at both sites.
The problem of parasite identification has varying levels of importance in these three scenarios:
-
(a) In a clear presence/absence contrast there are no parasite identification issues. Any parasite-based interpretations are likely to be robust unless they fall foul of other considerations such as longevity of the parasite (e.g. Lester and MacKenzie, Reference Lester and MacKenzie2009).
-
(b) Where prevalences or intensities of a parasite differ significantly between sites, the possibility of misidentification exists, but if an interpretation of biological meaning (i.e. population differences) can be construed from the data then the possibility that more than one species is present need not materially affect the interpretation and mistaken identification will not necessarily lead to an under-interpretation of biological difference between populations.
-
(c) Where specimens are assessed as being single parasite species, and not differing in their prevalence and intensity between sites, there is significant potential for loss of information if cryptic species have not been recognized. If the identification is mistaken and separate species are involved then this may lead to an interpretation of absence of difference with respect to stock discrimination where in fact important differences may exist.
The identity problem
One important consideration in the use of parasites in stock assessment is that there is an important difference between parasite distinction and identification. As taxonomists, we find it disappointing (or at least a compelling illustration of the challenge that still awaits) that numerous parasite species in biological tagging studies remain unidentified to species (e.g. Henriquez et al. Reference Henriquez, Gonzalez, Licandeo and Carvajal2011; MacKenzie et al. Reference MacKenzie, Brickle, Hemmingsen and George-Nascimento2013; Zischke et al. Reference Zischke, Griffiths, Tibbetts and Lester2013). However, from the perspective of stock discrimination, we acknowledge that the key to reliable analysis is distinction rather than identification. That is, we need to be confident that what is identified as a single species really is a single species rather than necessarily having the correct formal name for it. Having made that allowance, we still maintain strongly that full identification of parasite species is an important example of the principle that science should be replicable. In our view, all parasitologists should aspire to having their parasites named either by their own efforts or by recruiting taxonomists to their study (and perhaps by funding them!).
Having established that it is important for parasites in stock assessment studies to have their distinctiveness established, we must face the fact that this is not easy. Fishes have a huge array of parasites from many phyla and certainly no single worker can claim to be an expert on the identification of them all. Parasites are, in general, notoriously difficult to identify because of their size, frequently because of the paucity of reliable taxonomic characters (Poulin, Reference Poulin2011), and, in the context of biological tagging studies, the fact that samples are not necessarily in optimal condition for identification. These difficulties are exacerbated by the fact that new species are being described continually for almost all groups of parasites of fishes. The important theoretical concept that all taxonomic work and, as a result, all identifications, remain hypotheses is especially true for parasites.
Cryptic species
The core identification issue considered here is that of cryptic species, an issue that has become significant in most animal groups in which it has been explored. Thus, it has recently become clear that African elephants are two species instead of one (Ishida et al. Reference Ishida, Oleksyk, Georgiadis, David, Zhao, Stephens, Kolokotronis and Roca2011); manta rays are now recognized as two species instead of one (Marshall et al. Reference Marshall, Compagno and Bennett2009); and bone fishes (Albulidae) are now considered to comprise perhaps eight species instead of one (Colborn et al. Reference Colborn, Crabtree, Shaklee, Pfeiler and Bowen2001). An exact definition of cryptic species remains mildly controversial (Pérez-Ponce de León and Nadler, Reference Pérez-Ponce de León and Nadler2010). The ‘pure’ definition is of species that cannot be distinguished on the basis of their morphology despite molecular evidence that they are specifically distinct and perhaps biological evidence in terms of distribution and behaviour. This sharp definition merges into a range of less rigorous concepts which essentially capture the idea that two species (or whole complexes of species) are closely related and difficult to distinguish. Often such species, in the light of reciprocal illumination from molecular studies, can indeed be distinguished by morphology (e.g. Jousson and Bartoli, Reference Jousson and Bartoli2002; Hunter et al. Reference Hunter, Ingram, Adlard, Bray and Cribb2010). We suspect that a posteriori distinction is actually usually possible and we do note that some groups of parasites seem to be designated as being cryptic without any evidence of a serious effort to distinguish them on the basis of their morphology. Even taxa studied only as larval stages, for which taxonomic distinction can be expected to be considerably more difficult than in the corresponding adult parasites, are often characterized as comprising cryptic species (e.g. Miura et al. Reference Miura, Kuris, Torchin, Hechinger, Dunham and Chiba2005; Leung et al. Reference Leung, Keeney and Poulin2009; Detwiler et al. Reference Detwiler, Bos and Minchella2010; Locke et al. Reference Locke, McLaughlin and Marcogliese2010). However, given that taxonomy is done as a service to all of biology, we see no value in invoking an especially strict definition for cryptic species. Quite reasonably, for those interested in using parasites as biological tags, cryptic species are simply those that cannot be readily and reliably distinguished by their morphology.
Although combinations of apparently cryptic species might be distinguished by detailed morphological study, it has become the norm to seek distinction from molecular data. Molecular exploration of species level distinctions of parasites commenced with allozyme electrophoresis studies (e.g. Baverstock et al. Reference Baverstock, Adams and Beveridge1985; Bray and Rollinson, Reference Bray and Rollinson1985; Reversat et al. Reference Reversat, Leducq, Marin and Renaud1991). At present cryptic species differences are explored overwhelmingly through nuclear and mitochondrial DNA sequencing as exemplified by the many reports discussed below. Available markers provide varying levels of resolution (Vilas et al. Reference Vilas, Criscione and Blouin2005; Yao et al. Reference Yao, Song, Liu, Luo, Han, Li, Pang, Xu, Zhu, Xiao and Chen2010) and the selection of the marker tends to depend on the question being posed and, in some cases, on the nature of relevant pre-existing data. There is no accepted standard of a level of molecular variation that should be taken to equate with recognition of separate species (Nolan and Cribb, Reference Nolan and Cribb2005), and thought and practice in this area are certainly still evolving (Nadler and Pérez-Ponce de León, Reference Nadler and Pérez-Ponce de León2011). Most authors tend, we think reasonably, to take a case by case approach as to what variation might be considered inter- and intraspecific. One simple yardstick employed in some of our work is to consider any level of genetic distinction that exceeds that between recognizable morphospecies as prima facie evidence of the presence of separate and potentially cryptic species.
Forms of cryptic species
As we have seen above, cryptic species have the capacity to reduce the information generated from biological tagging studies and, at the worst, to lead to the false interpretation of a homogeneous population where in reality there is significant heterogeneity. Given this potential significance, it is important to understand how and why cryptic species may appear. Pairs or complexes of cryptic species are, as far as we are aware, always closely related, implying recent common ancestors. Thus, cryptic species pairs are usually ‘sibling’ or ‘sister’ species and if a complex of cryptic species occurs they have been known as ‘species flocks’. It is, therefore, unsurprising that they are difficult to distinguish. The cryptic pairs/complexes may have a range of origins, or at least presentation in the field, and these in turn have dramatically varying levels of importance for stock discrimination studies.
Firstly, numerous combinations of cryptic species have been identified in association with separate sympatric host species, for examples in the Trematoda (Jousson et al. Reference Jousson, Bartoli and Pawlowski2000; Hunter et al. Reference Hunter, Ingram, Adlard, Bray and Cribb2010; Carreras-Aubets et al. Reference Carreras-Aubets, Repulles-Albelda, Kostadinova and Carrasson2011; Calhoun et al. Reference Calhoun, Curran, Pulis, Provaznik and Franks2013; Curran et al. Reference Curran, Tkach and Overstreet2013); and Monogenea (Huyse and Volckaert, Reference Huyse and Volckaert2002; Bueno-Silva et al. Reference Bueno-Silva, Boeger and Pie2011). Glennon et al. (Reference Glennon, Perkins, Chisholm and Whittington2008) reported several cases of cryptic monogenean species in association with separate and allopatric ray species. Martínez-Aquino et al. (Reference Martínez-Aquino, Reyna-Fabián, Rosas-Valdez, Razo-Mendivil, Pérez-Ponce de León and García-Varela2009) found evidence of cryptic species within the acanthocephalan Neoechinorhynchus golvani associated with separate lineages of fresh-water cichlid and brackish-water eleotrid fishes in Mexico. We have found no clear-cut examples of cestodes or nematodes being considered cryptic species in such circumstances. Usually (as in most of the examples given above) the ‘cryptic species’ have proven distinguishable on morphological grounds given the reciprocal illumination of molecular study. Exceptions in this respect are certain sympatric species of Euryakaina (Trematoda: Cryptogonimidae) which are associated with different species of lutjanids on the Great Barrier Reef (GBR); these currently remain distinguishable only with the use of molecular approaches (Miller et al. Reference Miller, Adlard, Bray, Justine and Cribb2010a ). In addition to these studies where the term ‘cryptic’ is invoked, it is almost axiomatic that parasite taxonomists have a high expectation of finding separate species in separate hosts, so that they remain on the alert for them and describe and report them without necessarily invoking the term ‘cryptic species’. Indeed for some groups, the expectation of strict specificity is so high that when host sharing is found it is reported on specifically (e.g. Schoelinck et al. Reference Schoelinck, Cruaud and Justine2012).
Our reading of the literature suggests to us that species reported from multiple hosts which later prove to be a complex of species with stricter than originally-realized specificity is the dominant paradigm for the discovery of what are interpreted in the literature as cryptic parasite species (Miller et al. Reference Miller, Bray and Cribb2011). From the perspective of biological tagging studies, this is good news – what is possibly the largest source of cryptic species does not affect tagging studies. Biological tagging studies usually consider individual fish species rather than complexes of species. In this respect, a biological tagging study may well have an identification that eventually proves to be wrong because the parasite is being mistaken for a closely related species in another host species, but the differentiation of the parasite for the purposes of the study will remain informative.
Secondly, numerous groups of fish parasite species have been identified as comprising complexes (species flocks) of species that infect a single host species, often at a single locality. This pattern can be seen as being expressed on two levels – obvious and cryptic. Thus, the serranid Epinephelus maculatus has eight species of the monogenean genus Pseudorhabdosynochus (Justine, Reference Justine2007), the lutjanid Symphorus nematophorus has six species of the trematode genus Retrovarium (Miller and Cribb, Reference Miller and Cribb2007a ), and the ray Himantura uarnacoides has five species of the cestode genus Acanthobothrium (Reyda and Caira, Reference Reyda and Caira2006). In none of these cases, or in many comparable systems, are the species especially cryptic; in each case the requirement for identification is only for careful work. Such cases thus present no particular barrier to effective stock discrimination studies.
Quite different from the many cases of recognizable multiple congeners are the handful of cases where genuinely cryptic species are reported as occurring sympatrically in or on the same host species. We exclude from this category accounts of larval stages (including the zoonotically important Anisakidae – Suzuki et al. Reference Suzuki, Murata, Hosaka and Araki2010; Klimpel and Palm, Reference Klimpel, Palm and Mehlhorn2011) which cannot, in our view, be reasonably expected to be readily distinguishable. Among trematodes we are aware of two reports of such species. Two species of Hurleytrematoides (Monorchiidae) are each reported to form a complex of two morphologically indistinguishable sympatric species infecting the same chaetodontid fishes on the GBR and at Ningaloo Reef (McNamara et al. Reference McNamara, Miller and Cribb2014). Similarly, Hunter and Cribb (Reference Hunter and Cribb2012) reported two distinct but morphologically indistinguishable haplotypes of Transversotrema borboleta, which they thought probably represented distinct species, on an overlapping range of sympatric fishes on the GBR. Among the cestodes we are aware only of the spathebothriidean Didymobothrium rudolphii which has been shown to comprise two cryptic species (not given separate names but shown, a posteriori, to differ slightly from each other morphologically) in the same fish species and with an overlapping geographical distribution off the coast of Portugal (Marques et al. Reference Marques, Santos, Gibson, Cabral and Olson2007). Among acanthocephalans, Echinorhynchus gadi has been shown to comprise a complex of multiple species in the North Sea (Vainola et al. Reference Vainola, Valtonen and Gibson1994) of which two may occur in the same individual fishes (Wayland et al. Reference Wayland, Gibson and Sommerville2005).
Cases such as these certainly have the capacity to undermine biological tagging studies as it is at least challenging to determine which (if either) species is being compared with samples from elsewhere. Fortunately, the literature so far suggests that co-occurring and genuinely cryptic species are not common. However, equally, the phenomenon has perhaps not yet been much sought. It may be commoner than we realize.
Thirdly, a basis of the genesis of cryptic species is their appearance in geographically distinct populations of the same host species. Such cases presumably imply that the parasites are speciating faster than their hosts or that there has been local host-switching. The effect, closely similar parasite species occurring in the same host species, is at the heart of the potential problems caused by cryptic species for stock assessment using parasite biological tags. Our reading of the literature and our own studies suggests that this phenomenon remains understudied but that it is frequently being found when the possibility is explored. We thus now review what is known of the issue for one major parasite taxon, the Trematoda of fishes. The basis of the review is the consideration of studies where molecular approaches have been used to test the identity of parasite species in individual hosts over their range. We pay equal attention to studies that have found no genetic variation as to those that do.
Trematodes
Trematodes have the richest known fauna of all the major metazoan taxa of fishes. Because they are often small and perhaps harder to find than some other parasite groups, trematodes are not typically considered the most useful group for tagging studies. In addition, their frequently short lifespans can mitigate against their use as effective biological tags (Lester and MacKenzie, Reference Lester and MacKenzie2009). However, there are now numerous studies that have explored the identity of trematodes of fishes over substantial portions of their range using a variety of molecular markers and approaches and in this respect they are probably better known than any other taxon.
Species stable over range
Several molecular studies have explored the identity of trematodes of fishes over their range without finding any evidence of cryptic species. The outstanding case is that of the fish blood fluke Cardicola forsteri which, on the basis of ITS2 and partial 28S rDNA sequences, has been shown to have an essentially global distribution in its tuna hosts (Aiken et al. Reference Aiken, Bott, Mladineo, Montero, Nowak and Hayward2007). In addition, Cardicola orientalis has been reported with identical ITS2 and 28S rDNA sequences from southern Australia and Japan (Shirakashi et al. Reference Shirakashi, Tsunemoto, Webber, Rough, Ellis and Ogawa2013). A substantial body of work based mainly on ITS2 rDNA sequences from the tropical Indo-West Pacific (TIWP) (Table 1) has demonstrated that 38 species from nine families remain identical (usually in the same host species) over ranges of at least 1000 km (between Heron and Lizard Islands on the GBR) and up to 9950 km (straight line distance between Moorea in French Polynesia and Ningaloo Reef off Western Australia). ITS2 rDNA sequences can be considered a reliable (though not infallible) marker for the distinction of species (Yao et al. Reference Yao, Song, Liu, Luo, Han, Li, Pang, Xu, Zhu, Xiao and Chen2010) so these studies establish that it is perfectly possible for a wide taxonomic range of trematodes to retain their specific identity over wide geographical ranges and thus, presumably, serve as effective subjects for stock assessment.
Table 1. Trematode species reported with identical ITS2 rDNA sequences over ranges >1000 km in the tropical Indo-West Pacific. Maximum separation in km calculated as straight line in Google Earth
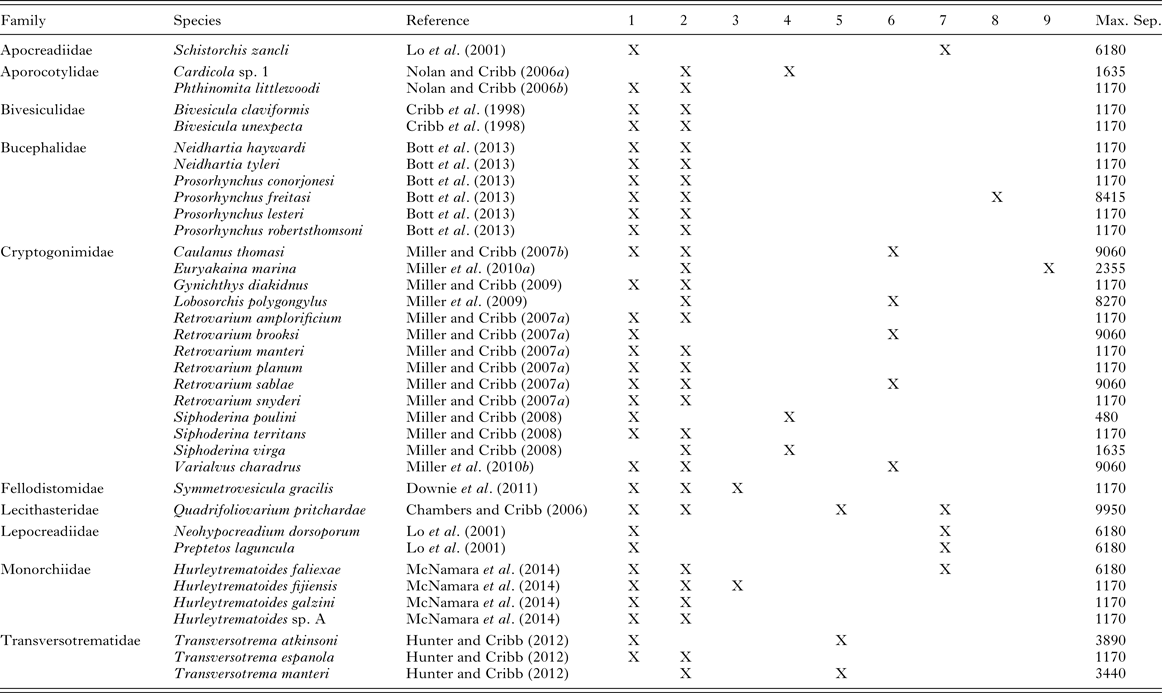
1. Heron Island, southern Great Barrier Reef; 2. Lizard Island, northern Great Barrier Reef; 3. Swain Reefs, central Great Barrier Reef; 4. Moreton Bay, southern Queensland, Australia; 5. Ningaloo Reef, Western Australia; 6. Maldives; 7. Moorea, French Polynesia; 8. Gambier Archipelago, French Polynesia; 9. New Caledonia.
Species unstable over range
In contrast to the studies showing species maintaining their identity over wide ranges of the TIWP, there are also, however, 11 species from four genera that show what has been interpreted as intraspecific geographical variation on the basis of ITS2 rDNA species (Table 2). The variation ranges from as low as a single base pair (e.g. Hurleytrematoides morandi, Transversotrema gigantica, Symmetrovesicula gracilis) to 22 base pairs (Table 2). These differences may reflect population distinctions which probably mean that they should not be considered a single unit in stock assessment. Two species, Hurleytrematoides faliexae and S. gracilis appear in both Tables 1 and 2, indicating that they remain identical between some combinations of localities but vary between some others. There are also cases where differences in ITS2 rDNA (and in some cases additional markers) from different localities are substantial and can best be interpreted as cryptic species pairs in separate parts of the distribution of the same fish species (Table 2). It is notable, and encouraging, from the analysis of ITS2 rDNA sequences from TIWP fishes that species that retain their genetic integrity over wide ranges significantly outnumber those that differ.
Table 2. Trematode species reported with variation in ITS2 rDNA sequences over ranges >1000 km in the tropical Indo-West Pacific. All species interpreted, at least provisionally, as individual species
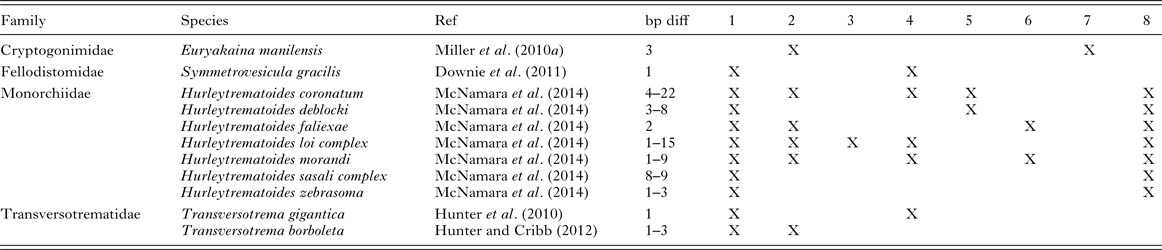
1. Heron Island, southern Great Barrier Reef; 2. Lizard Island, northern Great Barrier Reef; 3. Swains Reefs, central Great Barrier Reef; 4. Ningaloo Reef, Western Australia; 5. Maldives; 6. Moorea, FP; 7. New Caledonia; 8. Palau.
Several further examples require case-by-case understanding of the nature of the system and the available data to fully appreciate how they may impinge on the problem of cryptic species. Miller and Cribb (Reference Miller and Cribb2007b ) observed that two genotypes were associated with phenotypically similar specimens of Latuterus restricted to the GBR and the Maldives respectively. A posteriori analysis of the associated morphotypes revealed distinct morphological differences and these differences, in combination with the consistent genetic differences, led to the recognition of two distinct species in the system. As such, these species should not be considered cryptic but a warning that superficially similar species may be easily overlooked.
In the most comprehensive single molecular analysis of trematodes of marine fishes from a wide geographical range, McNamara et al. (Reference McNamara, Miller and Cribb2014) used ITS2 rDNA and mtCO1 sequences to explore representatives of 16 morphospecies of Hurleytrematoides (Monorchiidae) from chaetodontid fishes from one site in the Indian Ocean and five sites in the Pacific Ocean. The eight species for which CO1 sequences were obtained from multiple localities all varied between every combination of localities and for ITS2 sequences eight of ten species differed between at least some localities. Both markers gave a spectrum of variation, broadly consistent between the two, from what could only be interpreted as intra-specific variation through to what might easily be interpreted as inter-specific variation if a yardstick of the dissimilarity between distinct morphospecies was used. Re-examination of the specimens revealed no morphological basis for subdivision of the morphospecies so that the variation was certainly cryptic. No new species of Hurleytrematoides were named because the level of variation seen was continuous with what could be better interpreted as intraspecific variation. Species of Hurleytrematoides are thus poorly represented in Table 1 (genetically stable species) and over-represented in Table 2 (genetically unstable species). There is no particular reason evident for the variability of the species in this genus, demonstrating that there is as yet no reliable pattern to the appearance of cryptic species.
Freshwater systems might be expected to encourage a more rapid rate of speciation (and thus the appearance of cryptic species) than the sea because of the limited opportunity for exchange between river systems. From limited studies this appears to be the case. Criscione and Blouin (Reference Criscione and Blouin2007) used microsatellite markers and mtDNA sequence data to show that the opecoelid Plagioporus shawi had distinctions congruent with ‘evolutionarily significant units’ of its salmon hosts over a relatively small geographic range. Rosas-Valdez et al. (Reference Rosas-Valdez, Choudhury and Pérez-Ponce de León2011) provided strong evidence, based on analysis of 28S ribosomal RNA and mtCO1 genes, that the gorgoderid Phyllodistomum ictaluri is a complex of at least four species in North America, three of which infect allopatric populations of one catfish species. An even more complex system has been described by Razo-Mendivil et al. (Reference Razo-Mendivil, Vazquez-Dominguez, Rosas-Valdez, Pérez-Ponce de León and Nadler2010) for the apocreadiid genus Crassicutis in cichlid fishes of Mexico where analysis of ITS1 and mtCO1 sequences suggested the presence of seven cryptic species infecting an overlapping range of 12 cichlid species in multiple localities. Although biologically fascinating, these cases are of little concern to stock assessment studies as in general the distinctions reported above relate to different drainages which could be expected to be treated as separate stocks.
Overall, therefore, trematodes of fishes demonstrate a remarkable range of effects from apparent homogeneity across essentially cosmopolitan ranges through to complex population structures over small ranges. At present the balance of interpretations supports the idea that genetic identity is usually maintained across substantial ranges in the sea (probably much less so in fresh water). However, there have been insufficient studies to make this conclusion especially robust and certainly when more sensitive markers are employed more genetic structuring tends to be found.
Glass ¾ full
The partly cryptic complexity that is being revealed rapidly now for aquatic helminth parasites comes as no real surprise – the question is how to react to it. For taxonomists there is the issue that the potential fauna to be characterized is growing larger faster than we are describing it – we seem to be chasing the sun! In addition, we face renewed problems of deciding just what constitutes a species. None of this angst need concern biological taggers. Their problem is to be confident in their parasite identifications. There is no doubt that cryptic species are a real consideration in this process, but we are happy to at least be able to report that one of the major sources of cryptic species (those associated with separate host species), is of no special concern to them. Certainly cryptic parasite species in individual host species at individual sites is a potential problem (although so far a small one) and cryptic parasite species in individual host species at different localities is a significant one.
Overall, we judge that the glass is ¾ full or better, for two reasons. In the first place we can observe that a failure to recognize cryptic species will not of itself lead to spurious recognition of fish stocks; indeed it seems likely that at least some studies have successfully distinguished stocks while unwittingly failing to recognize all the species in the animal under study. Second, it is clear that parasites have more information to offer than we have realized previously because their taxonomy and distributions are more complex than we thought. Of course, tapping this information requires more work than a quick identification through a stereo microscope; samples will need to be sequenced which adds to the expense and time of such studies but with potentially rich rewards, although the introduction of new generations of sequencers should reduce these problems. Increasingly, however, molecular analysis of parasite samples at the infra-specific level is being incorporated into integrated stock assessment programmes (Criscione et al. Reference Criscione, Cooper and Blouin2006; Mattiucci et al. Reference Mattiucci, Farina, Campbell, MacKenzie, Ramos, Pinto, Abaunza and Nascetti2008; Baldwin et al. Reference Baldwin, Rew, Johansson, Banks and Jacobson2011) so that already the opportunities are being grasped. The future of these approaches thus seems bright.
ACKNOWLEDGEMENTS
Thanks are due to the many colleagues and students who have been involved in this research.
FINANCIAL SUPPORT
Part of the research discussed in this paper was funded by grants from the Australian Research Council or the Australian Biological Resources Study.





