Maltreatment includes physical, sexual, and emotional abuse and neglect (Leeb, Paulozzi, Melanson, Simon, & Arias, Reference Leeb, Paulozzi, Melanson, Simon and Arias2008). In 2015, 683,000 children in the United States were victims of maltreatment (US Department of Health & Human Services, 2017), and while international estimates of maltreatment are variable, the World Health Organization notes that one in four adults report being physically abused as children (World Health Organization, 2017). While there are many ways by which maltreatment may exert its influence on child development, one avenue is the capacity of maltreatment to “get under the skin” (Lupien, King, Meaney, & McEwen, Reference Lupien, King, Meaney and McEwen2001), becoming embedded at multiple levels of biological organization, from genetic expression to cortical structure and function (Hertzman, Reference Hertzman, Adler, Marmot, McEwen, Stewart, Adler, Marmot and Stewart1999).
There is abundant evidence that speaks to the pernicious nature of maltreatment's influence in both the short and long term. Children who have been maltreated exhibit poorer performance on tasks of essential cognitive abilities, including working memory (DePrince, Weinzierl, & Combs, Reference DePrince, Weinzierl and Combs2009), attention (Nolin & Ethier, Reference Nolin and Ethier2007; Porter, Lawson, & Bigler, Reference Porter, Lawson and Bigler2005), and inhibitory control (Pollak et al., Reference Pollak, Nelson, Schlaak, Roeber, Wewerka, Wiik and Gunnar2010), and also display lower levels of academic achievement (Carrey, Butter, Persinger, & Bialik, Reference Carrey, Butter, Persinger and Bialik1995; DeBellis, Hooper, Spratt, & Woolley, Reference DeBellis, Hooper, Spratt and Woolley2009; Perez & Widom, Reference Perez and Widom1994). Similar deficits in executive functions (Aas et al., Reference Aas, Steen, Agartz, Aminoff, Lorentzen, Sundet and Melle2012) and educational attainment (Jaffee et al., Reference Jaffee, Ambler, Merrick, Goldman-Mellor, Odgers, Fisher and Arseneault2018; Majer, Nater, Lin, Capuron, & Reeves, Reference Majer, Nater, Lin, Capuron and Reeves2010; Navalta, Polcari, Webster, Boghossian, & Teicher, Reference Navalta, Polcari, Webster, Boghossian and Teicher2006) have been observed among adults who experienced maltreatment as children. Nor are the effects of maltreatment limited to cognitive development. Children who experience maltreatment exhibit worse health outcomes than their peers, including higher rates of obesity and poorer cardiovascular health (Rogosch, Dackis, & Cicchetti, Reference Rogosch, Dackis and Cicchetti2011; Shalev et al., Reference Shalev, Moffitt, Sugden, Williams, Houts, Danaese and Caspi2012), and these problems are also observed among adults who were maltreated as children (Miller, Chen, & Parker, Reference Miller, Chen and Parker2011; Shalev et al., Reference Shalev, Moffitt, Sugden, Williams, Houts, Danaese and Caspi2012; Tyrka et al., Reference Tyrka, Price, Kao, Porton, Marsella and Carpenter2010). Mental health is also adversely impacted by maltreatment. Maltreatment is associated with higher rates of depression (Kessler, Davis, & Kendler, Reference Kessler, Davis and Kendler1997; Nanni, Uher, & Danese, Reference Nanni, Uher and Danese2012), bipolar disorder (Agnew-Blais & Danese, Reference Agnew-Blais and Danese2016), psychotic disorders (Varese et al., Reference Varese, Smeets, Drukker, Lieverse, Lataster, Viechtbauer and Bentall2012), posttraumatic stress disorder (PTSD) (Widom, Reference Widom1999), as well as increased likelihood of substance use (Klanecky, McChargue, & Bruggeman, Reference Klanecky, McChargue and Bruggeman2012; Madruga et al., Reference Madruga, Laranjeira, Caetano, Ribeiro, Zaleski, Pinsky and Ferri2011), self-injury (Lang & Sharma-Patel, Reference Lang and Sharma-Patel2011), and suicide in adulthood (Angelakis, Gillepsie, & Panagioti, Reference Angelakis, Gillepsie and Panagioti2018).
These pervasive, adverse effects of maltreatment have spurred research devoted to understanding the etiology of maltreatment's effects, including the neurophysiological embedding of maltreatment. Given maltreatment's nature as a toxic stressor (National Scientific Council on the Developing Child, 2005), researchers have placed particular emphasis on the activity of the systems that mediate the neurophysiological response to stress – the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenal (HPA) axis – and indeed dysregulation of these systems has been linked to many of the sequalae of maltreatment. For example, dysregulated ANS activity has been linked to poorer performance on measures of executive functions (Forte, Favieri, & Casagrande, Reference Forte, Favieri and Casagrande2019) and deficits in higher level cognitive functions among individuals with neurological impairments (Bassi & Bozzali, Reference Bassi and Bozzali2015); HPA axis function has also been associated with poorer performance on executive functions tasks in children (Blair et al., Reference Blair, Granger, Willoughby, Mills-Koonce, Cox and Greenberg2011; Wagner et al., Reference Wagner, Cepeda, Krieger, Maggi, D'Angiulli, Weinberg and Grunau2016) and lower levels of overall cognitive function in children (Suor, Sturge-Apple, Davies, Cicchetti, & Manning, Reference Suor, Sturge-Apple, Davies, Cicchetti and Manning2015) and adults (Lee et al., Reference Lee, Glass, McAtee, Wand, Bandeen-Roche, Bolla and Schwartz2007). Dysregulated ANS activity has been implicated in gastrointestinal illnesses (Kolacz & Porges, Reference Kolacz and Porges2018; Kolacz, Kovacic, & Porges, Reference Kolacz, Kovacic and Porges2019), while dysregulated HPA axis activity is associated with obesity in children (Rosmalen et al., Reference Rosmalen, Oldehinkel, Ormel, de Winter, Buitelaar and Verhulst2005; Veldhorst et al., Reference Veldhorst, Noppe, Jongejan, Kok, Mekic, Koper and van den Akker2014) and adults (Jackson, Kirschbaum, & Steptoe, Reference Jackson, Kirschbaum and Steptoe2017). Dysregulated ANS activity has also been linked to poorer mental health, including depression in adolescents and adults (Kemp et al., Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt2010; Koenig, Kemp, Beauchaine, Thayer, & Kaess, Reference Koenig, Kemp, Beauchaine, Thayer and Kaess2016), as well as anxiety and PTSDs (Chalmers, Quintana, Abbott, & Kemp, Reference Chalmers, Quintana, Abbott and Kemp2014). Similarly, dysregulation of the HPA axis activity is associated with internalizing behaviors in children (Laurent, Gilliam, Wright, & Fisher, Reference Laurent, Gilliam, Wright and Fisher2015) and depression in adults (Knorr, Vinberg, Kessing, & Wetterslev, Reference Knorr, Vinberg, Kessing and Wetterslev2010; Vreeburg et al., Reference Vreeburg, Hoogendijk, van Pelt, DeRijk, Verhagen, van Dyck and Penninx2009), as well as a host of mental illnesses that typically manifest in adulthood: bipolar (Watson, Gallagher, Ritchie, Ferrier, & Young, Reference Watson, Gallagher, Ritchie, Ferrier and Young2004), psychotic (Karanikas, Antoniadis, & Garyfallos, Reference Karanikas, Antoniadis and Garyfallos2014) and PTSDs (Goenjian et al., Reference Goenjian, Najarian, Pynoos, Steinberg, Manoukian, Tavosian and Fairbanks1994), substance use (Sinha, Reference Sinha2008), nonsuicidal self-injurious behaviors (Larkin, Di Blasi, & Arensman, Reference Larkin, Di Blasi and Arensman2014), and suicide (O'Connor, Ferguson, Green, O'Carroll, & O'Connor, Reference O'Connor, Ferguson, Green, O'Carroll and O'Connor2016).
The association between maltreatment and dysregulated neurophysiological activity, on one hand, and dysregulated neurophysiological activity and poorer cognitive function and reduced physical and mental health, on the other, outlines a model linking maltreatment to these outcomes via the dysregulation of the ANS and HPA axis. The purpose of this paper is to assess the evidence regarding the first association in this model by systematically reviewing studies that have examined the relation between maltreatment and the activity of these systems in childhood, in an effort to address two questions: (a) Does the literature support a particular account of ANS or HPA axis function under conditions of homeostasis and stress? That is, are these systems characteristically hyper- or hypoactive among children who have experienced maltreatment? And (b) to the extent that they are, can findings that are at odds with this account be explained either by methodological differences across studies or “third variables” such as gender, psychopathology, and genetics?
Maltreatment and the ANS
The ANS: Structure, function, and role in the stress response
The ANS lies outside (or is peripheral to) the central nervous system, and is divided into two branches: the parasympathetic nervous system (PNS) and the sympathetic nervous system (SNS). Through efferent fibers originating in the brain and spinal cord, both branches of the ANS innervate tissues and organs throughout the body, collectively known as visceral effectors. Parasympathetic input to these effectors promotes anabolic activities such as extracting, conserving, and storing energy or allowing organs to rest and repair. Thus, parasympathetic input causes the bronchial muscles to constrict (limiting oxygen consumption), decreases the rate and strength of the heart's contraction (limiting blood flow), increases motility, tone, and gastric secretions of the stomach and intestines (encouraging digestion), and promotes glycogenesis in the liver (the storage of energy as glucose). However, under conditions of stress the activity of the PNS is reduced (or withdrawn), and the activity of the SNS increased. The net function of sympathetic activation is to free and direct metabolic resources in order to support active defense behaviors. Sympathetic input dilates the bronchial muscles, allowing for increased oxygen intake, increases heart rate while dilating the vessels supplying blood to the skeletal muscles, inhibits digestion by reducing the motility, tone, and gastric secretions of the stomach and intestines, and promotes the liver's conversion of glycogen into glucose.
Altered ANS function following maltreatment
For most children, homeostatic ANS function will feature relatively high levels of PNS activity and relatively low levels of SNS activity; under situations of challenge, PNS activity will decrease and SNS activity will increase. But maltreatment may be expected to prompt alterations in ANS function under conditions of both homeostasis and stress. Under homeostatic conditions the most likely alteration of ANS activity for a maltreated child would be an increase in SNS activity, relative to a child not exposed to maltreatment, facilitating increased levels of vigilance to threat. Increases in SNS activity might be accompanied by decreases in PNS activity, in part due to the inhibitory inter-connections between the SNS and PNS, and in part because the context of maltreatment may offer reduced opportunities for states of calm engagement that are supported by relatively high levels of PNS activity. This configuration of ANS activity would be adaptive for a child who had been maltreated, at least in the short-term. However, in the long-term these elevated levels of SNS activity would strain children's cardiovascular function, a type of physiological burden that is often referred to as allostatic load (McEwen, Reference McEwen2005).
Under stressful conditions, two patterns of altered ANS activity are equally plausible. On one hand, exposure to a toxic stressor may recalibrate the autonomic stress response to a state of hyperreactivity, prompting large decreases in PNS activity and large increases in SNS activity in response to stress. While frequent recruitment of autonomic resources may impose a heavy allostatic load in the long term, in the immediate context these resources would support defensive behaviors necessary to ameliorate or escape an experience that may, in extreme cases, be life threatening. On the other hand, the experience of maltreatment may, over time, lead to down-regulation or hyporeactivity of the autonomic stress response. Such a response would feature smaller decreases in PNS activity and diminished increases in SNS activity. This could be indicative of a stress response that has become habituated to extreme adversity, or it could reflect the child's perception that a challenge posed by maltreatment is insurmountable. The latter possibility would recommend tonic immobility (playing dead), a last line of behavioral defense that is supported by an extreme reduction in SNS activity and that may, in part, be manifest in dissociative disorders associated with maltreatment (Putnam, Reference Putnam1993). The adaptivity of this hyporeactive recalibration would be inverse to that of a hyperreactive recalibration with respect to time: while hyporeactivity might avoid the long-term allostatic load imposed by frequent and large activations of the autonomic stress response, they also raise the possibility that in the short-term a child might not have the metabolic resources to support defensive or flight behaviors when they are absolutely needed.
Maltreatment and the HPA axis
The HPA axis: Structure, function, and role in the stress response
The HPA axis comprises three structures: the hypothalamus, the pituitary, and the adrenal glands. By secreting corticotropin-releasing factor, neurons of the paraventricular nucleus of the hypothalamus signal the pituitary to secrete adrenocorticotropic hormone, which in turn prompts the synthesis and release of glucocorticoids – most notably, cortisol – into the blood stream. Cortisol and other glucocorticoids bind to glucocorticoid receptors throughout the body, increasing the levels of glucose in the blood (Munck & Náray-Fejes-Tóth, Reference Munck, Náray-Fejes-Tóth and DeGroot1995) and suppressing immune function (Wiegers & Reul, Reference Wiegers and Reul1998). Understanding the physiological function of the HPA axis does not a priori suggest what role it would play in the stress response. The “classical” account, first formulated by Hans Seyle (Reference Seyle1950), focuses on the metabolism-enhancing functions of the HPA axis, a view that has become so well entrenched in the psychophysiological literature that activation of the HPA axis has become nearly synonymous with stress (Gunnar, Reference Gunnar1992). However, the prolonged gap between the onset of a stressor and an HPA axis response led Munck, Guyre, and Hollbrook (Reference Munck, Guyre and Hollbrook1984) to propose a “revisionist” account, wherein the role of the HPA axis is to suppress previously activated neurophysiological defense mechanisms, including those associated with the SNS. Under certain conditions, both arguments may be correct. For brief stressors with a sudden onset, activation of the HPA axis is of limited utility, as the stressor will pass before the effects of activation are realized. But, as Sapolsky, Romero, and Munck (Reference Sapolsky, Romero and Munck2000) point out, in social species such as our own, stressors are predictable, and thus activation of the HPA axis in anticipation of stressor onset can serve as a preparation for subsequent activation.
Altered HPA axis function following maltreatment
Given its role in the stress response, it is not surprising that research has assessed the association between maltreatment and alterations of HPA axis activity. Like research on alterations in ANS activity, studies of the HPA axis have examined altered function under conditions of both homeostasis and stress. However, unlike studies of ANS activity, investigations of HPA axis activity have had to accommodate the more complex profile of homeostatic HPA axis function, which features circadian or diurnal rhythms. The terms “basal” and “baseline” activity are alternatively used to refer to this homeostatic function. Elevated levels of morning HPA axis activity may be considered hyperactive, as may elevated levels of HPA axis activity prior to the onset of a stressor, regardless of when that activity is measured. In all but the youngest children, diurnal levels of HPA axis activity follow a predictable pattern, in which levels of activity peak shortly after waking and then gradually decrease over the course of the day. Therefore, attenuation of this decrease – a flatter diurnal trajectory – may also be classified as hyperactivity. However, lower levels of morning activity, when combined with flattened diurnal trajectories, can result in higher cumulative levels of HPA axis activity over the course of the day, and thus this pattern of HPA axis activity may too be indicative of hyperactivity (Miller, Chen, & Zhou, Reference Miller, Chen and Zhou2007).
The experience of maltreatment is most likely to engender hyperactivity across different components of homeostatic HPA axis activity. As noted above, one of the functions of the HPA axis may be to mobilize metabolic resources in anticipation of stressors that are predictable (Sapolsky et al., Reference Sapolsky, Romero and Munck2000). Maltreated children – particularly those who suffer frequent or repeated maltreatment – inhabit an environment in which highly stressful events are disproportionately likely to occur. As in the case of the ANS, elevated homeostatic activity of the HPA axis may be adaptive, in that it provides metabolic resources to support vigilance and active defense behaviors. However – and also as in the case of the ANS – in the long-term elevated levels of HPA axis activity may impose a heavy allostatic load, inhibiting immune and cognitive function.
Maltreatment may also have the capacity to alter HPA axis reactivity to stress. One possibility is that maltreatment results in hyperreactivity of the HPA axis, or hypercortisolism. According to the glucocorticoid cascade hypothesis (Sapolsky, Krey, & McEwen, Reference Sapolsky, Krey and McEwen1986), more frequent and pronounced increases in HPA axis activity and concomitant elevations in cortisol damage hippocampal receptors, interfering with the negative feedback loop that signals the paraventricular nucleus of the hypothalamus to cease secretion of corticotropin releasing factor. Hyperreactivity of the HPA axis may be adaptive for the maltreated child in the short term, providing resources to mitigate situations of extreme stress, but in the long term may impose an allostatic load. A second possibility is that maltreatment leads to hyporeactivity of the HPA axis, or hypocortisolism. According to this account, repeated exposure to the severe stress of maltreatment results in habituation of the HPA axis, which manifests as a blunted response to stress. The adaptive value of this alteration would be protection from the adverse effects of repeated or large spikes in HPA axis activity, but to the extent that this prevented a child from recruiting the full range of metabolic resources in the face of a truly existential threat, this alteration would be maladaptive.
Current study
Although these accounts of the potential associations between maltreatment and the altered function of the ANS and HPA axis are plausible, as of yet they have not been systematically evaluated in light of the literature. In the remainder of this paper we present the method and results for a systematic review of the associations between maltreatment and ANS and HPA axis activity in childhood. The results of our review are divided into two sections: the first reviews the literature on maltreatment and ANS function; while the second examines maltreatment and HPA axis activity. The separation of two systems does not accurately reflect reality – wherein the activity of these systems is closely coordinated – but it does reflect the literature, in which the activity of one system is measured typically in isolation.
Method
Search strategy
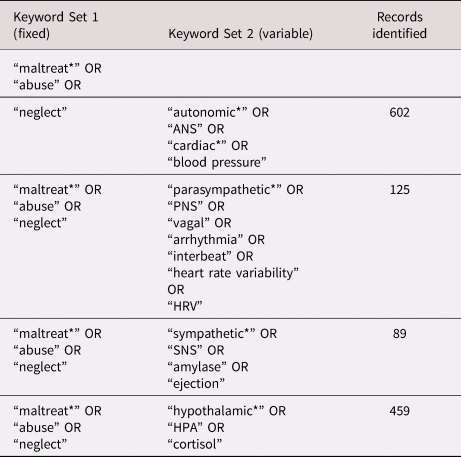
A protocol for reviewing the literature was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Shamseer et al., Reference Shamseer, Moher, Clarke, Ghersi, Liberati, Petticre and Stewart2015). PsycInfo and Medline were searched concurrently using four different combinations of keywords. The first combination comprised [“maltreat*” OR “abuse” OR “neglect” AND “autonomic*” OR “ANS” OR “cardiac” OR “blood pressure”]. The second combination retained [“maltreat*” OR “abuse” OR “neglect”] but replaced the second set of keywords with [“autonomic,” etc.] with a new set designed to yield studies that focused on the PNS. The third and fourth combinations retained the first set of keywords but replaced the second set with new keywords focused on the SNS and HPA axis, respectively. No date limitations were placed on the search results. The final search was completed on September 15, 2019. See Table 1 for a summary of keywords used in each iteration of the search.
Table 1. Summary of studies included in the review
Study selection
Together, the four iterations of the search yielded 1,275 papers; removing duplicate records reduced this number to 1,110 (for a diagram of the number of papers included at each stage of the review, see Figure 1). The records for these studies were compiled in a spreadsheet, and the titles and abstracts of each study were reviewed by the first author. Only those studies that met the following inclusion criteria were retained: (a) the paper had to report the results of an empirical study that examined the association between maltreatment (or the allegation of maltreatment) and some aspect(s) of ANS (including PNS and/or SNS) and/or HPA axis activity; (b) maltreatment could include any form of abuse or neglect perpetrated or alleged to have been perpetrated by a parent or primary caregiver, but excluded abuse perpetrated by others (e.g., a significant other), peer victimization, or exposure to violence, including inter-partner violence; (c) maltreatment must have occurred (or have been alleged to have occurred) during childhood (between ages 0 and 18 years); (d) the activity of neurophysiological system(s) must also have been measured during childhood; and (e) if maltreatment was included as part of an overall index of adversity the association between maltreatment (or its allegation) and neurophysiological activity must have been assessed independent of other aspects of adversity. This final criterion resulted in the exclusion of studies that examined the association between posttraumatic stress and neurophysiological activity except in those cases in which the trauma was due solely to maltreatment and/or the association between maltreatment and neurophysiological activity was examined independently of other factors that led to the posttraumatic stress.
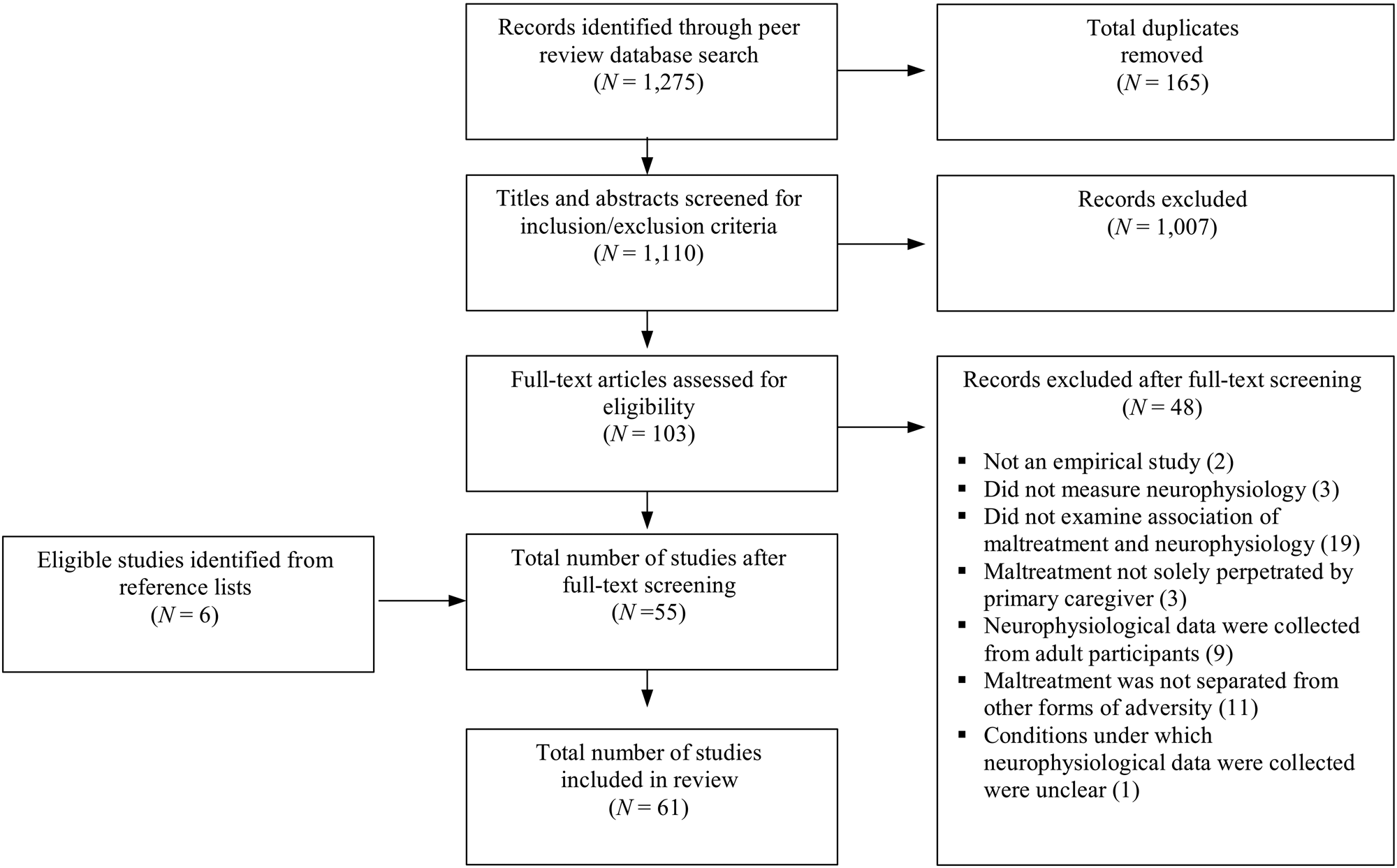
Figure 1. A depiction of the number of studies included at each stage of the literature review.
A series of report characteristics were also applied as inclusion criteria. To be included in the review, we required that a study was: (a) empirical (which excluded letters, commentary, and case studies); (b) published in English; and (c) published in a peer-reviewed journal. The decision to include only papers published in English was based on prior research that suggests doing so does not bias review results (Morrison et al., Reference Morrison, Polisena, Husereau, Moulton and Clark2012), while the decision to include only peer-reviewed studies provided some assurance as to the quality of studies without requiring the use of potentially problematic assessment tools (Siddaway, Wood, & Hedges, Reference Siddaway, Wood and Hedges2019).
The application of these criteria yielded 103 papers. These papers were subject to full-text review by the first author. Approximately 40% of these papers were selected at random for an independent review by the second or third author (who each reviewed approximately half of these papers). After applying the criteria to the full texts, 55 papers remained. One paper (Koopman et al., Reference Koopman, Carrion, Butler, Sudhakar, Palmer and Steiner2004) was excluded not on the basis of the criteria, but rather because it was not clear whether neurophysiological activity was collected under conditions of homeostasis or challenge; six others were excluded because they extended the results of other papers that reported an association between maltreatment and neurophysiological activity. In each case, the two reviewers agreed about which papers should be included. Finally, the reference lists of the remaining papers were examined and the inclusion criteria applied by the first author. This yielded an additional six studies for inclusion in the review, for a total of 61 studies.
Data extraction and analysis
For each of these studies, the following information was extracted by the second author and entered into a form that included the following fields: (a) the type(s) of maltreatment; (b) the age at which maltreatment occurred; (c) the source(s) of information about maltreatment, where reported; (d) the age(s) at which neurophysiological data were collected, the neurophysiological system(s) assessed and the method for doing so; (e) the size and composition of the sample; and, where applicable, (f) the nature of the stressor(s) used to elicit a neurophysiological response. This form had been developed by the first author, who trained the second and third authors in its use. As data were entered into the form, the first author checked the entries and discussed them as needed with the second and third authors to ensure the reliability of data extraction. Data were then converted into a format for presentation in Table 2, using the fields from the form as the column headings for the table.
Table 2. Summary of studies included in the review

a Specific types of abuse and/or neglect are listed for studies where that information was provided.
b Approximate age(s) at which maltreatment occurred.
c When provided by the authors, specific measures used for self-report are listed. When the authors specified the approach taken to classify children as maltreated using public records, that approach is listed.
d Hypothalamic–pituitary–adrenal (HPA) axis, autonomic nervous system (ANS), parasympathetic nervous system (PNS), sympathetic nervous system (SNS).
e When approximate times of collected were reported by the authors, those are included in the table. When mean times of collection are reported (regardless of whether these included indices of dispersion such as standard deviations) the mean time of collection is reported to the nearest hour.
As is evident from the information presented in the table, the studies included in the review were heterogeneous with respect to nearly all aspects of study design, from the type(s) of maltreatment to sample size and composition to the neurophysiological system(s) from which data were collected and under what conditions. The extent of the heterogeneity precluded the possibility of conducting a meta-analysis. We therefore provide a systematic narrative synthesis of the literature that characterizes different associations between maltreatment and neurophysiological function in the context of methodological variations across studies.
Results
Altered ANS function following maltreatment
Nine studies have examined autonomic function under homeostatic conditions among maltreated children, with five of these reporting evidence of hyperactivity. Gooding and colleagues reported that maltreated adolescents (13–17 years) exhibited significantly higher resting diastolic blood pressure (a global measure of ANS activity) relative to nonmaltreated children (Gooding, Milliren, Austin, Sheridan, & McLaughlin, Reference Gooding, Milliren, Austin, Sheridan and McLaughlin2016). Using more specific measures, Lunkenheimer and colleagues found that children (3–5 years) who had been physically abused exhibited lower levels of respiratory sinus arrhythmia (a measure of PNS activity; Lunkenheimer, Busuito, Brown, & Skowron, Reference Lunkenheimer, Busuito, Brown and Skowron2018); Skowron et al. (Reference Skowron, Loken, Gatzke-Kopp, Cipriano-Essel, Woehrle, Van Epps and Ammerman2011) reported a similar finding for children (3–5 years) who had been physically abused or neglected (Skowron et al., Reference Skowron, Loken, Gatzke-Kopp, Cipriano-Essel, Woehrle, Van Epps and Ammerman2011). McLaughlin et al. (Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015) found that Romanian children (13 years) who had been institutionalized exhibited shorter pre-ejection periods (an indication of higher SNS activity) at baseline compared to both noninstitutionalized children and children who had been moved from institutional to foster care. Among older females (12–16 years), one study found that a history of maltreatment was associated with lower levels of respiratory sinus arrhythmia, an index of PNS activity (Miskovic, Schmidt, Georgiades, Boyle, & MacMillan, Reference Miskovic, Schmidt, Georgiades, Boyle and MacMillan2009), although another study conducted with adolescent females (14–19 years) reported a trend towards higher levels of respiratory sinus arrhythmia among those who had been maltreated in the past year (Shenk, Putnam, & Noll, Reference Shenk, Putnam and Noll2012).
While this is the only study of which we are aware to have yielded contrary findings, Gordis and colleagues did not find significant differences in respiratory sinus arrhythmia or skin conductance levels (a measure of SNS activity) between children and adolescents (9–16 years) who were maltreated and their peers (Gordis, Feres, Olezeski, Rabkin, & Trickett, Reference Gordis, Feres, Olezeski, Rabkin and Trickett2010), just as Ford and colleagues found no differences in resting heart rate between abused children (6–19 years) and their peers (Ford, Fraleigh, Albert, & Connor, Reference Ford, Fraleigh, Albert and Connor2010). Similar findings were reported by Keeshin and colleagues, in that maltreatment was not associated with levels of saliva alpha amylase (an index of ANS activity) among adolescents (12–17 years), although there was an association between saliva alpha amylase and the severity of PTSD symptoms, which may correspond to the severity of maltreatment (Keeshin, Strawn, Out, Granger, & Putnam, Reference Keeshin, Strawn, Out, Granger and Putnam2015).
Of the 12 studies that examined the function of the ANS among maltreated children in response to stress, seven reported evidence of hyporeactivity. Using the Trier Social Stress Task (TSST; Kirschbaum, Pirke, & Hellhammer, Reference Kirschbaum, Pirke and Hellhammer1993), McLaughlin and colleagues found that a history of maltreatment was associated with reduced ANS reactivity, indexed by cardiac output, a global measure of overall ANS function, among adolescents (13–17 years; McLaughlin, Sheridan, Alves, & Mendes, Reference McLaughlin, Sheridan, Alves and Mendes2014a). Studies employing other global measures (heart rate and blood pressure) have yielded similar results. Leitzke and colleagues found that children and adolescents (9–14 years) subjected to physical abuse did not exhibit an increase in systolic blood pressure in response to the TSST, whereas children who had not been maltreated did (Leitzke, Hilt, & Pollak, Reference Leitzke, Hilt and Pollak2015). Analogous patterns of hyporeactivity in response to the TSST have been observed among maltreated adolescents (Gooding et al., Reference Gooding, Milliren, Austin, Sheridan and McLaughlin2016; McLaughlin et al., Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015) when diastolic blood pressure was used as a measure of ANS activity. Using more specific measures, McLaughlin and colleagues also found that maltreated adolescents exhibited blunted pre-ejection period response to the TSST relative to controls (McLaughlin et al., Reference McLaughlin, Sheridan, Alves and Mendes2014a, Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015).
Among studies using stressors other than the TSST, two have yielded evidence that also supports the hyporeactivity account. Ford and colleagues reported that physically abused children and adolescents (6–19 years) were more likely to exhibit decreases in the global ANS measures of heart rate and systolic blood pressure in response to a blood draw procedure than their peers (Ford et al., Reference Ford, Fraleigh, Albert and Connor2010), though it is important to note these changes may have been indicative of syncope (fainting), which can accompany a blood draw in some individuals. Similarly, Pollak and colleagues reported that maltreated children (4–5 years) exhibited reduced SNS reactivity and heart rate deceleration in response to angry background conversations (Pollak, Vardi, Bechner, & Curtin, Reference Pollak, Vardi, Bechner and Curtin2005). However, two studies employing stressors other than the TSST support an account of hyperreactive ANS function. Shenk and colleagues found that female adolescents (13–23 years) who had been sexually abused were more likely than controls to display reductions of respiratory sinus arrhythmia in response to a timed cognitive task (Shenk, Noll, Putnam, & Trickett, Reference Shenk, Noll, Putnam and Trickett2010), a finding similar to that reported by Rinnewitz et al. (Reference Rinnewitz, Koenig, Parzer, Brunner, Resch and Kaess2018), who observed a positive association between the severity of maltreatment and the magnitude of the reduction in heart rate variability in response to a cold pressor task among a sample of maltreated adolescent females (12–17 years) exhibiting nonsuicidal self-injurious behaviors (Rinnewitz et al., Reference Rinnewitz, Koenig, Parzer, Brunner, Resch and Kaess2018). Oosterman and colleagues reported mixed findings, reporting that while neglected children (26–88 months) exhibited larger increases in the pre-ejection period in response to the Strange Situation (Ainsworth, Blehar, Waters, & Wall, Reference Ainsworth, Blehar, Waters and Wall1978) compared to children who had not been neglected, they also exhibited smaller decreases in respiratory sinus arrhythmia (Oosterman, De Schipper, Fisher, Dozier, & Schuengel, Reference Oosterman, De Schipper, Fisher, Dozier and Schuengel2010). Two other studies found no association between maltreatment and the response to different challenges (conflict videos in the case of Gordis et al., Reference Gordis, Feres, Olezeski, Rabkin and Trickett2010 and a teaching task in the case of Skowron et al., Reference Skowron, Loken, Gatzke-Kopp, Cipriano-Essel, Woehrle, Van Epps and Ammerman2011). Figure 2a summarizes the literature on altered ANS function under conditions of maltreatment.

Figure 2a. A graphical summary of the literature examining the association between maltreatment and autonomic nervous system function.

Figure 2b. A graphical summary of the literature examining the association between maltreatment and HPA axis function under conditions of homeostasis.
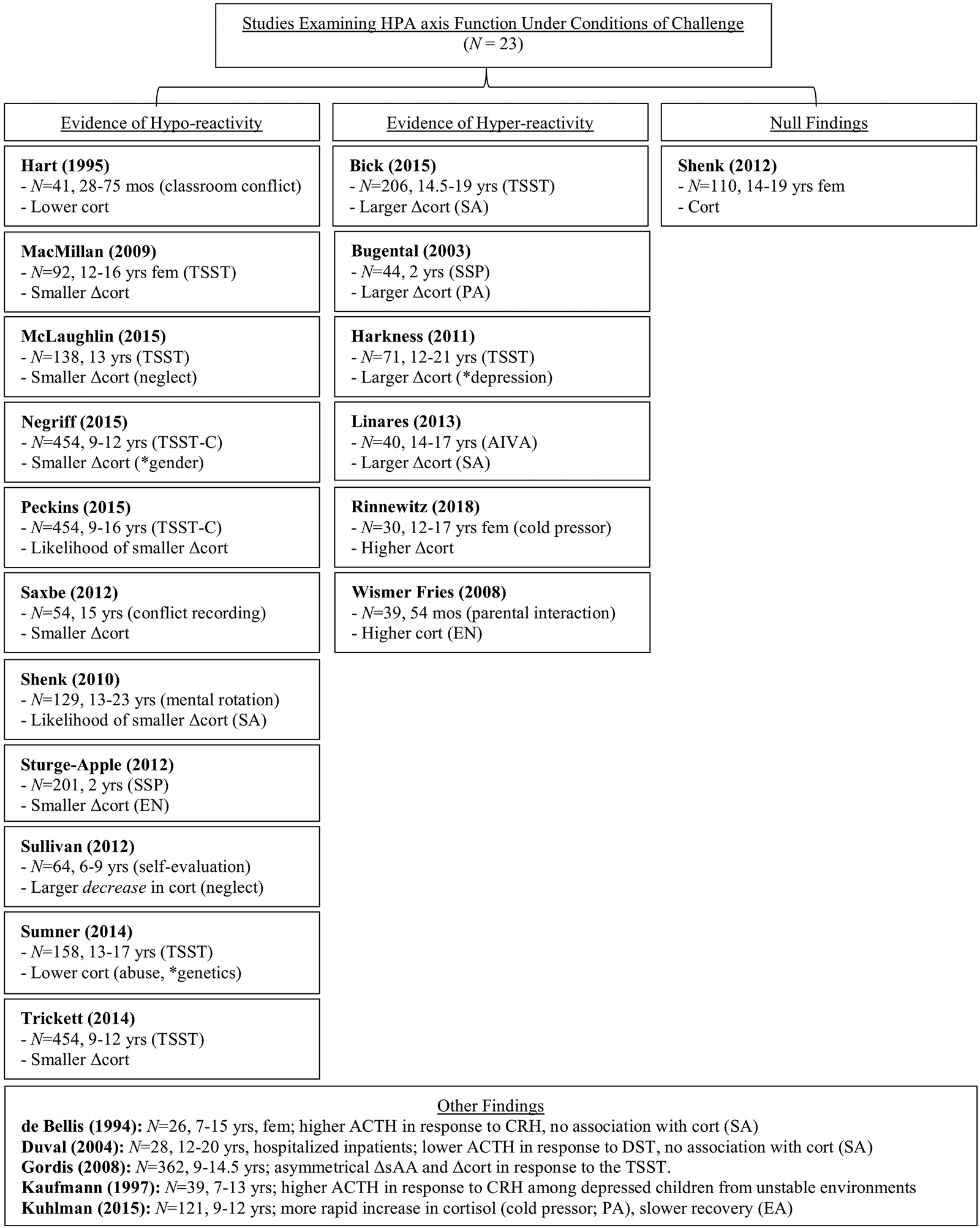
Figure 2c. A graphical summary of the literature examining the association between maltreatment and HPA axis function under conditions of challenge.
Altered HPA axis function following maltreatment
Altered HPA axis function under conditions of homeostasis
Thirty-seven studies have examined HPA axis function under conditions of homeostasis. Thirteen of these examined HPA axis activity prior to the onset of stressor (i.e., baseline function), of which six reported an association between maltreatment and HPA axis hyperactivity. In two of these studies, frequent maternal emotional withdrawal (Bugental, Martorell, & Barraza, Reference Bugental, Martorell and Barraza2003) and maternal emotional neglect (Sturge-Apple,, Davies, Cicchetti, & Manning, Reference Sturge-Apple, Davies, Cicchetti and Manning2012) were associated with elevated levels of salivary cortisol prior to participation in the Strange Situation Procedure (Ainsworth et al., Reference Ainsworth, Blehar, Waters and Wall1978) among young children (aged 2 years). Studies of older children have yielded similar results: Bick and colleagues reported that adolescents (14.5–19 years) who experienced moderate to severe physical neglect exhibited significantly higher levels of cortisol prior to participating in the Trier Social Stress Task for Children (TSST-C; Buske-Kirschbaum et al., Reference Buske-Kirschbaum, Jobst, Wustmans, Kirschbaum, Rauh and Hellhammer1997) compared to adolescents with no or minimal levels of physical neglect (Bick et al., Reference Bick, Nguyen, Leng, Piecychna, Crowley, Bucala and Grigorenko2015), while school-aged children (6–9 years) who had been physically neglected exhibited higher cortisol levels prior to participating in a self-evaluation task than children who had not been neglected (Sullivan, Bennett, & Lewis, Reference Sullivan, Bennett and Lewis2013). In a study of children and adolescents (ages 8–17 years), Şimşek and colleagues found that those who had been sexually abused exhibited higher levels of resting cortisol than children and adolescents who had not been abused (Şimşek, Yüksel, Kaplan, Uysal, & Alaca, Reference Şimşek, Yüksel, Kaplan, Uysal and Alaca2015). Harkness and colleagues examined psychopathology together with maltreatment status, and found that depressed adolescents who had been maltreated exhibited higher cortisol levels prior to the TSST than comparably depressed adolescents who had not been maltreated (Harkness, Stewart, & Wynne-Edwards, Reference Harkness, Stewart and Wynne-Edwards2011). Although six studies that examined baseline cortisol found no evidence of differences as a function of maltreatment (DeBellis et al., Reference DeBellis, Chrousos, Dorn, Burke, Helmers, Kling and Putnam1994; MacMillan et al., Reference MacMillan, Georgiades, Duku, Shea, Steiner and Schmidt2009; McLaughlin et al., Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015; Shenk et al., Reference Shenk, Putnam and Noll2012; Sumner, McLaughlin, Walsh, Sheridan, & Koenen, Reference Sumner, McLaughlin, Walsh, Sheridan and Koenen2014; Usta, Tuncel, Akbas, Aydin, & Say, Reference Usta, Tuncel, Akbas, Aydin and Say2016), two of these did report differences in biomarkers related to cortisol. De Bellis and colleagues reported lower levels of adrenocorticotropic hormone among sexually abused children and adolescents (7–15 years), while Utsa and colleagues found higher cortisol-to-dehydroepiandrosterone sulphate ratios among sexually abused adolescents with symptoms of posttraumatic stress (Usta et al., Reference Usta, Tuncel, Akbas, Aydin and Say2016).
One study that examined baseline HPA axis activity did so by assessing cortisol levels at the same time of day over the course of 20 weeks, and focused on the degree of variability in cortisol levels over time. The authors found that there was a greater degree of between-person variability in cortisol among children and adolescents (5–13 years) who had been maltreated than among their comparison-group peers. Moreover, among children who had been maltreated, the experience of fewer types of maltreatment, less severe maltreatment, and less recent maltreatment were all associated with greater within-person variability in cortisol levels over time (Doom, Cicchetti, & Rogosch, Reference Doom, Cicchetti and Rogosch2014).
Ten studies that have examined the association between maltreatment and HPA axis function have focused on diurnal activity. Eight of these reported an association between maltreatment and diurnal hyperactivity. For example, Bernard and colleagues found that infants and toddlers (3–31 months) who had been maltreated exhibited lower waking cortisol levels and flattened slopes of cortisol throughout the day, which, as discussed above, is indicative of hyperactivity (Bernard, Butzin-Dozier, Rittenhouse, & Dozier, Reference Bernard, Butzin-Dozier, Rittenhouse and Dozier2010). Bernard also found that maltreated children living with their birth parents exhibited flatter slopes than those living with foster parents. These findings are similar to those reported by Gunnar and colleagues: children (6–12 years) who had been Romanian orphans and who had been adopted after 8 months of age exhibited higher diurnal cortisol levels than orphans who had been adopted before 4 months of age. Moreover, among the Romanian orphans, those who remained in institutionalized care for longer periods of time exhibited higher diurnal cortisol levels (Gunnar, Morison, Chisholm, & Schuder, Reference Gunnar, Morison, Chisholm and Schuder2001). Puetz and colleagues observed similar results, reporting that school-aged children (8–14 years) who had been maltreated exhibited lower waking cortisol levels and flattened diurnal cortisol slopes, relative to their comparison-group peers (Puetz et al., Reference Puetz, Zweerings, Dahmen, Ruf, Scharke, Herpertz-Dahlmann and Konrad2016). Among younger children (3–5 years), de Punder and colleagues found that the total amount of cortisol produced over the course of the day was positively correlated with the number and cumulative severity of instances of abuse children had experienced (de Punder et al., Reference de Punder, Overfeld, Dörr, Dittrich, Winter, Kubiak and Heim2016). Similar findings were reported by Doom and colleagues, though the authors also found that among maltreated females (8–11 years), more recent or less pervasive maltreatment was associated with higher cortisol levels (Doom, Cicchetti, Rogosch, & Dackis, Reference Doom, Cicchetti, Rogosch and Dackis2013). Finally, as was the case in studies examining cortisol levels prior to the onset of a stressor, psychopathology may reinforce the association between maltreatment and diurnal HPA axis activity. In a series of studies with a sample of school-aged children (6–13 years), Cicchetti and colleagues found that those who had been maltreated and who displayed comorbid internalizing (Cicchetti, Rogosch, Gunnar, & Toth, Reference Cicchetti, Rogosch, Gunnar and Toth2010; Cicchetti, Rogosch, & Oshri, Reference Cicchetti, Rogosch and Oshri2011) or externalizing symptoms (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2001a) exhibited flattened diurnal cortisol patterns relative to controls. Moreover, among school-aged children (6–11 years) who had been maltreated, those with comorbid depression were more likely than those without depression to display an increase in cortisol levels over the course of the day, rather than the typical decrease (Hart, Gunnar, & Cicchetti, Reference Hart, Gunnar and Cicchetti1996). Two of the ten studies that examined the association between maltreatment and diurnal activity found evidence of hypoactivity. Kertes and colleagues found that maltreatment, operationalized as cumulative deprivation prior to placement in foster care, was associated with higher levels of HPA axis activity after children (7–11 years) had been living with their foster families for at least three years (Kertes, Gunnar, Madsen, & Long, Reference Kertes, Gunnar, Madsen and Long2008). Kočovská reported that children (9–12 years) who had been maltreated prior to adoption and who exhibited reactive attachment disorder were compared to children who had not been maltreated, adopted, or diagnosed with an attachment disorder (Kočovská et al., Reference Kočovská, Wilson, Young, Wallace, Gorski, Follan and Minnis2013).
An additional 14 studies have focused on waking or morning levels of cortisol as an aspect of homeostatic HPA axis activity. Five of these reported an association between maltreatment and hyperactivity. Young children (54 months) who had been severely emotionally neglected while in institutional care exhibited higher morning levels of cortisol relative to their peers who had not been maltreated (Wismer Fries, Shirtcliff, & Pollak, Reference Wismer Fries, Shirtcliff and Pollak2008). Among school-aged children (6.5–11 years), exposure to maltreatment (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2001b) and neglect prior to adoption (Kertes et al., Reference Kertes, Gunnar, Madsen and Long2008) were associated with elevated waking cortisol levels, while a history of neglect was associated with higher waking cortisol among adolescents (14–19 years; Gerra et al., Reference Gerra, Zaimovic, Castaldini, Garofano, Manfredini, Somaini and Donnini2010). Finally, one longitudinal study found sexually abused females exhibited higher levels of waking cortisol at their initial assessment (which occurred when participants were between 6 and 16 years of age) than girls who had not been abused, and also maintained higher levels of waking cortisol as over the 13 years of the study (Trickett, Noll, Susman, Shenk, & Putnam, Reference Trickett, Noll, Susman, Shenk and Putnam2010). However, two studies have not found an association between maltreatment and waking cortisol levels. For example, Cicchetti and colleagues found no difference in morning cortisol levels among maltreated infants (11–15 months) and their peers (Cicchetti et al., Reference Cicchetti, Rogosch and Oshri2011), while Kuhlman and colleagues reported similar findings among children and adolescents (9–16 years) who had been physically or emotionally abused (Kuhlman, Geiss, Vargas, & Lopez-Duran, Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015). Duval and colleagues reported that there were no differences in morning cortisol between adolescents (12–20 years) with PTSD due to sexual abuse and their peers who were not diagnosed with PTSD, but it is important to note that these adolescents were hospitalized at the time of the study (Duval et al., Reference Duval, Crocq, Guillon, Mokrani, Monreal, Bailey and Macher2004).Reference Schulz, Kirschbaum, Pruessner and Hellhammer1988
Moreover, the results of two other studies suggest that certain types of maltreatment may be associated with lower waking or morning cortisol levels, and that gender and psychopathology may be implicated in the broader association between maltreatment and cortisol levels. For example, while Trickett and colleagues found that sexually abused girls (ages 6–16 years) exhibited higher waking cortisol than girls who had not been abused (Trickett et al., Reference Trickett, Noll, Susman, Shenk and Putnam2010), King and colleagues observed that in a mixed-gender sample of children (ages 5–7), sexual abuse was associated with lower waking cortisol levels compared to controls (King, Mandansky, King, Fletcher, & Brewer, Reference King, Mandansky, King, Fletcher and Brewer2001). Moreover, Alink and colleagues found that among school-aged children (6–9 years), the experience of maltreatment was associated with lower morning cortisol levels measured one year later, but that this relation was fully mediated by externalizing behaviors (Alink, Cicchetti, Kim, & Rogosch, Reference Alink, Cicchetti, Kim and Rogosch2012). Differential results by maltreatment type were reported by Bruce and colleagues in their work with a single sample of young children (3–6 years): children who had been exposed to emotional abuse exhibited higher morning cortisol levels relative to their peers who had not been maltreated, whereas those who had experienced physical neglect exhibited lower levels (Bruce, Fisher, Pears, & Levine, Reference Bruce, Fisher, Pears and Levine2009).
In four studies (two of which used the same data) cortisol was measured at multiple time points in the morning, allowing for an examination of the association between maltreatment and the cortisol awakening response (CAR). This response is characterized by an initial elevation in cortisol levels that occurs within 1–2 hr after waking, and that typically precedes the diurnal decline in HPA axis activity observed throughout the remainder of the day (Pruessner et al., Reference Pruessner, Wolf, Hellhammer, Buske-Kirschbaum, von Auer, Jobst and Kirschbaum1997). According to the CAR anticipation hypothesis, larger CARs in anticipation of daily stressors may be adaptive (Schulz, Kirschbaum, Pruessner, & Hellhammer, Reference Schulz, Kirschbaum, Pruessner and Hellhammer1998). In two of these four studies, both of which were conducted with a single sample of maltreated adolescents, Kaess and colleagues examined maltreated adolescents’ CARs when they were 14–16 years old. Overall, higher levels of maltreatment in childhood were associated with smaller awakening responses in adolescence (Kaess et al., Reference Kaess, Whittle, Simmons, Jovev, Allen and Chanen2017). However, in subsequent analyses of these same data, this association was found to hold only among those participants who were found to have a larger pituitary gland in early adolescence (11–13 years; Kaess, Whittle, O'Brien-Simpson, Allen, & Simmons, Reference Kaess, Whittle, O'Brien-Simpson, Allen and Simmons2018). Two other studies have also reported an association between maltreatment and the awakening response that was contingent on other factors. Keeshin and colleagues observed an inverse relation between the magnitude of the awakening response and the severity of both posttraumatic stress symptomatology following maltreatment and traumatic experiences prior to maltreatment among adolescents (12–17 years; Keeshin, Strawn, Out, Granger, & Putnam, Reference Keeshin, Strawn, Out, Granger and Putnam2014). In contrast, Quevedo and colleagues reported an association between maltreatment and larger awakening responses, but only among adolescents (11–19 years) who were depressed (as compared to adolescents who had not been maltreated; Quevedo, Doty, Roos, & Anker, Reference Quevedo, Doty, Roos and Anker2017). Among depressed adolescents, the association between maltreatment and a larger awakening response was contingent upon elevated levels of dorsal anterior cingulate cortical activity during negative self-description (Quevedo et al., Reference Quevedo, Doty, Roos and Anker2017).
Although the studies reviewed in this section thus far varied substantially in their methodology, those that assessed cortisol did so by assaying bodily fluids (typically saliva and occasionally blood). However, two studies examined cortisol concentrations in hair, which is thought to reflect cumulative or life stress (Karlén, Ludvigsson, Frostell, Theodorsson, & Faresjö, Reference Karlén, Ludvigsson, Frostell, Theodorsson and Faresjö2011). Consistent with this account, do Prado and colleagues found that adolescents who experienced maltreatment early in childhood exhibited higher levels of hair cortisol than their peers (do Prado, Grassi-Oliveira, Daruy-Filho, Wieck, & Bauer, Reference do Prado, Grassi-Oliveira, Daruy-Filho, Wieck and Bauer2017). However, in another study, White et al. (Reference White, Ising, Klitzing, Sierau, Michel, Klein and Stalder2017) reported that children who had been maltreated exhibited lower cortisol concentrations than their peers, a finding the authors linked to variations in the chronicity and number of types of maltreatment (White et al., Reference White, Ising, Klitzing, Sierau, Michel, Klein and Stalder2017).
Altered HPA axis function under conditions of challenge
Twenty-three studies have examined the association between maltreatment and HPA axis activity under conditions of challenge with children across a range of ages and employing a variety of stressors. Two of these studies were conducted with school-aged children and adolescents using the TSST as the stressor and reported an association between maltreatment and hyporeactivity of the HPA axis. Trickett and colleagues observed a blunted cortisol response to the TSST among maltreated school-aged children (9–12 years), relative to controls, and found that this attenuated response was especially pronounced among children who had been sexually and/or physically abused (Trickett, Gordis, Peckins, & Susman, Reference Trickett, Gordis, Peckins and Susman2014). In their work with Romanian adolescents (age 13 years), McLaughlin and colleagues reported similar findings: adolescents who had remained in institutional care as young children exhibited a blunted cortisol response to the TSST compared to their peers who had been moved to foster care (McLaughlin et al., Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015).
Four additional studies of school-aged children and adolescents that employed the TSST have also found evidence of hyporeactivity, but in those studies these findings were contingent upon age, gender, or genetic factors. For example, in a longitudinal study, school-aged children who had been maltreated were no more likely to exhibit a blunted cortisol response to the TSST-C (the variant of the TSST for children) than controls when cortisol was initially measured at 9–12 years. However, at the second and third measurements, taken 1 and 3 years later, maltreated children did exhibit a blunted response, though they did not do so at the final assessment, which occurred 7 years after the first (Peckins, Susman, Negriff, Noll, & Trickett, Reference Peckins, Susman, Negriff, Noll and Trickett2015). Two studies suggest that hyporeactivity is contingent upon the combination of age and gender. In an exclusively female sample, adolescents (12–16 years) who had been maltreated exhibited a blunted cortisol response to the TSST relative to controls (MacMillan et al., Reference MacMillan, Georgiades, Duku, Shea, Steiner and Schmidt2009). However, within a mixed-gender sample of school-aged children (9–12 years), Negriff and colleagues found that maltreatment was associated with blunted cortisol response to the TSST among males (9–12 years), but not among females of the same age (Negriff, Saxbe, & Trickett, Reference Negriff, Saxbe and Trickett2015). Finally, one study that accounted for genetic factors found that while maltreated adolescents (13–17 years) exhibited blunted responses to the TSST relative to their peers at the trend level, only among those maltreated adolescents with one or more G alleles of the rs110402 polymorphism on the gene CRFR1 (which codes for corticotropin releasing factor receptor 1) was the magnitude of the response significantly smaller (Sumner et al., Reference Sumner, McLaughlin, Walsh, Sheridan and Koenen2014).
Five studies employing a variety of stressors other than the TSST have also yielded evidence of HPA axis hyporesponsivity among maltreated children and adolescents. For example, Saxbe and colleagues found that adolescents (age 15 years) who had experienced higher cumulative family aggression (physical abuse) produced less cortisol across the course of a laboratory-based conflict discussion with their parents when that discussion featured higher levels of conflict (Saxbe, Margolin, Spies Shapiro, & Baucom, Reference Saxbe, Margolin, Spies Shapiro and Baucom2012). Hyporesponsivity to conflict was also observed among maltreated children (age 28–75 months) by Hart and colleagues, who reported that on days featuring high levels of peer social conflict, children who had been maltreated displayed lower cortisol levels relative to their peers. Moreover, whereas children who had not been maltreated displayed higher levels of cortisol on high-conflict days over the course of a month, maltreated children displayed similar cortisol levels regardless of daily conflict (Hart, Gunnar, & Cicchetti, Reference Hart, Gunnar and Cicchetti1995). Shenk and colleagues found that females (age 13–23 years) who had been sexually abused were significantly more likely to exhibit a blunted cortisol response to a timed mental rotation task than controls 7 years after maltreatment occurred (Shenk et al., Reference Shenk, Noll, Putnam and Trickett2010), while Sullivan and colleagues observed that physically neglected children (6–9 years) exhibited a steeper decline in cortisol levels during a series of self-evaluation tasks than their peers (Sullivan et al., Reference Sullivan, Bennett and Lewis2013). Among younger children, Sturge-Apple, Davies, Cicchetti, and Manning (Reference Sturge-Apple, Davies, Cicchetti and Manning2012) found maternal emotional neglect predicted a smaller cortisol response to the Strange Situation among maltreated toddlers (age 2 years) than their peers.
In contrast, six of the 23 studies that have examined HPA axis activity in response to challenge have yielded evidence that the HPA axis is hyperreactive to stress. For example, Bick et al. (Reference Bick, Nguyen, Leng, Piecychna, Crowley, Bucala and Grigorenko2015) found that adolescents (14.5–19 years) who had been sexually abused exhibited greater cortisol reactivity to the TSST than their peers at a level approaching significance. Harkness and colleagues reported similar results: maltreated adolescents (12–21 years) who were depressed exhibited larger cortisol responses (as indexed by the area under the curve) to the TSST than depressed adolescents who had not been maltreated (Harkness et al., Reference Harkness, Stewart and Wynne-Edwards2011). Evidence of hyperreactivity has also been found using stressors other than the TSST. For example, whereas Saxbe and colleagues found that adolescents (15 years) who had experienced physical abuse exhibited a reduced cortisol response to a high-conflict discussion with their parents (Saxbe et al., Reference Saxbe, Margolin, Spies Shapiro and Baucom2012), Wismer Fries and colleagues noted larger cortisol responses during interactions between young children (54 months) who had been neglected while in institutionalized care and their foster mothers than those observed among controls (Wismer Fries et al., Reference Wismer Fries, Shirtcliff and Pollak2008). And whereas Trickett and colleagues found that children (9–12 years) who had been physically or sexually abused exhibited especially small reactions to the TSST (Trickett et al., Reference Trickett, Gordis, Peckins and Susman2014), Linares and colleagues observed that adolescents (14–17 years) who had been sexually abused exhibited the largest cortisol response to completing the Attitudes toward Interpersonal Violence Assessment (Reese-Weber, Reference Reese-Weber2008) relative to their peers and after controlling for physical abuse by their partner (Linares, Shrout, Nucci-Sack, & Diaz, Reference Linares, Shrout, Nucci-Sack and Diaz2013). A similar finding was reported by Rinnewitz et al. (Reference Rinnewitz, Koenig, Parzer, Brunner, Resch and Kaess2018): more severe maltreatment was associated with larger increases in cortisol in response to a cold pressor task among female adolescents (12–17 years) who exhibited nonsuicidal self-injurious behaviors (Rinnewitz et al., Reference Rinnewitz, Koenig, Parzer, Brunner, Resch and Kaess2018). Burgental and colleagues reported that toddlers (2 years) who had experienced frequent corporal punishment displayed larger cortisol responses to the Strange Situation (Ainsworth et al., Reference Ainsworth, Blehar, Waters and Wall1978) than their peers (Bugental et al., Reference Bugental, Martorell and Barraza2003), in contrast to Sturge-Apple et al. (Reference Sturge-Apple, Davies, Cicchetti and Manning2012), who found that toddlers who had experienced emotional neglect exhibited smaller cortisol responses to the Strange Situation.
Duval and colleagues found that hospitalized adolescents diagnosed with PTSD due to sexual abuse exhibited lower levels of adrenocorticotropic hormone (but not cortisol) following a dexamethasone suppression test than their hospitalized peers without PTSD (Duval et al., Reference Duval, Crocq, Guillon, Mokrani, Monreal, Bailey and Macher2004); de Bellis and colleagues reported similar findings for adrenocorticotropic hormone (but again, not cortisol) following an infusion of corticotropin-releasing hormone (DeBellis et al., Reference DeBellis, Chrousos, Dorn, Burke, Helmers, Kling and Putnam1994). Although these are not stressors per se, the administration of pharmacological agents is a commonly used approach to assess the reactivity of the HPA axis. Finally, two studies found no association between maltreatment and the magnitude of the HPA axis response to challenge (Kuhlman et al., Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015; Shenk et al., Reference Shenk, Putnam and Noll2012), though Kuhlman and colleagues did observe more rapid increases in cortisol in response to the cold pressor task among adolescents who had been physically abused and slower recovery following the task among emotionally abused adolescents (Kuhlman et al., Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015). The literature on altered HPA axis function following maltreatment is summarized in Figures 2b and 2c.
Discussion
Summary and interpretation
Summary: Altered ANS function following maltreatment
Five of the nine studies that examined the association between maltreatment and ANS function under homeostatic conditions reported hyperactivity. These studies varied in the type(s) of maltreatment, how maltreatment was measured (self-report or public records), sample age and composition, and the way in which autonomic function was assessed. The one study that reported an association between maltreatment and ANS hypoactivity (Shenk et al., Reference Shenk, Putnam and Noll2012) did not differ substantially along these dimensions, and indeed was quite similar in terms of sample composition (adolescent females) and method of ANS measurement (RSA) to another study reporting hyperactivity (Miskovic et al., Reference Miskovic, Schmidt, Georgiades, Boyle and MacMillan2009). Therefore, it is not possible to attribute these differences to methodological causes.
Seven of the 12 studies that examined the association between maltreatment and the autonomic response to stress reported evidence of hyporeactivity. These studies varied widely in types of maltreatment and the ways in which autonomic response was indexed, but with two exceptions (Ford et al., Reference Ford, Fraleigh, Albert and Connor2010; Pollak et al., Reference Pollak, Vardi, Bechner and Curtin2005) they featured samples comprising adolescents. In this respect they were similar to the two studies that reported an association between maltreatment and ANS hyperreactivity (those by Rinnewitz et al., Reference Rinnewitz, Koenig, Parzer, Brunner, Resch and Kaess2018 and Shenk et al., Reference Shenk, Noll, Putnam and Trickett2010). What appears to differentiate these two studies from those that reported ANS hyporeactivity is the nature of the stressor employed. In all but one case (Ford et al., Reference Ford, Fraleigh, Albert and Connor2010), the studies that reported hyporeactivity employed a psychosocial stressor, either the TSST (in four studies) or a conflict recording or video (in two cases). However, the two studies that reported hyperreactivity used a physical (Rinnewitz et al., Reference Rinnewitz, Koenig, Parzer, Brunner, Resch and Kaess2018) or cognitive challenge (Shenk et al., Reference Shenk, Noll, Putnam and Trickett2010) as the stressor. The nature of the stressor may also account for the complex pattern of results reported by Oosterman et al. (Reference Oosterman, De Schipper, Fisher, Dozier and Schuengel2010), in which smaller decreases of respiratory sinus arrhythmia (indicative of PNS hyporeactivity) and larger increases in pre-ejection period (corresponding to SNS hyperreactivity) were observed among young children who had been neglected. The particular stressor employed in this study – caregiver separation – was unique, and while speculative, one could argue that the hyperactive SNS response exhibited by children with a history of neglect may be attributable to sensitization among these children to disengaged caregiver behavior.
Summary: Altered HPA-axis function following maltreatment
Among the 37 studies that examined the function of the HPA axis under conditions of homeostasis, 17 reported an association between maltreatment and elevated levels of HPA-axis function, with particularly consistent findings among studies that defined homeostatic function as HPA axis activity prior to the onset of a stressor (i.e., baseline function) or activity measured over the course of the day. None of the 13 studies that examined the association between maltreatment and baseline function reported evidence of hypoactivity; six reported an association between maltreatment and hyperactivity. Although these studies varied considerably in how maltreatment was measured and the ages of the participants (which ranged from 2–21 years across studies), they were consistent in two respects: in each case, salivary cortisol was used to index HPA axis activity, and, in four of the six studies, physical or emotional neglect was the type of maltreatment associated with hyperactivity. While the four studies that reported no association between maltreatment and HPA axis activity also measured that activity via salivary cortisol, two of these (McLaughlin et al., 2014a; Sumner et al., Reference Sumner, McLaughlin, Walsh, Sheridan and Koenen2014) did not include children who had been neglected, raising the question of whether the association between maltreatment and baseline HPA axis activity may vary by maltreatment type as an avenue for future research (see below). Seven of the ten studies that examined diurnal function also reported evidence of hyperactivity, and these studies varied widely in the type(s) of maltreatment assessed, the manner in which maltreatment was reported, and the size and composition of the sample. The two studies that reported evidence of hypoactivity (Kertes et al., Reference Kertes, Gunnar, Madsen and Long2008; Kočovská et al., Reference Kočovská, Wilson, Young, Wallace, Gorski, Follan and Minnis2013) did not differ from the other studies in these respects, but they did differ substantially in other ways. In the case of Kertes and colleagues, there was at least a three-year gap between the experience of maltreatment (prior to foster placement) and the assessment of HPA axis activity (following foster placement); for Kočovská et al. (Reference Kočovská, Wilson, Young, Wallace, Gorski, Follan and Minnis2013), children who had been maltreated and adopted were compared against children who had not been adopted. Therefore, in both cases variations in children's experience in foster care settings following maltreatment may explain findings of hypoactivity.
The literature is less consistent where homeostatic function is defined as waking or morning cortisol. Six studies – five of which examined waking levels of cortisol and one of which assessed the CAR – found evidence of HPA axis hyperactivity; three studies (two waking, one CAR) found evidence of hypoactivity, and two studies found no association between maltreatment and waking cortisol levels. Although these studies varied widely in terms of type(s) of maltreatment, reporting method, and sample size and composition, there is no apparent link between a particular aspect of this variation and a given pattern of findings. There is, however, a potential methodological explanation for the studies that reported an association between maltreatment and hypoactivity (Alink et al., Reference Alink, Cicchetti, Kim and Rogosch2012; Kaess et al., Reference Kaess, Whittle, Simmons, Jovev, Allen and Chanen2017; King et al., Reference King, Mandansky, King, Fletcher and Brewer2001). In all three of these studies, only those samples required to index waking cortisol or the CAR were collected. It is therefore possible that these lower, early levels of cortisol were followed by elevated levels throughout the remainder of the day, which, together with lower waking cortisol, would constitute a flattened diurnal slope indicative of hyperactivity.
The literature indicates that the HPA axis is hyporeactive to stress, with 11 of the 23 studies that have examined the association between maltreatment and reactivity yielding evidence of hyporeactivity, compared to six that reported evidence of hyperreactivity. The literature is most consistent among studies that used the TSST (or the TSST-C) to assess reactivity among school-age children or adolescents; six of the eight studies that fit this description reported hyporeactivity. Comparing studies in which the stressor and/or ages of children in the sample varied were more likely to yield discrepant results.
Consider, for example, findings reported by Trickett et al. (Reference Trickett, Gordis, Peckins and Susman2014), wherein physically or sexually abused children (ages 9–12) exhibited a blunted response to the TSST, and those findings reported by Linares et al. (Reference Linares, Shrout, Nucci-Sack and Diaz2013), in which adolescents (ages 14–17) who had been sexually abused displayed a hyperreactive response to the Attitudes toward Interpersonal Violence Assessment (Reese-Weber, Reference Reese-Weber2008). These differences might be attributable to the different nature of the stressors employed in the two studies – a social-evaluative task (the TSST) versus a questionnaire (the Attitudes toward Interpersonal Violence Assessment) – or the way in which physical abuse was handled in the analyses: as a component of maltreatment by Trickett or covariate controlled for by Linares.
However, it is also possible that these differences instead reflect genuine heterogeneity in the association between types of maltreatment and altered patterns of HPA axis reactivity to stress that may be, in part, contingent upon age; adolescence may constitute a developmental shift, in which the association between sexual abuse and reactivity changes from hypo- to hyperreactivity. Extending this account to include the interaction between age and gender may help to explain discrepancies in the association between maltreatment and waking or morning cortisol. Though Trickett et al. (Reference Trickett, Noll, Susman, Shenk and Putnam2010) found that sexually abused girls who ranged in age from 6–16, but who were, on average, pre-adolescents (M age = 11 years), exhibited higher waking cortisol levels than girls who had not been abused, King et al. (Reference King, Mandansky, King, Fletcher and Brewer2001) reported that sexual abuse was associated with lower waking cortisol levels in a mixed-gender sample of younger children (ages 5–7). It is therefore possible that adolescence is a point of inflection in the association between sexual abuse and homeostatic function of the HPA axis (defined as waking or morning cortisol), and that the timing of this switch is contingent upon gender, and, specifically, the gender-associated differences in the onset of adrenarche.
Interpretation
As this summary makes clear, the literature regarding the neurophysiological embedding of maltreatment is complex. Nevertheless, it supports an account in which maltreatment is associated with hyperactivity of the ANS and HPA axis under conditions of homeostasis and hyporeactivity to stress. While the fact that a single account of altered function applies to both systems is surprising given the methodological variability across studies, this account makes good neurophysiological and evolutionary sense.
Consider first the consistencies between this account and our understanding of neurophysiological function as it occurs in situ: while our review reflected the literature, in that it devoted separate sections to the ANS and HPA axis, within any individual these systems operate in close concert, and their coordination is facilitated by mutually inhibitory and excitatory connections among systems. Therefore, while Gordis and colleagues found that maltreated children were more likely to exhibit “asymmetrical” patterns of multisystem neurophysiological activity, in general patterns of multisystem activity are likely to be congruent or symmetrical, both under conditions of homeostasis and challenge (Gordis, Granger, Susman, & Trickett, Reference Gordis, Granger, Susman and Trickett2008). Our review indicates that these idiographic patterns of multisystem activity are mirrored at the level of the sample.
This same person-centered perspective can also elucidate why homeostatic hyperactivity of the ANS or HPA axis may be associated with hyporeactivity to challenge: within an individual, homeostatic ANS or HPA axis activity provides a restrictive or permissive context for the activity of that system in response to stress. For example, hyperactivity of the ANS or HPA axis under homeostatic conditions may restrict the reactivity of that system in response to challenge, in accordance with the law of initial values and together with the fact that activity is bound by certain physiological limits. Within the meta-sample of studies reviewed here, we may be observing the same phenomenon at work, as indicated by studies that report hyperactivity under homeostatic conditions and hyporeactivity of the ANS (e.g., Gooding et al., Reference Gooding, Milliren, Austin, Sheridan and McLaughlin2016) or HPA axis (e.g., Sturge-Apple et al., Reference Sturge-Apple, Davies, Cicchetti and Manning2012).
From an evolutionary perspective, hyperactivity of the ANS and HPA axis under conditions of homeostasis may serve two adaptive purposes: first, increased activity of the sympathetic branch of the ANS and the HPA axis provides the metabolic resources required to cope with an environment in which threats are extreme and severe – that is, the environment of maltreatment. Second, ANS hyperactivity corresponds to reduced activity of the PNS. As noted above, higher levels of homeostatic PNS activity foster a state of calm engagement with the environment, and, as such, higher levels of baseline PNS activity are generally considered a promotive factor for development. However, there is evidence that the association between high levels of baseline PNS activity and developmental outcomes is contingent upon the nature of the developmental environment, such that in environments marked by higher levels of adversity – including environments characterized by negative parenting behaviors – high levels of baseline PNS activity are associated with poorer developmental outcomes (cf., Conradt, Measelle, & Ablow, Reference Conradt, Measelle and Ablow2013). This is entirely consistent with polyvagal theory (Porges, Reference Porges1992): if higher levels of PNS activity foster engagement with the developmental environment and that environment is marked by adversity, higher PNS activity is essentially opening the child to the full experience of that adversity.
Reductions in PNS activity as part of an overall pattern of ANS hyperactivity – and the concomitant closing off of the child to the environment – would therefore be adaptive for a child exposed to maltreatment, at least in the short term. The long-term problem with reduced homeostatic PNS activity is that the PNS evolved to support interaction with the environment under nonthreatening conditions by modulating metabolic output, and one way to accomplish this is through the reduction of parasympathetic activity. A maltreated child, for whom levels of homeostatic PNS activity are already reduced, has an inhibited ability to further decrease PNS activity, and therefore must draw on other systems to facilitate environmental interaction – namely, the SNS and HPA axis – even in the absence of threat. While this may be effective in the short term, this strategy cuts against the grain of our evolutionary heritage, in which the resources of the SNS and HPA axis are recruited only after those offered by recruiting the PNS are exhausted (Jackson, Reference Jackson and Taylor1958). If a maltreated child is drawing on the resources of the SNS and HPA axis in their interactions with the environment under homeostatic conditions, the ranges of potential system response under conditions of challenge will be reduced. By again applying a person-centered perspective we can see that the blunted neurophysiological response to stress common among maltreated children may be less an adaptation to maltreatment than a consequence of how maltreatment recalibrates homeostatic neurophysiological function.
As we noted at the outset of this paper, the capacity of maltreatment to become embedded in the activity of multiple neurophysiological systems may partially explain its adverse effects on child development. The alterations in neurophysiological function reported in the research reviewed here suggest a multitude of specific pathways that may link maltreatment to developmental outcomes. For example, maltreatment is associated with hyperactivity of the HPA axis activity under conditions of homeostasis, resulting in elevated levels of cortisol. Elevated cortisol levels may imperil neurological development, particularly in areas of the brain that develop more slowly over childhood, such as the prefrontal cortex (Gunnar & Quevedo, Reference Gunnar and Quevedo2007; Heim & Nemeroff, Reference Heim and Nemeroff2001), and indeed maltreatment is associated with reduced volume in this area of the brain (DeBellis, Reference DeBellis2001). The prefrontal cortex is the putative seat of executive functions, and children who have been maltreated exhibit poorer performance on measures of this core set of cognitive abilities (DePrince et al., Reference DePrince, Weinzierl and Combs2009; Nolin & Ethier, Reference Nolin and Ethier2007; Pollak et al., Reference Pollak, Nelson, Schlaak, Roeber, Wewerka, Wiik and Gunnar2010; Porter et al., Reference Porter, Lawson and Bigler2005). Thus, it is possible to delineate a path from maltreatment to HPA axis hyperactivity and elevated levels of cortisol to impeded development of the prefrontal cortex to poorer performance on measures of executive functions, or more generally from maltreatment to neurophysiological function to neurological development to cognitive outcomes. Although this path is certainly suggested by prior research, to date no study of which we are aware has empirically assessed its viability. An important direction for future research is to specify and then test these extended mediation models using longitudinal data, and the literature provides ample basis on which to formulate models connecting maltreatment to a multitude of adverse outcomes associated with maltreatment, from psychopathology (through altered HPA axis activity, immune function, and inflammatory response) to emotion regulation (through ANS hyporesponsivity and reward processing).
Future directions
Although at present the literature supports a certain association between maltreatment and altered neurophysiological function, that literature is hardly exhaustive; as can be seen in Figure 3, our knowledge about the association between maltreatment and neurophysiological function for any particular period of development is based on results from a small number of studies. Clearly there is a need for additional research designed to replicate and expand upon the findings presented in this paper in order to advance our understanding of the association between maltreatment and neurophysiological function. Here we offer two sets of suggestions that may help build that understanding as efficiently as possible, as well as one particular question for future research to address.

Figure 3. A graphical summary of the literature reviewed in this paper. For each study, the top, gray bar indicates the approximate ages at which maltreatment was reported to have occurred, while the bottom bar indicates the range of ages at which the function of the ANS (dark gray, white numerals), HPA axis (white, black numerals), or both (light gray, black numerals) were assessed. The bounds of the range during which neurophysiological function was assessed are indicated in Arabic numerals.
One suggestion concerns the analyses we conduct and how we report findings. Given their sample sizes, most studies of maltreatment focus on overall relations between maltreatment and neurophysiological function (i.e., main effects), rather than exploring relations that may be contingent on third factors (interaction effects). However, modest conditional relations between maltreatment and neurophysiological function that do not achieve statistical significance (r ~ [.10–.30]) due to power constraints may nevertheless be of practical significance for other researchers; so too might the knowledge that the relation between maltreatment and neurophysiological function is essentially orthogonal in the presence of a particular third factor. It would therefore benefit the field if investigators reported the results of exploratory data analyses in supplementary materials, even – or perhaps especially – when the results of those analyses are not significant. The exact analyses performed would of course depend on the study, but reporting associations between maltreatment and neurophysiological function by the type, severity, and recency of maltreatment, as well as relevant third factors, would be ideal.
The second suggestion concerns how we collect data. One suggestion is for researchers to come to some agreement regarding which stressors to employ in their studies. When different studies employ different stressors, comparing results across studies becomes difficult, even when children are of similar ages. While it may not be necessary for researchers to agree on a specific task or procedure, selecting tasks of a similar nature would facilitate the comparison of results. For example, the results of two studies that employ social evaluation tasks, even if both are not the TSST, may be quite comparable, whereas the results obtained using a social evaluation task and a stressor of another nature – be it a timed cognitive task, a conflict discussion, or completing a sensitive questionnaire – may not be. Another suggestion would be for researchers to simultaneously measure the function of the PNS, SNS, and HPA axis. It is common to study these systems in isolation, and the literature reflects that fact. But, as noted above, the activity of these systems is highly coordinated, and therefore studying the activity of one system without examining the function of the remaining systems may lead researchers to draw erroneous conclusions about the activity of the measured system with relation to maltreatment. In short, the activity of the unmeasured system or systems constitutes a crucial third factor that may moderate the relation of maltreatment and the activity of the measured system (see Holochwost, Kolacz, & Mills-Koonce, Reference Holochwost, Kolacz and Mills-Koonceunder review, for an elaboration of this argument).
Finally, there is one specific question for future research highlighted by the literature. Where both ANS and HPA axis activity are concerned, the results of prior research suggest that possibility that certain specific types of maltreatment – and, in particular, neglect and abuse – may be associated with different alterations in neurophysiological function. As McLaughlin and colleagues note, it is possible that abuse, which constitutes a threat or presence of a negative environmental input, may be associated with different neurophysiological sequalae than neglect or deprivation, which may be defined as the absence of positive environmental inputs (McLaughlin, Sheridan, & Lambert, Reference McLaughlin, Sheridan and Lambert2014b). Although this framework is compelling and well-grounded in research with animal models, its validation would require comparing the results of studies in which the broad type of maltreatment varied but other aspects of maltreatment, as well as other key third factors such as child age and gender, were either held constant procedurally or adjusted for statistically. At present, the literature does not include a sufficient number of studies that fit this description and would therefore allow this framework to be tested, but as the literature continues to expand testing this explanation for different patterns of neurophysiological function will become possible.
Implications for policy and practice
The findings of this review suggest that maltreatment has the capacity to dysregulate multiple neurophysiological systems, and that the sequalae of childhood maltreatment may persist into adulthood. This underscores the need for policy and practice to focus on “upstream” efforts designed to prevent maltreatment from occurring in the first place. Home visitation is the most commonly employed approach in prevention efforts (Klika, Lee, & Lee, Reference Klika, Lee, Lee, Klika and Conte2018), and while key differences exist across home visiting models, there is general consensus that intervening with families as early possible is critical to building parents’ capacity to create a safe, stable, and nurturing environment.
A number of different home visitation approaches have been found to re-organize neurophysiological systems to more regulated patterns of activity (Boparai et al., Reference Boparai, Au, Koita, Oh, Briner, Harris and Bucci2018; Slopen, McLaughlin, & Shonkoff, Reference Slopen, McLaughlin and Shonkoff2014). However, while these approaches are widely considered examples of innovative practice, they have not as of yet been adopted for broad implementation by policy makers and practitioners. This may reflect a commendable desire to await consistent results from replication before implementing a given program at scale, as well as realities of bureaucratic inertia and budgetary constraints. But to some extent the fact that innovative approaches with strong evidence of effect have not been widely implemented is attributable to the barriers that impede the translation of research findings into policy and practice.
These barriers include policy makers’ and practitioners’ limited access to subscription-based academic journals and even more restricted time to read the articles that appear in them. To their credit, publishers have worked to remove some of these barriers by making certain papers open access, while universities have created centers to distill and disseminate research findings. But these efforts cannot overcome the misalignment between academic research conducted in highly controlled settings with relatively small convenience samples and the needs of policy makers and practitioners for findings that are generalizable to the families in their communities. One solution is to use extant data; it is not uncommon for states and large municipalities to have access to data from larger and more representative samples than any researcher could reasonably hope to collect. However, state and local agencies often lack the time to plumb these data for the relevant findings they may hold, and, in some cases, procedures to ensure that the data being collected are of acceptable quality for research purposes. These are areas in which researchers can provide guidance, but to do so effectively will require forging long-term, collaborative relationships among researchers, practitioners, and policy makers. Creating and sustaining these relationships will require investments on the part of professional organizations and funders, but those investments will have the potential to pay dividends in the form of improved outcomes for children and families.









