The phrenic nerve is one of the most important nerves in the human body due to its pivotal role in the respiratory process. This nerve innervates the diaphragm to enable its primary motor functions, therefore, its injury can lead to dysfunction or paralysis of this major respiratory muscle. While a number of diseases can lead to phrenic nerve injury including diabetes, herpes zoster virus, invasion of the nerve by a tumour, and neurological conditions like multiple sclerosis and muscular dystrophy, the most common cause of PNI is cardiac or thoracic surgery, accounting for around 64% of phrenic nerve injuries.Reference Mandoorah and Mead1
Approximately 23 neonates per 1000 are diagnosed with CHD requiring surgical intervention.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2 Surgical procedures involving the heart and surrounding structures in young patients carry the risk of resulting in harmful complications due to the small size and close proximity of structures within the surgical field. Diaphragmatic paralysis is one of the most common complications with a prevalence of 1.9–12.9% as seen in recent prospective studies.Reference de Leeuw, Williams, Freedom, Williams, Shemie and McCrindle3 The incidence of these injuries is higher following some procedures such as the Fontan operation, Blalock–Taussig–Thomas Shunt, arterial switch operations, correction of Tetralogy of Fallot, and ventricular septal defect closure.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2
As neonates and infants are primarily diaphragmatic breathers with intercostal muscles playing little role in the respiratory process, they often present with more severe clinical symptoms in comparison to older children and adults.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2 Early signs of nerve injury can be more non-specific – such as unexplained shortness of breath, orthopnoea, fatigue, and insomnia.Reference Mandoorah and Mead1 However, over time, DP can lead to more severe consequences such as recurrent pneumonia, atelectasis, respiratory distress, and difficulty or failure to wean off mechanical ventilation.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2 This reliance on mechanical ventilation is particularly seen as a result of bilateral DP which causes a 60% reduction in an infant’s respiratory function.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 There are many methods to detect DP, and the most commonly used modalities in practice are bedside ultrasonography and fluoroscopy.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 The management plan for this condition remains controversial and mainly revolves around the optimal preservation of respiratory function, often through the implementation of prolonged mechanical ventilation or transthoracic diaphragmatic plication.Reference Joho-Arreola, Bauersfeld, Stauffer, Baenziger and Bernet5 The aim of this review is to explore the pathophysiology and risk factors of PNI, the diagnostic criteria, and the available management options in order to appropriately guide the preservation of diaphragmatic and respiratory function in children.
Pathophysiology
The common occurrence of PNI resulting from surgery for CHD correlates directly with the anatomical position of the phrenic nerve and the structures adjacent to its route (Fig 1).

Figure 1. A diagram showing the pathways of the right and left phrenic nerves.Reference Oliver and Ashurst6
Consisting of motor, sensory, and sympathetic nerve fibres, the phrenic nerve begins its descent from the anterior rami of the C3, C4, and C5 nerve roots.Reference Oliver and Ashurst6 As the longest branch of the cervical plexus, it begins its journey from the neck and travels from a lateral position medially over the anterior scalenus anterior muscle.Reference Ostrowska and de Carvalho7 The nerve then descends vertically behind the prevertebral fascia posterior to the sternocleidomastoid, internal jugular vein, thoracic duct, and several other important structures in the neck before finally entering the thorax through the superior thoracic aperture.Reference Ostrowska and de Carvalho7
The right and left phrenic nerves differ slightly in their routes. The shorter and more vertical right nerve descends lateral to the brachiocephalic nerve and the superior caval vein towards the pericardium covering the right atrium and inferior caval vein.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 The left nerve travels laterally to the aortic arch and descends anteriorly between the mediastinal and pericardial pleura. It then crosses anteriorly to the internal thoracic artery as it reaches the pericardium on the opposing side covering the left ventricle.Reference Mandoorah and Mead1,Reference Talwar, Agarwala, Mittal, Choudhary and Airan4,Reference Ostrowska and de Carvalho7 The right phrenic nerve innervates the right diaphragmatic dome lateral to the inferior caval vein, and the left nerve supplies the left diaphragmatic dome. The two phrenic nerves together provide motor innervation to the muscle resulting in the inspiratory and expiratory movements required for successful respiration.Reference Oliver and Ashurst6
Due to the unique course of the left phrenic nerve, its exposure increases its susceptibility to damage from nerve hypothermia through ice-cold slush that is used to protect the myocardium during cardiac surgery.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 This nerve may also be damaged by prolonged stretching of the pericardium, nerve stretch via retractors, or during dissection/removal of structures on the left side such as the thymus or the vertical vein.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 The right phrenic nerve can easily be severed or subjected to electrocoagulation injury, as is often the case if the superior caval vein is being dissected.Reference Oliver and Ashurst6 Damage to the nerve can present as diaphragmatic dysfunction or unilateral/bilateral diaphragmatic paralysis. This often manifests as respiratory distress in children.Reference Mandoorah and Mead1
Incidence and risk factors
Cardiac surgery for CHD is one of the most common causes of PNI in children, with an incidence between 0.3% and 12.8%.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2 Recent studies have demonstrated an incidence of post-operative diaphragmatic paralysis due to PNI between 0.46% and 5.5% of patients.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2,Reference Zhang, Xu, Li, Yang, Sheng and Jun8–Reference Akay, Ozkan and Gultekin10 The degree of risk of PNI may depend on the type of surgical procedure that patients receive. The risk is higher in CHD corrective surgeries as they generally require open-heart surgery, which is extensive and involves more structural changes to repair defects when compared to palliative surgery.Reference Joho-Arreola, Bauersfeld, Stauffer, Baenziger and Bernet5 The surgical procedures that are commonly responsible for causing diaphragmatic paralysis are the Fontan, Blalock-Taussig-Thomas shunt, arterial switch operation, and Tetralogy of Fallot repair.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 A study by Akbariasbagh et al reported a prevalence rate of PNI in 16.6% of patients as a complication of these procedures.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2 Furthermore, Joho-Arreola et al also reported an incidence of 17.6%, 12.8%, and 10.8%, respectively, for patients who received the aforementioned procedures.Reference Joho-Arreola, Bauersfeld, Stauffer, Baenziger and Bernet5
Although these surgical procedures account for the highest incidence of PNI, several other procedures can also cause this injury. For instance, Akay et al demonstrated that PNI was most common in patients following Tetralogy of Fallot (31.5%), Blalock-Taussig-Thomas shunt (11.1%), and VSD closure with pulmonary artery patch plasty (11.1%).Reference Akay, Ozkan and Gultekin10 In addition, a study conducted by Georgiev et al highlighted that the highest number of patients (16.2%) who suffered from diaphragmatic palsy had undergone the arterial switch procedure, followed by 12.8% that had Tetralogy of Fallot repair and 12.2% that had superior cavopulmonary connection surgery.Reference Georgiev, Konstantinov, Latcheva, Mitev, Mitev and Lazarov9 Other risk factors that can increase a patient’s susceptibility to PNI include cyanotic heart defects (which require open-heart surgery to correct, risking phrenic nerve damage) as well as having undergone previous cardiac surgery. Indeed, there is a statistically significant increase in diaphragmatic injury in patients with cyanotic CHDs compared to non-cyanotic CHDs.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2 Previous cardiac surgery can increase the risk of complications as the resultant adhesions and fibrosis can make future interventions more technically challenging.Reference Akbariasbagh, Reza Mirzaghayan, Akbariasbagh, Shariat and Ebrahim2
Diagnosis
Diagnosis of PNI is a complex and challenging process, without universally accepted guidelines. Many of the clinical signs of PNI are non-specific. Therefore, it is important that clinicians keep a high index of suspicion for patients undergoing cardiac surgery. Most traumatic phrenic nerve palsies are unilateral and are mainly seen incidentally on routine follow-up chest X-rays. However, it is important to carry out a thorough history and clinical examination of high-risk patients (such as those post-cardiac surgeries) to identify the presence of signs and symptoms. These include shortness of breath, persistent tachypnoea, difficulty with feeding, recurrent pneumonia, and atelectasis. Additionally, PNI may also result in diaphragmatic paralysis, which can cause respiratory distress due to the paradoxical movement of the diaphragm on the injured side. These patients may also find it difficult to wean off ventilation, which can prolong the duration of their intensive care and hospital stay.Reference Zhang, Xu, Li, Yang, Sheng and Jun8
Physical examination of the patient may show decreased breath sounds on the affected side, dullness to percussion, and paradoxical movement of the epigastrium during respiration and dullness on percussion.Reference Mandoorah and Mead1 If the patient’s history and clinical examination suggest PNI, investigations must be carried out to confirm the diagnosis. As mentioned, in cases of unilateral diaphragm paralysis, a chest X-ray may show an abnormally raised diaphragm on the affected side. However, chest X-ray only has a positive predictive value of 33% for the diagnosis of diaphragmatic palsy and therefore should not be used to make a definitive diagnosis.Reference Dubé and Dres11
Pulmonary function tests are commonly used in order to assess diaphragmatic weakness and palsy. Cases of unilateral weakness usually result in a mild decrease in forced vital capacity, but total lung capacity and functional residual capacity may remain within the normal range. Supine positioning of the patient can result in a further decrease in forced vital capacity by 10–20%.Reference Dubé and Dres11
Ultrasound examination is a simple and non-invasive method of assessing diaphragmatic dysfunction and can be carried out at the bedside. It can be used to evaluate the diaphragmatic thickness and inspiratory diaphragm thickening fraction, amongst other variables. Ultrasound has been shown to be more sensitive than fluoroscopy in the diagnostic process.Reference Houston, Fleet, Cowan and McMillan12 Furthermore, there is widespread availability of ultrasonography in most units, which helps to explain why it remains one of the most frequently used imaging modalities for PNI.Reference Zambon, Greco, Bocchino, Cabrini, Beccaria and Zangrillo13
Phrenic nerve stimulation is the gold-standard imaging modality for the diagnosis of PNI and diaphragmatic weakness. Stimulation of the phrenic nerve results in negative pressure generated by the diaphragm, which can be measured using the transdiaphragmatic pressure.Reference Dubé and Dres11 Transcutaneous phrenic nerve stimulation is performed at the level of the neck on the affected side, producing an involuntary contraction of the diaphragm. However, some patients may find this uncomfortable, and it may prove to be technically difficult to perform in patients with anatomical variations.Reference Dubé and Dres11
Fluoroscopy is another diagnostic investigation that may be utilised for the diagnosis of PNI. It is an imaging modality that uses X-rays to allow for instantaneous assessment of internal structures. X-ray beams are constantly discharged and displayed on a screen and a real-time, dynamic image is produced.14 Linhart et al evaluated whether fluoroscopy of spontaneous breathing was more sensitive than phrenic nerve stimulation for detecting PNI during cryoablation for paroxysmal AF.Reference Linhart, Nielson and Andrié15 Out of 133 patients undergoing cryoablation who were either monitored with fluoroscopy or phrenic nerve stimulation, the study found that all cases of phrenic nerve palsy were detected by fluoroscopy before phrenic nerve stimulation, thereby deeming fluoroscopy to be more sensitive.Reference Linhart, Nielson and Andrié15 Its use in this regard may potentially be extended to patients suspected of having PNI post-cardiac surgery.
There has also been the development of diaphragmatic electromyographic monitoring modalities to allow for the prediction of PNI. Lakhani et alReference Lakhani, Saiful, Parikh, Goyal, Bekheit and Kowalski16 evaluated whether the use of a modified lead I and recordings of diaphragmatic compound action potentials would predict PNI. It was found that patients who suffered PNI were found to have a significant decrease in CMAP when compared to those who did not suffer PNI (from 0.33 mV ± 0.14 mV to 0.09 mV ± 0.05 mV). The authors, therefore, concluded that CMAP recording is a reliable method of assessing and predicting PNI.Reference Lakhani, Saiful, Parikh, Goyal, Bekheit and Kowalski16
Prevention and management
Successful prevention of PNI requires an in-depth understanding of the anatomy of this important structure and the aspects of the surgical procedure that cause injury to the PN. This allows the surgeon to modify techniques such as using cold liquid saline rather than ice slush, and the use of insulation pads to protect the PN from ice slush, thereby preventing hypothermic injury to the PN. Careful identification of the PN prior to opening up the pericardium and ensuring the opening itself and the use of cautery is kept at a distance from the PN also aid in preventing mechanical and thermal injury. Direct mechanical trauma and indirect injury due to the stretching of the PN from the use of sternal retractors have been mentioned, but preventing this is challenging due to the essential nature of these instruments.Reference Aguirre, Sinha, Zimmet, Lee, Kwa and Rosenfeldt20
Management of PNI in children post-CHD surgery can either be conservative with prolonged ventilatory support or invasively in the form of diaphragmatic plication surgery, with the principal aim of preserving respiratory function.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 For patients with incidental asymptomatic post-operative PNI, supportive treatment with muscle strengthening training and physiotherapy input is the mainstay. These patients benefit from regular follow-up to identify any deterioration in clinical status and referral for surgical treatment early. For symptomatic patients, supportive ventilation may comprise a 4–6-week trial of continuous positive airway pressure during which injury to the phrenic nerve may spontaneously recover.Reference Haller, Pickard and Tepas17 However, while this approach may avoid subjecting the patient to surgical risk, there are several disadvantages. First, spontaneous recovery is often unpredictable – studies have shown recovery time to range widely from between 5 days and 12 months – and in 16% of cases, recovery never occurs.Reference Watanabe, Trusler, Williams, Edmonds, Coles and Hosokawa18,Reference Iverson, Mittal, Dugan and Samson19 Aguirre et al also highlight the significant cost that arises from keeping a patient in an ICU bed on supportive ventilation for prolonged periods, which can be up to $3000 (AUD) per day.Reference Aguirre, Sinha, Zimmet, Lee, Kwa and Rosenfeldt20
Meanwhile, diaphragmatic plication surgery aims to flatten the dome of the diaphragm to provide the lungs with greater volume for expansion.Reference Kaufman21 and is especially effective in unilateral diaphragmatic paralysis.Reference Lemmer, Stiller and Heise22 This is thought to be because the downward movement of the healthy side of the diaphragm during inspiration produces negative intrathoracic pressure, and the abdominal contents are drawn into the paralysed side of the thorax due to the paradoxical upward movement of the paralysed side. Plication corrects this paradoxical movement, allowing lung expansion on the affected side, thereby improving respiratory kinetics for gas exchange.Reference Schwartz and Filler23,Reference Ciccolella, Daly and Celli24 The diaphragm can be approached for plication from either the thorax or the abdomen via open or minimally invasive surgery.Reference Aguirre, Sinha, Zimmet, Lee, Kwa and Rosenfeldt20 If diaphragmatic paralysis is bilateral then a transverse upper abdominal incision is used, however, if it is unilateral, then a transthoracic repair through a posterolateral thoracotomy via the seventh intercostal space is preferred. In addition, there are two main plication techniques. One involves passing sutures through the diaphragm three or four times, tying them together, and repeating this across the diagram. The other involves using Babcock forceps to hold up the diaphragm, then placing a row of interrupted sutures at the base and folding down the held-up section, which is then sutured a second time to create three overlapping layers in the thinned out central portion of the diaphragm. Both methods create a tense and firm diaphragm, eliminating paradoxical movement (Fig 2).Reference Talwar, Agarwala, Mittal, Choudhary and Airan4
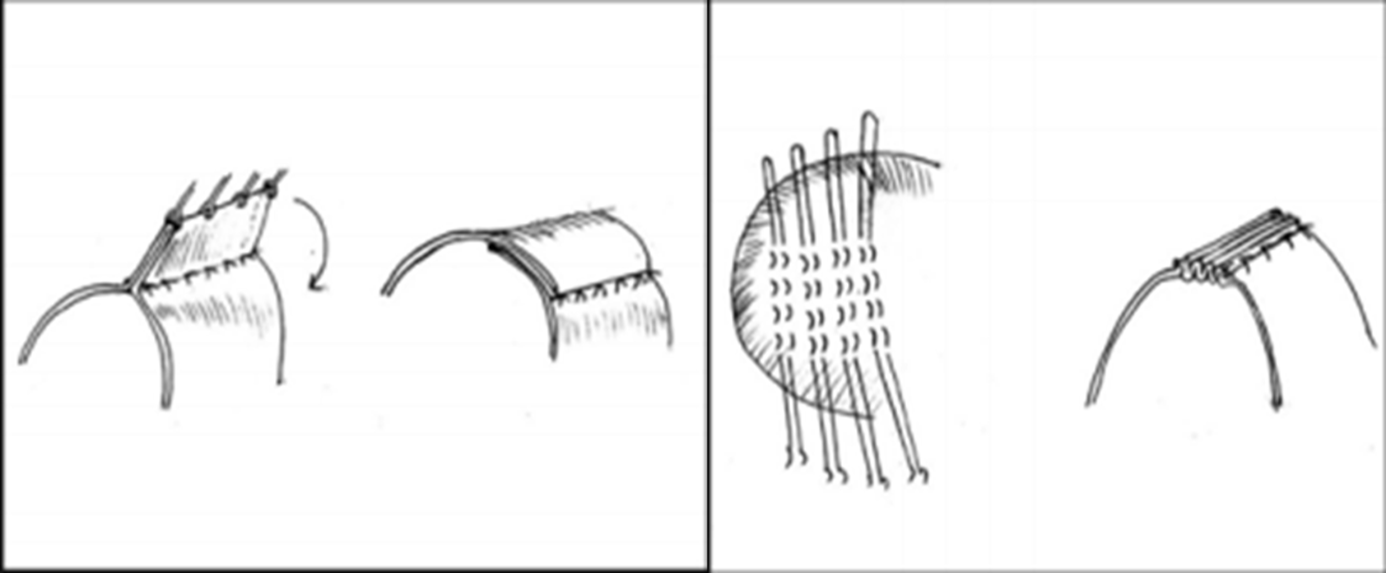
Figure 2. Techniques one and two, respectively, for diaphragmatic plication.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4
While supportive ventilation was once the favoured treatment option for PNI post-CHD surgery, diaphragmatic plication is now the most widely accepted treatment, especially in children under the age of 1.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 In patients with confirmed diaphragmatic paralysis and respiratory insufficiency, diaphragmatic plication has been shown to be the best option to wean patients from mechanical ventilation, reduce the risk of pulmonary infections, and shorten hospital stay.Reference Joho-Arreola, Bauersfeld, Stauffer, Baenziger and Bernet5,Reference Georgiev, Konstantinov, Latcheva, Mitev, Mitev and Lazarov9,Reference Lemmer, Stiller and Heise22,Reference Tönz, von Segesser, Mihaljevic, Arbenz, Stauffer and Turina25,Reference Yellin, Lieberman and Barzilay26 Moreover, Kizilcan et alReference Kizilcan, Tanyel, Hiçsönmez and Büyükpamukçu27 found normal placement of the plicated diaphragm in a 1–11-year follow-up in 12 patients after plication surgery and satisfactory diaphragm function in 9 of the 12.Reference Kizilcan, Tanyel, Hiçsönmez and Büyükpamukçu27 There is, however, conflicting advice in the literature regarding the optimal timing of plication surgery – some studies recommending it to be undertaken as soon as PNI is diagnosed,Reference Stauffer and Rickham28 while others propose a 1–6-week period of supportive ventilation to allow the possibility of spontaneous recovery before performing surgery.Reference Lakhani, Saiful, Parikh, Goyal, Bekheit and Kowalski16,Reference Watanabe, Trusler, Williams, Edmonds, Coles and Hosokawa18,Reference Schwartz and Filler23,Reference Tönz, von Segesser, Mihaljevic, Arbenz, Stauffer and Turina25,Reference Serraf, Planche, Lacour Gayet, Bruniaux, Nottin and Binet29,Reference Mickell, Oh, Siewers, Galvis, Fricker and Mathews30,Reference Hamilton, Tocewicz, Elliott, de Leval and Stark31 Nonetheless, if the diaphragm atrophies then undertaking plication too late can impair outcomes. Therefore, a decision for early surgical intervention must be made on paediatric patients where surgery would be most beneficial. Key indications for undertaking plication surgery include the respiratory status of the patientReference de Leeuw, Williams, Freedom, Williams, Shemie and McCrindle3 and age, as older children are more likely to spontaneously recover with supportive ventilation due to their lower reliance on diaphragmatic breathing.Reference Tsakiridis, Visouli and Zarogoulidis32 Thus, children who are able to breathe spontaneously without the need for oxygen supply or clinical signs of respiratory insufficiency may not require plication, whereas newborns or infants without pulmonary risk factors who cannot be weaned from mechanical ventilation should be considered for plication surgery urgently after the diagnosis is confirmed.Reference Lemmer, Stiller and Heise22 Furthermore, in exceptional circumstances such as children with univentricular physiology, the risks of performing a thoracic procedure are significantly lower than leaving the patient intubated for several weeks with higher-than-normal pulmonary resistance.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 A full list of indications described in the literature is shown in Table 1.
Table 1. Indications for diaphragmatic plication for PNI post-CHD surgeryReference Talwar, Agarwala, Mittal, Choudhary and Airan4,Reference Aguirre, Sinha, Zimmet, Lee, Kwa and Rosenfeldt20

The use of minimally invasive surgical techniques for diaphragmatic plication adults has been described in several studies.Reference Tsakiridis, Visouli and Zarogoulidis32,Reference Stamenovic33 However, literature detailing their use in children is limited.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 The benefits of using such techniques are well documentedReference Lewis, Caccavale, Sisler and Mackenzie34,Reference Mack, Aronoff, Acuff, Douthit, Bowman and Ryan35 and include reduced pain, improved cosmetic results, pulmonary function, and lower morbidity. In a review of video-assisted diaphragmatic plication in children, Hines et al describe that when plication is performed through a regular thoracotomy, the beneficial effects are delayed due to loss of pulmonary function associated with the pain of the thoracotomy, which is less so the case with minimally invasive surgery – as exemplified by the fact that two paediatric patients were discharged within just 24 hours of surgery.Reference Hines36 Though experience with using these techniques in children is limited, the demonstrated benefits suggest that their use to manage PNI post-CHD surgery will inevitably increase in the future.
Limitations
Current literature suggests that PNI is often an incidental finding in patients post-CHD surgery.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 Further research may be required to identify methods that help to detect PNI in the early stages after CHD surgery. Although routine follow-up chest X-ray has a high sensitivity for detecting incidental PNI’s, it only has a positive predictive value of 33%.Reference Dubé and Dres11 This highlights the need for further research to explore whether more accurate diagnostic modalities such as ultrasonography and pulmonary function tests should be used routinely after CHD surgery.
There is also limited data on the quality of life of patients after PNI; current literature has little evaluation of life expectancy or recovery (Table 1). Health-related quality of life in children is regarded as a complex, multidimensional, subjective concept that includes various aspects related to the patient’s health such as social, emotional, cognitive, and physical functioning.Reference Seid, Varni and Jacobs37 It may be expected that patients with diaphragmatic palsy would have a lower quality of life compared to patients who did not suffer from this complication. This is due to the fact that PNI can result in serious respiratory distress due to weakness of the intercostal muscles, mobile mediastinum, horizontal orientation of the rib cage, and recumbent position.Reference de Leeuw, Williams, Freedom, Williams, Shemie and McCrindle3 These clinical features have the potential to cause far-reaching effects on the overall well-being of the child. To investigate this further, future studies could perhaps involve the use of HRQoL questionnaires such as the cardiac disease-specific Paediatric quality of life inventory for children less than 5 years of age.Reference Germain, Aballéa and Toumi38 This step would allow for a better understanding of the length of time that the patient has been impacted by PNI and the overall impact it has had on their life.
Furthermore, with regards to the management of PNI, diaphragmatic plication is currently the most commonly used treatment strategy.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 However, there is a lack of consensus regarding when this procedure should be performed. Therefore, further studies may help identify the optimal timing of the procedure in order to improve patient outcomes.
Minimally invasive surgical techniques are being trialled as a new technique for diaphragmatic plication in children.Reference Talwar, Agarwala, Mittal, Choudhary and Airan4 However, the lack of literature means that it is difficult to conclusively determine whether this technique is superior to current methods. Further research into this promising new technique is therefore also warranted.Reference Hines36
Conclusion
PNI is a common and serious complication of CHD surgery. It results in diaphragmatic paralysis which is of particular clinical significance given that young children are primarily diaphragmatic breathers. Therefore, the diagnosis and management of PNI in the paediatric population are of major importance. Traditionally, a conservative management approach has been favoured, whereby patients remain on mechanical ventilation whilst spontaneous phrenic nerve recovery is awaited. However, increasingly, surgical diaphragmatic plication is performed due to the uncertainty of spontaneous recovery and the costs associated with prolonged mechanical ventilation. While current literature suggests that surgical management is preferred, there is a lack of consensus regarding the optimal timing of diaphragmatic plication. Further research is needed in both ascertaining the optimal timing of surgical intervention for positive outcomes and determining the benefits of using novel minimally invasive techniques over existing interventions in children (Tables 2 and 3).
Table 2. Summary of various studies detailing outcomes of patients after phrenic nerve injury caused by CHD surgery

Table 3. Summarising advantages and disadvantages of management options

Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
No ethical approval required.








