Introduction
The ʻuaʻu, or Hawaiian Petrel Pterodroma sandwichensis, is an endangered seabird endemic to the Hawaiian Islands. It is currently known to breed on the islands of Kauaʻi, Maui, Lānaʻi, and Hawaiʻi Island, and historically bred on Oʻahu and Molokaʻi (Pyle and Pyle Reference Pyle and Pyle2017; Simons and Bailey Reference Simons, Bailey, Poole and Gill2020). Breeding habitat between islands (and even within islands) can vary dramatically, with birds breeding in old lava flows at the summit of the highest mountains in Hawaii, in holes dug in fern-covered slopes, and under tree roots and in the sides of cliff ledges in wet montane forests dominated by ʻōhiʻa Metrosideros polymorpha trees (Brandt et al. Reference Brandt, Parrish and Hodges1995; Hu et al. Reference Hu, Glidden, Lippert, Schnell, MacIvor and Meisler2001; Pyle and Pyle Reference Pyle and Pyle2017; Raine et al. Reference Raine, Driskill, Rothe and Vynne2022b; Simons Reference Simons1985; VanZandt et al. Reference VanZandt, Delparte, Hart, Duvall and Penniman2014). On Kauaʻi, the species has experienced a sharp decline in recent decades, with the most recent population trend showing a 78% decline between 1993 and 2013 (Raine et al. Reference Raine, Holmes, Travers, Cooper and Day2017), although this trend has since flattened out at a much smaller population level (Raine and Rossiter Reference Raine and Rossiter2020). The species is now predominantly restricted to remote montane ranges in the interior of the island, particularly in the north-west (Raine et al. Reference Raine, Driskill, Rothe and Vynne2022b; Troy et al. Reference Troy, Holmes, Veech, Raine and Green2017). On Lānaʻi, ʻuaʻu are now found only in the interior of the island, primarily on uluhe Dicranopteris linearis covered slopes of the Lānaʻihale (Pyle and Pyle Reference Pyle and Pyle2017; VanZandt et al. Reference VanZandt, Delparte, Hart, Duvall and Penniman2014).
Across its range, the species faces numerous threats during the breeding season, including depredation by introduced species such as cats Felis catus, black rats Rattus rattus, mongoose Herpestes javanicus, pigs Sus scrofa (Hu et al. Reference Hu, Glidden, Lippert, Schnell, MacIvor and Meisler2001; Judge et al. Reference Judge, Lippert, Misajon, Hu and Hess2012; Raine et al. Reference Raine, Driskill, Vynne, Harvey and Pias2020; Simons Reference Simons1985), and Barn Owls Tyto alba (Raine et al. Reference Raine, Vynne and Driskill2019, Reference Raine, Driskill, Vynne, Harvey and Pias2020), and burrow takeovers by feral honey bees Apis mellifera (Raine et al. Reference Raine, Driskill, Rossiter, Rothe, Pias and Sprague2023b). Powerline collisions are also a major conservation issue (Travers Reference Travers, Young and Vanderwerf2022; Travers et al. Reference Travers, Driskill, Stemen, Geelhoed, Golden and Koike2021, Reference Travers, Driskill, Scott, Hanna, Flaska and Bache2023), as well as habitat modification within breeding colonies due to invasive plants such as strawberry guava Psidium cattleianum and Himalayan ginger Hedychium gardnerianum (Raine et al. Reference Raine, Driskill, Rothe and Vynne2022b; VanZandt et al. Reference VanZandt, Delparte, Hart, Duvall and Penniman2014) and, to a lesser extent, attraction of fledglings (and in certain scenarios, adults) to artificial lights (Raine et al. Reference Raine, Holmes, Travers, Cooper and Day2017, 2024b; Reed et al. Reference Reed, Sincock and Hailman1985; Telfer et al. Reference Telfer, Sincock, Byrd and Reed1987).
The ʻuaʻu also undoubtedly faces threats at sea that, while poorly known, are important issues for similar species of seabirds worldwide and could include marine pollution (Clark et al. Reference Clark, Carneiro, Pearmain, Rouyer, Clay and Cowger2023; Derraik Reference Derraik2002; Sileo et al. Reference Sileo, Sievert, Samuel and Fefer1989), overfishing (Morra et al. Reference Morra, Chikaraishi, Gandhi, James, Rossman and Wiley2019; Wiley et al. Reference Wiley, Ostrom, Welch, Fleischer, Gandhi and Southon2013), and the effects of climate change and bycatch (Gilman et al. Reference Gilman, Kobayashi and Chaloupka2008). These marine threats are particularly concentrated in areas used by fledglings during their first winter, which span the waters off the Philippines, South Korea, and Japan (Raine et al. Reference Raine, Driskill, Raine, Rothe, Rossiter and Anderson2023a). This combination of factors has led to the ʻuaʻu being listed as “Endangered” under both the IUCN Red Data List (Birdlife International 2018) and the Endangered Species Act (USFWS 1983).
Recent genetic studies have shown strong differentiation between ʻuaʻu populations on Hawaiʻi Island and Kauaʻi, possibly due to foraging segregation (Wiley et al. Reference Wiley, Welch, Ostrom, James, Stricker and Fleischer2010). The same was also found to be true when considering morphometrics, with birds within the east Maui population (breeding in Haleakalā National Park) being larger than those on Hawaiʻi and Kauaʻi (Judge et al. Reference Judge, Hu and Bailey2014). Furthermore, ʻuaʻu also varied in vocalisations between islands, with Lānaʻi birds sounding distinctly different from Kauaʻi birds (Judge Reference Judge2011). Initial comparisons of the breeding phenology of populations of ʻuaʻu on different islands found evidence of differences between east Maui birds and other breeding populations, with birds on east Maui breeding one month earlier than those on other islands (Judge Reference Judge2011). Differences in breeding phenology were also found for different island populations of the closely related Galapagos Petrel Pterodroma phaeopygia (Friesen et al. Reference Friesen, González and Cruz-Delgado2006; Tomkins and Milne Reference Tomkins and Milne1991). Both cases highlight the importance of focusing conservation efforts at an individual island level.
This study considers data from long-term colony monitoring studies of ʻuaʻu on two islands within the species breeding range – Kauaʻi and Lānaʻi. A 12-year data set was used, compiled from monthly monitoring visits across the entire breeding season at 1,325 burrows on Kauaʻi. For Lānaʻi, a seven-year data set was used, compiled from monthly visits across the entire breeding season at 420 burrows. As well as data collected from monthly monitoring visits, data were also collected from hundreds of cameras monitoring a subset of burrows on both islands to compare the breeding phenology of these two geographically isolated populations. To date, the only complete studies of the phenology of different island populations of this species have been presented for the island of Maui (for the population breeding at high altitude in Haleakalā National Park; Simons Reference Simons1985) and Hawaiʻi (Judge Reference Judge2011). Judge (Reference Judge2011) undertook an initial comparison of the phenology between Hawaiʻi Island birds and those on other islands by considering differences in fledging dates between islands (from a small number of burrows (n = 18) from a single year on Lānaʻi and indirect data from Kauaʻi in the form of fledgling fall-out data collected by the Save Our Shearwaters rescue and rehabilitation programme). Our assessment builds upon earlier efforts to describe the phenology of ʻuaʻu by using multiple sources of monitoring data collected at a large number of burrows on two islands across multiple years. Detailed information on breeding phenology is important for directing a wide range of conservation and management actions.
Methods
Study area
On Kaua‘i, monitoring work was undertaken at six seabird management sites. These were the Upper Limahuli Preserve (a 153-ha area owned by the National Tropical Botanical Gardens) and five sites in Hono O Nā Pali Natural Area Reserve (a large 1,448-ha area owned by the State of Hawaii): Pihea, Pōhākea, North Bog, Hanakāpīʻai, and Hanakoa (Figure 1). All are located within the north-western section of Kaua‘i, at an elevation between 600 m and 1,100 m above sea level. Habitat across all of these sites consists of intact wet montane forest, criss-crossed with deep drainages, narrow ridgelines, and steep valley walls, and dominated by native species such as ‘ōhi‘a (Metrosideros polymorpha), lapalapa (Cheirodendron platyphyllum), and tree ferns (Cibotium spp.) in the canopy and large patches of uluhe fern (both Dicranopteris linearis and D. pinnatum) in the understorey. All sites had active predator control operations in place, which reduced the chances of monitoring work in the colonies affecting depredation risk for birds breeding in the area. Monitoring at four sites started in 2012 and has continued to the present, while monitoring at Hanakāpīʻai and Hanakoa began in 2015.
Table 1. Key dates in breeding phenology for ʻuaʻu on Kauaʻi and Lānaʻi
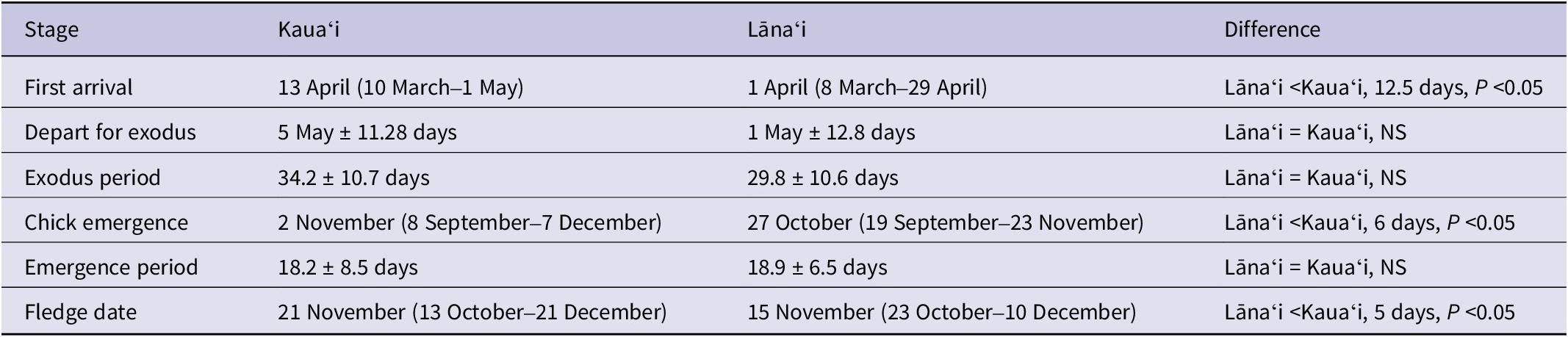
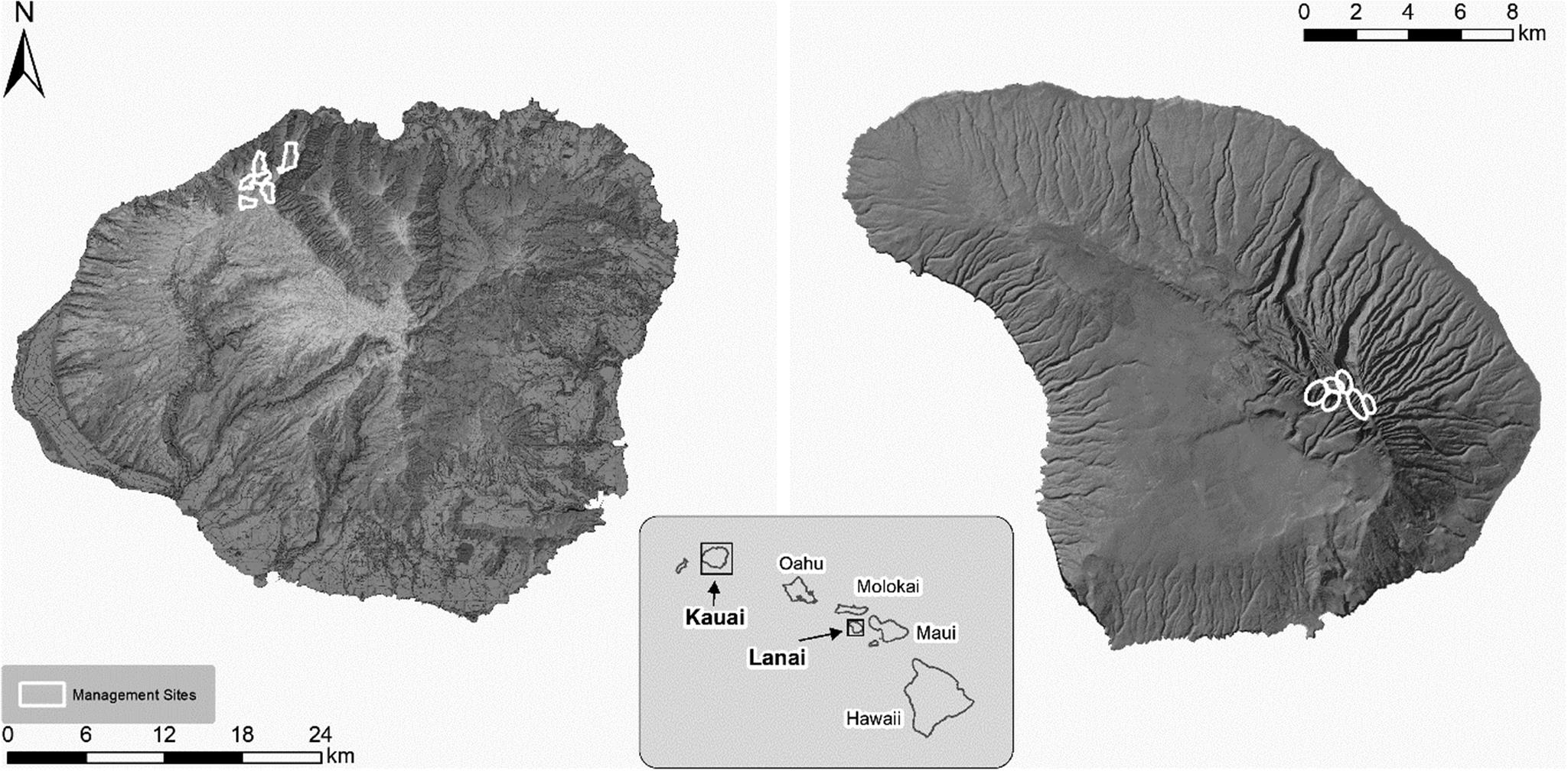
Figure 1. Location of study sites on the islands of Kaua‘i and Lānaʻi.
On Lānaʻi, colonies were monitored at multiple colonies, with the majority of data coming from three main sites: Hi‘i, North Hauola, and East Hauola (Figure 1). All are located on either side of the Lāna‘ihale to the east of Lānaʻi City, at an elevation between 650 m and 1,010 m above sea level. Habitat across all these sites consists of large patches of uluhe fern on steep slopes, interspersed with deep drainages and narrow ridgelines. Unlike the Kauaʻi sites, canopy was sparser with smaller areas of ‘ōhi‘a and other native trees as well as monoculture patches of the invasive strawberry guava. As with Kauaʻi, all sites had active predator control operations in place, which reduced the chances of monitoring work affecting depredation risk for birds breeding in the area. Monitoring on Lānaʻi started in 2015 and has continued to the present.
Monitoring
Seabird monitoring was undertaken at management sites using a combination of near-monthly burrow checks and motion-triggered cameras throughout the breeding season. Burrows were located through a combination of nocturnal auditory surveys and dedicated ground searching. Auditory surveys were conducted for two hours after dark and 1.5 hours starting two hours before dawn (with the two hours after dark incorporating the peak vocal activity for ʻuaʻu). Maps were produced from auditory surveys with activity polygons and the locations of concentrated ground calling, which helped focus diurnal ground searching efforts. All burrows located within each colony were marked with a unique identification tag (coloured and numbered cattle tags) and their locations were recorded using a hand-held GPS (Garmin Rino530HCx or Garmin Rino650). All burrows were then incorporated into the monitoring programme if they could be safely accessed on a regular basis.
Burrow checks started in mid-February before birds arrived to deploy cameras and continued near-monthly until December to recover monitoring equipment. If chicks were still in burrows during December, cameras were left in place until the following year to obtain data on late fledging events. Site access was either by foot (Pihea and all Lānaʻi sites) or helicopter (all other sites on Kauaʻi). During burrow checks, each burrow was inspected to assess breeding status. For deep burrows where direct visual inspection was not possible, a hand-held camera (Panasonic Lumix or Olympus Tough Stylus TG4/TG5/TG6) was used to take photographs into the back of the burrow to assess burrow contents. On Kauaʻi, a total of 1,325 unique ʻuaʻu burrows were monitored during the study period (2012–2023), with the largest number at Hanakāpīʻai (n = 451), North Bog (n = 322), and Hanakoa (n = 223). The breeding population across all six Kauaʻi management sites is estimated to be 2,491–4,237 breeding pairs (Raine et al. Reference Raine, Driskill and Rothe2024a). On Lānaʻi, a total of 420 unique ʻuaʻu burrows were monitored during the study period (2015–2022).
Motion-triggered cameras provided information about burrow activity at a higher temporal resolution compared with burrow checks. A subset of 30 or more burrows were monitored at each colony (2012–2023 on Kauaʻi and 2016–2022 on Lānaʻi) by cameras (mainly Reconyx Hyperfire PC900 and HP2X, although a small number of Reconyx Ultrafire XP9 were also used). Cameras were mounted on poles located 0.9–1.5 m away from the burrow entrance, with the camera pointed directly at the burrow mouth to catch all activity (both seabird and predator) at the burrow.
Burrows with a good field of view and only one entrance were preferentially chosen. Cameras were installed prior to the arrival of seabirds and removed at the end of the season after all chicks had fledged. Burrow activity monitoring for this paper was conducted only at burrows that had confirmed established breeders (burrows occupied by non-breeders, for example, were excluded). Birds were only considered to be established breeders if they were confirmed to have laid an egg at least once during their monitoring history. If burrows failed during the season, or the status of the birds using the burrow was uncertain, data collected from these burrows were removed from further consideration. If it was possible that burrow activity was missed by the camera (i.e. the camera was poorly positioned on a burrow and thus could have missed bird activity, the camera malfunctioned, or there were multiple entrances), then the data were excluded from further analysis.
Lastly, we include in this paper data collected from a small number (n = 4) of geolocators (Intigeo C65-SUPER light level recorder, Migrate Technology) deployed on breeding ʻuaʻu from Upper Limahuli (Kauaʻi) in 2022 and recovered in 2023. While these geolocators were deployed for a different study to identify wintering grounds, these devices provided important data on exodus periods and incubation shifts by two pairs of birds that we deemed useful for inclusion in this paper. Geolocators were attached to an individually numbered metal band on each bird using a cable tie and removed upon recapture. Tags weighed <1 g, meaning they were well below the recommended 3% tag/body weight threshold (Phillips et al. Reference Phillips, Xavier and Croxall2003), and in all cases birds returned from wintering grounds and successfully raised a chick in both years, indicating that the tags did not affect their survival or reproductive potential. Each bird was also sexed by taking a small blood sample from the tarsus during geolocator attachment which was then analysed at the Bishop Museum (Honolulu, Hawaii, USA).
Data analysis
Cameras
All photographs taken by burrow cameras were individually viewed and digitally coded once teams were back in the office. When reviewing photographs from both islands, we assessed burrow activity by cataloguing the date and time of (1) adult arrivals at the burrow entrance, (2) adult departures from the burrow, (3) adult burrow maintenance activity, (4) chick emergence (where the chick emerged from its burrow for the first time), (5) chick exercising bouts, and (6) chick fledging. Adults and chicks were differentiated by the presence of down, the state of the feathers if no down was visible (adults have clearly worn and sun-bleached feathers, whereas chicks have “clean” dark feathers), and behaviour (chicks engaged in prolonged exercise bouts and spent a lot of time exploring their surroundings whilst adults were focused on returning to feed their chick and then immediately departing). Arrival, departure, emergence, and fledging events were only included if the viewer was confident that the bird was actually undertaking the specified behaviour – if the behaviour was not clear, then it was not included in the analysis. Arrivals were considered valid if it was the first sighting of a bird on camera at night going directly into its burrow.
For first arrival date at a burrow in a given year, data were only considered if a camera was in position in front of the burrow before the 68th Julian day of the year (which corresponds to the earliest recorded first arrival ever recorded at either island), per this study. Cameras deployed after this date were excluded, as they could have missed the first arrival. The resulting data set was assessed for outliers according to inter-quartile range, with the upper quartile (late arrivals) being manually investigated for signs of camera trigger malfunction. All data were inspected to ensure continuous functioning of the camera according to battery life, as well as appropriate camera placement, and the absence of multiple entrances that could obfuscate an arrival. Departures were considered valid if an adult was sighted leaving its burrow and walking out of the camera’s field of view and never returning for the rest of the night. After 2018, due to the time-consuming nature of digitising large volumes of camera images, data collected on seabird activity were restricted to the following: (1) date and time of first arrival at the burrow by an adult for that year; (2) chick emergence date and time; (3) chick fledging date and time.
The number of cameras and the duration and seasonal timing of burrow monitoring varied within and across seasons, depending on research and management priorities, which impacted the sample size for analyses presented in this paper. Analyses that required uninterrupted camera monitoring thus have reduced sample sizes. We used changes in adult bird activity at the burrow to determine the transition dates between different phases of breeding (e.g. end of exodus and the start of egg laying/incubation). We cross-referenced the camera data results with data collected by staff conducting burrow checks.
As we had more comprehensive digitised data for cameras on Kauaʻi, we conducted additional analysis on this data set following our previous analysis of another endangered seabird on Kauaʻi – the ʻaʻo or Newell’s Shearwater Puffinus newelli (Raine et al. Reference Raine, Driskill, Rothe and Vynne2022b). Annual total visitation rates of adult breeding birds at ʻuaʻu burrows were also considered (available data from 2014–2017 only). For this analysis, only burrows that fulfilled all the following criteria were used: (1) cameras were in place before first arrival and left in place for the whole season; (2) cameras were positioned in such a way that all arrivals and departures were reliably caught on camera; (3) the burrow had confirmed breeding; (4) breeding was successful. Visitation rates were then calculated as total visits per burrow per day of active camera recordings.
Lastly, breeding probability in any given year was calculated for burrows of confirmed breeding pairs, with key caveats. These caveats were as follows: (1) the burrow had to belong to a confirmed breeding pair; (2) there had to be at least two consecutive years of monitoring at the burrow after breeding had been confirmed; (3) both adults were still presumed to be alive at the end of the prior breeding season (i.e. there was no confirmed depredation event of an adult at the burrow that year, the chick fledged as expected, and for burrows with cameras, adult activity patterns suggested that both adults were visiting the burrow and feeding the chick up to fledging). Breeding probability was then calculated for this subset of birds as the number of years with a confirmed breeding attempt divided by the number of years the burrow was monitored.
Geolocators
To establish the duration of exodus periods and incubation shifts by individual birds of known sex, we analysed the light levels recorded from geolocators deployed on two breeding pairs on Kauaʻi in 2022 and recovered in 2023. Geolocators record ambient light levels. While the birds were in their burrows, geolocators recorded the same light levels during the day as they recorded at night. This is in contrast to the typical light level patterns recorded when a bird was at sea, which showed expected diurnal patterns (i.e. an increase in light starting at sunrise, relatively small changes throughout the day, and a decrease in light level at sunset to a flat low level all night). By cross-referencing these periods with patterns of burrow activity (arrivals and departures of birds) on burrow cameras, these low-light, multi-day periods were confirmed to be reflective of time spent by each bird inside its burrow and could thus be used to calculate exodus periods and incubation shifts for known individuals.
All statistics were carried out in R statistical software version 3.6.1 (R Core Team 2024). Means are presented with standard deviation. For comparisons between islands, a series of linear mixed-effects or fixed-effects models were fitted with year and burrow as predictors to control for any potentially confounding effect of either of these variables. In each case linear model assumptions were assessed for normality and homoscedasticity of residuals. Where necessary, a transformation was applied to the independent variable in question.
Results
Throughout the breeding season there were clear fluctuations in adult activity at burrows with key inflection points evident throughout the season (Figure 2). Below we describe the activity patterns evident for both Kauaʻi and Lānaʻi in each breeding stage from arrival, through pre-lay exodus, incubation, chick rearing, chick emergence, and fledging. Due to the asynchronous breeding season that we describe for this species in this study, it is important to note that breeding phase and total season length can vary greatly at the scale of the colony level compared with that of individual burrows. We therefore present results at both the colony and individual burrow scale.
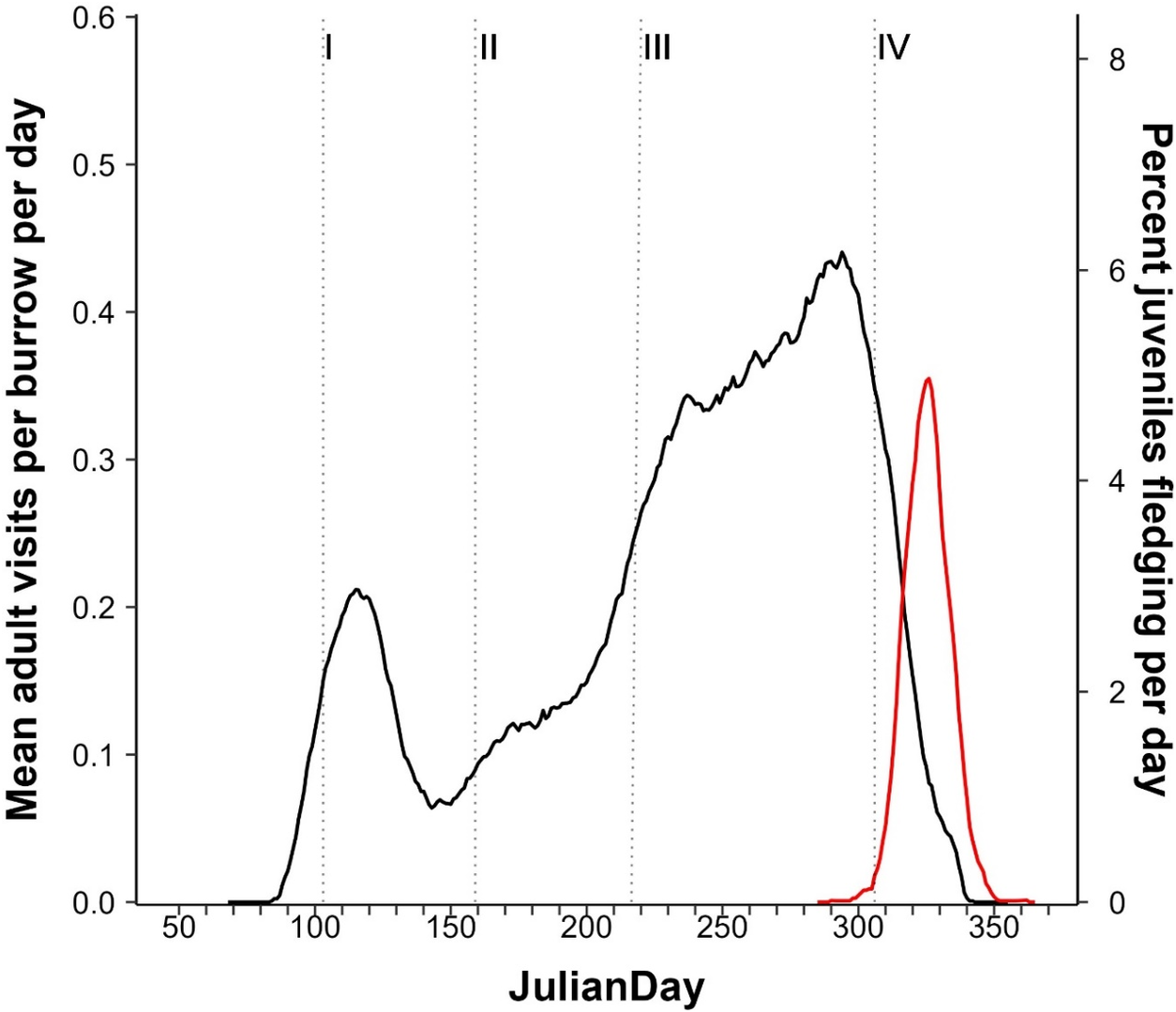
Figure 2. Mean visits per burrow (black line) per day at the colony level (all confirmed breeding pairs monitored by cameras between 2014 and 2017, Kauaʻi only). Movement patterns represent the overall movement into and out of the colony of breeding birds across all burrows combined, which incorporates the asynchronous breeding patterns of individual pairs. The percentage of juvenile fledglings on Kauaʻi (red line) is also presented by day of year. Dotted lines represent the averages for critical stages of the phenology, as follows: I = first arrival; II = return from pre-lay exodus, commencement of incubation; III = hatching, start of chick rearing; IV = first emergence of chicks.
Arrival
On Kauaʻi, the first arrival of adult ʻuaʻu at their burrows was on average 13 April (n = 482, earliest 10 March, latest 1 May – a difference of 53 days between the earliest and latest first arrival across all years). Eighty-five per cent of arrivals occurred within a two-week period centred on the mean, and 97.5% of arrivals occurred within a one-month period centred on the mean. At the beginning of the season, activity around burrows covered a period of several days, during which time birds were recorded on camera cleaning the entrances of dead leaves and twigs, as well as pushing out old nesting material or debris from within burrow chambers and sometimes dragging in fresh vegetative material (such as uluhe fern and ʻōhelo ʻai Vaccinium reticulatum). During this period, mean burrow visitation rates were 0.43 ± 0.18 visits per night with this breeding phase lasting a mean of 19.3 ± 6.78 days (see Figure 3).
Figure 3. Mean visits per day at the burrow level. Movement patterns represent movement into and out of individual burrows by breeding pairs, which excludes the effects of asynchronous movement patterns evident in Figure 2 at the colony level. I = arrival to pre-lay exodus; II = pre-lay exodus; III = incubation; IV = chick rearing. Width of bar is indicative of length of each breeding phase.
On Lānaʻi, the first arrival of ʻuaʻu adults at burrows was on average 1 April (n = 570, earliest 8 March, latest 29 April – also a difference of 53 days between the earliest and latest first arrival across all years). Here 81.9% of arrivals occurred within a two-week period centred on the mean, and 97.2% of arrivals occurred within a one-month period centred on the mean. Comparing Kauaʻi and Lānaʻi for all years in which data existed for both islands, there was a significant difference between the two islands with adults on Lānaʻi arriving on average 12.6 days earlier (linear mixed-effects regression model, est.: 12.63; 95% CI: 13.61, -11.65, P <0.0001).
We also considered whether there was any relationship between first arrival and moon phase. First arrival date at the burrow was not influenced by moon illumination (R = -0.1, P = 0.12). Due to the birds’ asynchronous arrival, first arrivals were spread relatively equally across moon phase. Moon illumination at first day of arrival varied annually from zero or near zero to full moon, with annual mean first arrival varying around half-moon illumination (e.g. 2020 mean illumination = 49 ± 35%, min. = 1, max. = 100).
Pre-lay exodus
On Kauaʻi, after arrival and the following short period of burrow preparation, adults departed on an extended pre-lay exodus. The average day that birds left on exodus was 5 May ± 11.3 days and the average exodus period (taken as the amount of time that both birds were away from the burrow) was 34.2 ± 10.7 days (min. = 16, max. = 49). Mean visitation rates across this period approached zero (mean burrow visitation rates = 0.02 ± 0.02 visits per day, see Figure 3). On Lānaʻi, the average day that birds left on exodus was 1 May ± 12.8 days and the average exodus period (taken as the amount of time that both birds were away from the burrow) was 29.8 ± 10.6 days (min. = 11, max. = 48). There was no significant difference between the islands for exodus duration (linear mixed-effects regression model, est.: 4.94; 95% CI: 10.48, 0.54, P >0.05). Geolocator data provided detailed information on the exodus period for two pairs of ʻuaʻu on Kauaʻi in 2023. For the first pair, exodus duration was 48 days for the male and 49 days for the female, while for the second it was 52 days for the male and 53 days for the female. In both burrows, the male of the pair left the burrow a day before the female for exodus, but both members of each pair arrived back from exodus on the same day.
Incubation
Most of the data available to assess incubation came from the larger Kauaʻi data set. Kauaʻi birds returned to their burrows after pre-lay exodus on 8 June ± 10.4 days (earliest 8 May, latest 25 June), while on Lānaʻi birds returned on 31 May ± 12.9 days (earliest 26 April, latest 28 June). As females are assumed to lay their eggs within a day of returning from exodus (Harrison Reference Harrison1990; see also information from burrow camera detailed below), then this would be the average egg lay date as well. At a subset of burrows on Kauaʻi where the burrow was shallow enough to see an egg being incubated (n = 68), the earliest a bird was confirmed incubating an egg was 7 June and the latest 7 August. For Lānaʻi, of all data where a bird was confirmed on an egg (n = 56), the average date for recording a bird incubating an egg was 21 June with the earliest being 26 May and the latest 18 August.
In 2023 a camera (set to record video) was set up on Kauaʻi on a ʻuaʻu burrow at the Pihea management site. It was positioned so that it was looking directly into the burrow chamber of a ʻuaʻu pair and captured the moment that the egg was laid. For this burrow, the female arrived at the burrow at 20h36 on 12 June. Upon arrival, the female spent over half an hour cleaning and reorganising the nest cup before laying the egg between 21h09 and 21h24. After laying the egg, the female commenced incubation. The male arrived two days later on 14 June at 20h50 and took over incubation from the female three minutes later. The female departed the burrow shortly thereafter at 21h06.
Given the difficulty of determining incubation periods from camera activity, we examined incubation length at two scales, i.e. changes in colony mean activity and individual burrow activity, to estimate incubation duration with all available data. At the colony scale (Figure 2), adult activity patterns on cameras indicated that the mean laying date and mean start of hatch (see below) were 58 days apart. At the individual burrow scale, mean burrow visitation rates were 0.19 ± 0.09 visits per day during the full incubation period, and (as detected by changes in activity outside the burrow) lasted an estimated 56.7 ± 2.1 days (see Figure 3), before increasing activity was detected (in terms of burrow arrivals and departures), indicating hatch and the end of incubation.
Geolocator data provided detailed information on the incubation shifts for two pairs of ʻuaʻu on Kauaʻi in 2023. For the first pair, the egg was laid on 27 June (assuming the first day back in the burrow by the female after exodus was the lay date). The male then carried out the first incubation shift for 16 days, was replaced by the female for 16 days, and took the third incubation shift for 17 days. The female returned for the final incubation shift of six days before the chick hatched on 20 August. The second pair followed a similar pattern. In this case the egg was laid on 13 June (following the same assumptions). The male then carried out the first incubation shift for 19 days, was replaced by the female for 16 days, took the third incubation shift for 19 days, and was replaced by the female for the final incubation shift of one day before the chick hatched on 7 August. For both pairs, the incubation period was 55 days in length.
Egg dimensions were calculated from abandoned eggs found outside ʻuaʻu nests on Kauaʻi (n = 46). Eggs were 61.5 ± 2.6 mm long and 43.6 ± 2.1 mm wide, with a length/width ratio (as a measure of roundness) of 1.4 ± 0.08.
Chick rearing
On Kauaʻi, from 13 July onwards, adult visitations at burrows increased dramatically, presumably indicating the beginning of chick hatching, with a mean hatching date of 4 August, followed by chick provisioning (see Figure 2).
The same camera which caught the egg lay in 2023 also captured the entire hatching process for the same pair. The first noise from the chick (peeping) was heard from under the adult at 15h43 on 4 August. The chick’s bill was first visible emerging from the egg 14 hours and 10 minutes later at 05h53 on 5 August and the chick fully emerged from the egg a further 2 hours and 28 minutes later at 08h21, after which the adult immediately began preening it. Throughout the hatching process (which lasted 17 hours and 38 minutes in total), the adult assisted the chick by clipping and removing sections of eggshell with its beak for at least three minutes over the duration of this period. The chick received its first meal from the female already in the burrow 3 hours and 22 minutes after hatching at 11h43, during which it was given 11 feeds over an 8-minute period, followed by a further feeding session 17h29.
Adult activity at burrows began to decrease rapidly from 21 October onwards as the fledging period approached. The last adult observation at an active burrow with a chick that fledged was on average 6 November (earliest = 24 September, latest = 24 November). The last adult observation was on average 13.5 days before the date that the chick fledged (min. = 31 days before fledging, max. = 1 day before fledging, SD = 6.25 days, n = 80). At the individual burrow scale, the chick rearing period (date of hatching to last visit by adult) lasted 94.5 ± 8.7 days (see Figure 3), with mean burrow visitation rates being 0.46 ± 0.13 visits per day during this period. Due to asynchronous breeding, at the colony level the chick rearing period extended across the colony to encompass 131 days (Figure 2).
On Lānaʻi, the last adult observation at an active burrow with a chick that fledged was on average 2 November (earliest = 19 October, latest = 24 November). The last adult observation was on average 12.7 days before the date that the chick fledged (min. = 22 days before fledging, max. = 1 day after fledging, SD = 6.3 days, n = 26). There was no significant difference between islands for the interval between last adult observation and fledge date at a burrow (linear regression model, est.: 1.44; 95% CI: 1.59, 4.48, P >0.05).
Chick emergence and fledging
On Kauaʻi, mean chick emergence was the 2 November (n = 411, earliest 8 September, latest 7 December). On Lānaʻi, chick emergence started on average on 27 October (n = 467, earliest 19 September, latest 23 November). There was a significant difference between first emergences on Lānaʻi and Kauaʻi (linear mixed-effects regression model, est.: 5.71; 95% CI: 7.43, -3.96, P <0.0001) with Lānaʻi chicks emerging on average 5.7 days earlier than those on Kauaʻi. However, there was no difference between islands for the length of the emergence period (linear mixed-effects regression model, est.: 0.60; 95% CI: 0.79, 2.00, P >0.05).
On Kauaʻi, a subset of monitored burrows was assessed for chick exercising activity (consisting of burrows in which both chick emergences and fledges were recorded, and in which the camera was functioning for the entire duration of this period). An exercise event was considered to be any amount of time that a chick was outside its burrow, and behaviours included wing flapping, stretching, exploring surroundings, etc. Chicks completed on average 43.3 ± 16.5 exercise events (min. = 23, max. = 106) between emergence and fledging, for an average total of 23.7 ± 18.4 hours (range = 4.0–1,15.3 hours) spent outside their burrows across the entire exercise period. The average amount of time a chick spent outside its burrow during exercise events was 32.9 ± 61.7 minutes (range = 6 seconds–12.1 hours).
Average fledge date on Kauaʻi was 21 November (n = 531, earliest 13 October, latest 21 December: a difference of 68 days between the earliest and latest recorded fledge date, Figure 2). Here, 73.9% of fledgings occurred within a two-week period centred on the mean (14–28 November), and 95.1% of fledgings occurred within a one-month period centred on the mean (7 November–5 December). Average fledge date on Lānaʻi was 15 November (n = 426, earliest 23 October, latest 10 December: a difference of 48 days between the earliest and latest recorded fledge date). Here, 65.5% of fledgings occurred within a two-week period centred on the mean (8–22 November), and 93.7% of fledgings occurred within a one-month period centred on the mean (1–29 November). In terms of an inter-island comparison, there was a significant difference between Lānaʻi and Kauaʻi (linear mixed-effects regression model, est.: 7.64; 95% CI: 7.43, -5.16, P <0.0001) with Lānaʻi chicks fledging on average 6.4 days earlier than those on Kauaʻi.
With respect to the timing of a fledging event, birds on Kauaʻi fledged on average 116 ± 127 minutes after sunset (median: 66.3 minutes, interquartile range [IQR]: 85.2 minutes, earliest fledge 17h41, 12 minutes before sunset, latest fledge 07h52, 838 minutes after sunset) (Figure 4). On Lānaʻi, birds fledged on average 98.7 ± 1,137 minutes after sunset (median: 58.4 minutes, IQR: 71.8 minutes, earliest fledge 17h39, 15 minutes before sunset, latest fledge 05h59, 724 minutes after sunset). A linear regression of log-transformed response (minutes after sunset) indicated no significant difference between islands when controlling for year (est: -0.12, 95% CI: 0.30, 0.04, P >0.05). Time and night of fledging were also considered in relation to moon illumination, as fallout of this species on Kaua‘i is strongly correlated to moon phase (Telfer et al. Reference Telfer, Sincock, Byrd and Reed1987), with fallout typically occurring on dark nights with no moon illumination. Despite the correlation of moon phase and fallout previously reported in the literature, moon illumination did not influence the timing or night of birds fledging with fledging equally spread across moon phases (r = -0.035, P = 0.33).
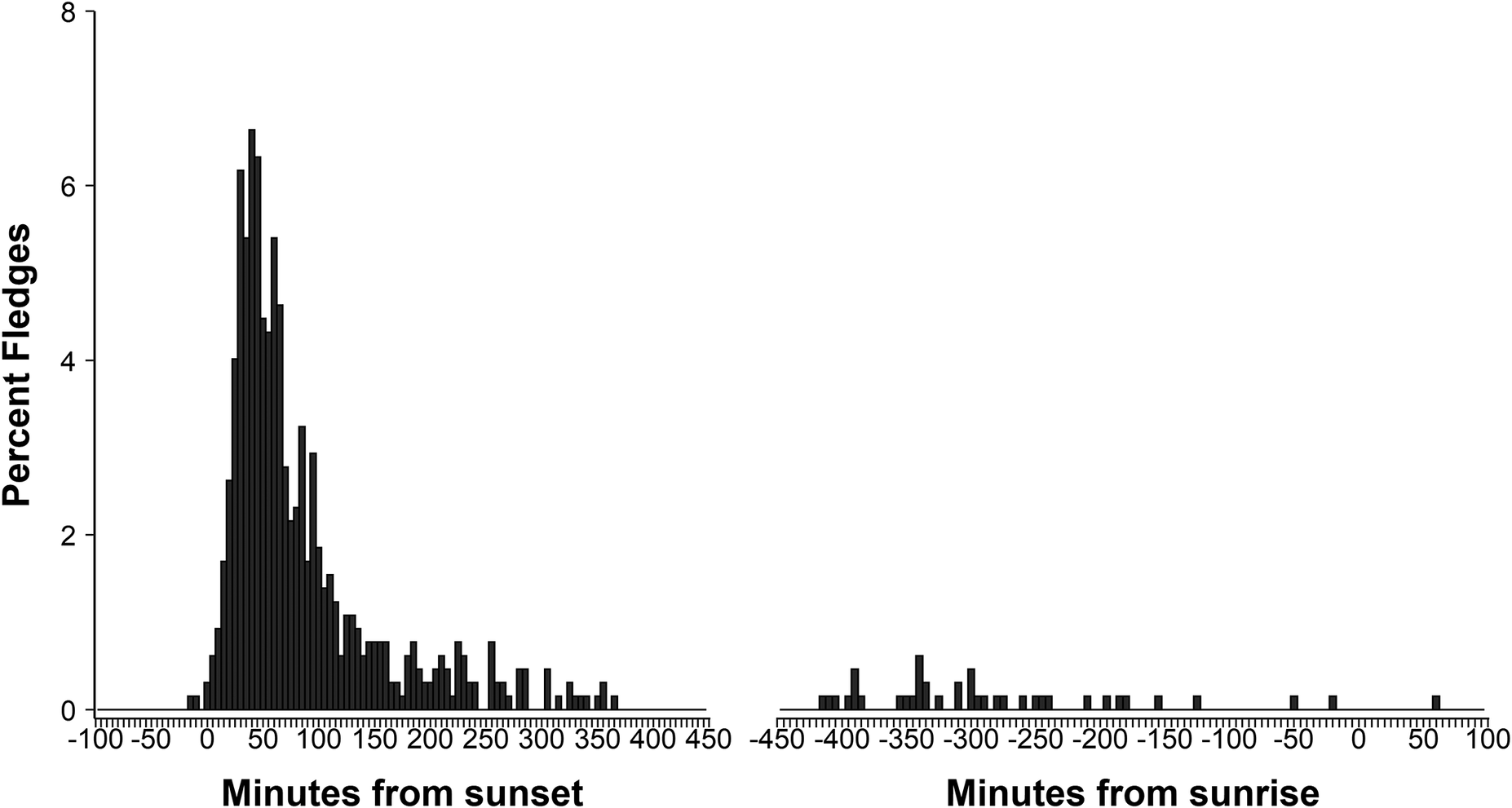
Figure 4. Timing of fledging for ʻuaʻu chicks from their burrows (on Kauaʻi and Lānaʻi combined).
Nightly temporal patterns
The nightly arrival and departure patterns of breeding adult birds were also considered across the breeding season. Overall, arrival and departure transit activity occurred throughout the night: 72% of birds arrived at the burrow prior to midnight and 55% departed the burrow after midnight (Figure 5). While birds arrived throughout the night, there was a peak in the earlier part of the night, and 50% of all arrivals occurred over a 249-minute period (85–334 minutes after sunset) with a median arrival time of 163 minutes after sunset. Departures were more evenly spread across the night than were arrivals, with small peaks in the evening and the morning.
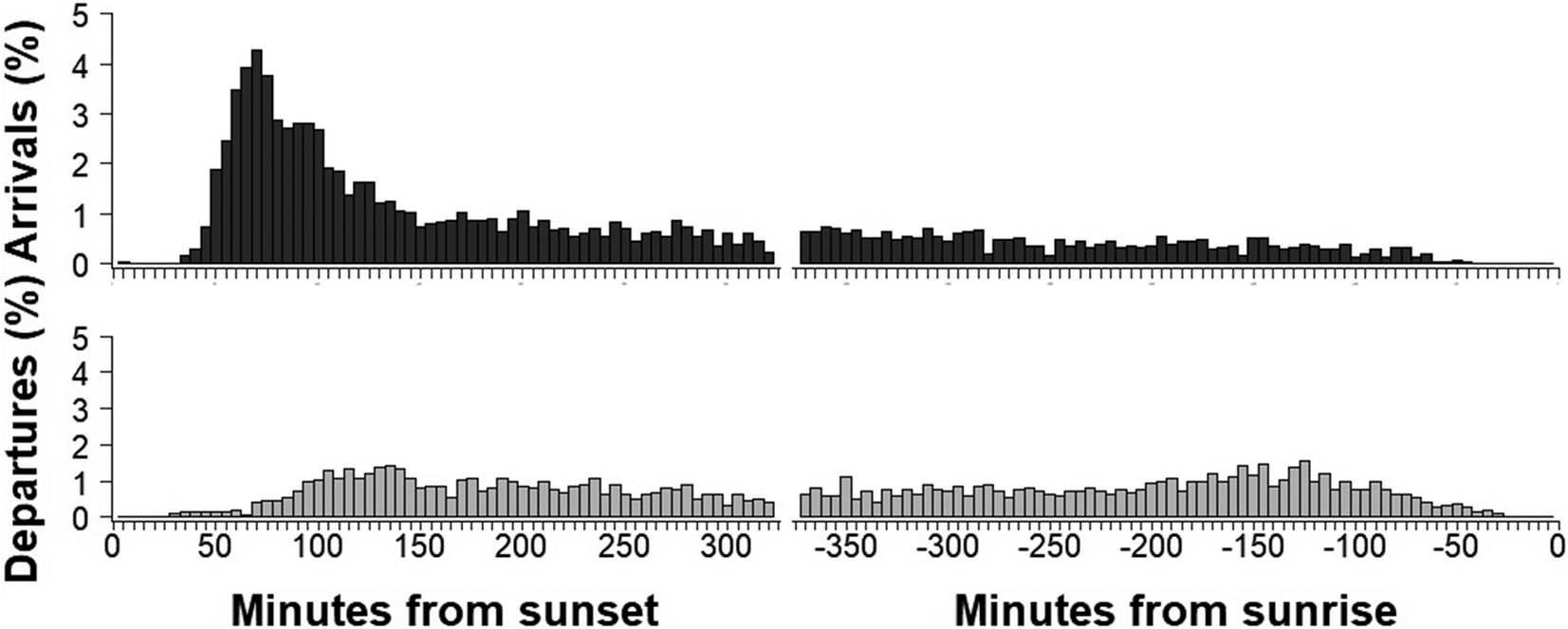
Figure 5. Nightly patterns for breeding birds arriving and departing from the burrow.
Breeding season length and visitation patterns
Considering the earliest adult arrival at a burrow for the start of the breeding season (10 March) to the last time a chick fledged (21 December), the maximum number of days that ʻuaʻu from breeding pairs are present on Kaua‘i each year is 287 days. At the individual burrow scale, the season length was 215.7 ± 13.4 days (see Figure 3), with 61.2 ± 9.5 days from first arrival to laying and 154.5 ± 11.7 days from incubation through to fledging. At the individual burrow scale, the annual adult visitation rate was 63.1 ± 15.9 visits per burrow across the whole season. For Lānaʻi, considering the earliest adult arrival at a burrow for the start of the breeding season (8 March) to the last time a chick fledged (10 December), the maximum number of days that ʻuaʻu from breeding pairs are present on Lānaʻi each year is 278 days.
Annual breeding probability
Breeding probability data were also available for a subset of breeding pairs (n = 799) on Kaua‘i that met the caveats described in the methods. The breeding probability for confirmed breeders in any given year was 0.994. The burrows with the most breeding attempts – 6+ years – never skipped a year of breeding. Of all burrows analysed, only 19 pairs skipped a year (2.3% of all pairs), with one pair skipping two years over the course of monitoring and the rest only once. In 20% of these cases, the pair skipped a year after a failed breeding attempt.
Breeding phenology for ʻuaʻu on Kaua‘i and Lāna‘i
Based on the data presented above, the breeding phenology of the ʻuaʻu on Kaua‘i is as follows (see also Figure 6, Table 1). Breeding birds arrive in the middle of April, undergo an exodus of approximately one month during May, and return to their burrows to lay their eggs in the beginning of June. Incubation lasts for 56.7 ± 2.1 days, during which time adults undertake incubation shifts that last just over two weeks at a time. Incubation continues through July with average hatch date being 4 August. The chick-rearing period ends on average two weeks before the chick fledges. Fledging starts in mid-October, peaks in mid-November, with the last birds fledging in the third week of December. On Lānaʻi, the breeding season starts two weeks earlier and ends one week earlier. Breeding birds are asynchronous, with a 68-day (Kauaʻi) and 48-day (Lānaʻi) gap between first and last fledging birds, although 95.1% (Kauaʻi) and 93.7% (Lānaʻi) of birds fledge within a one-month period centred on the mean. After the breeding season, all ʻuaʻu depart breeding sites and head to their wintering grounds.
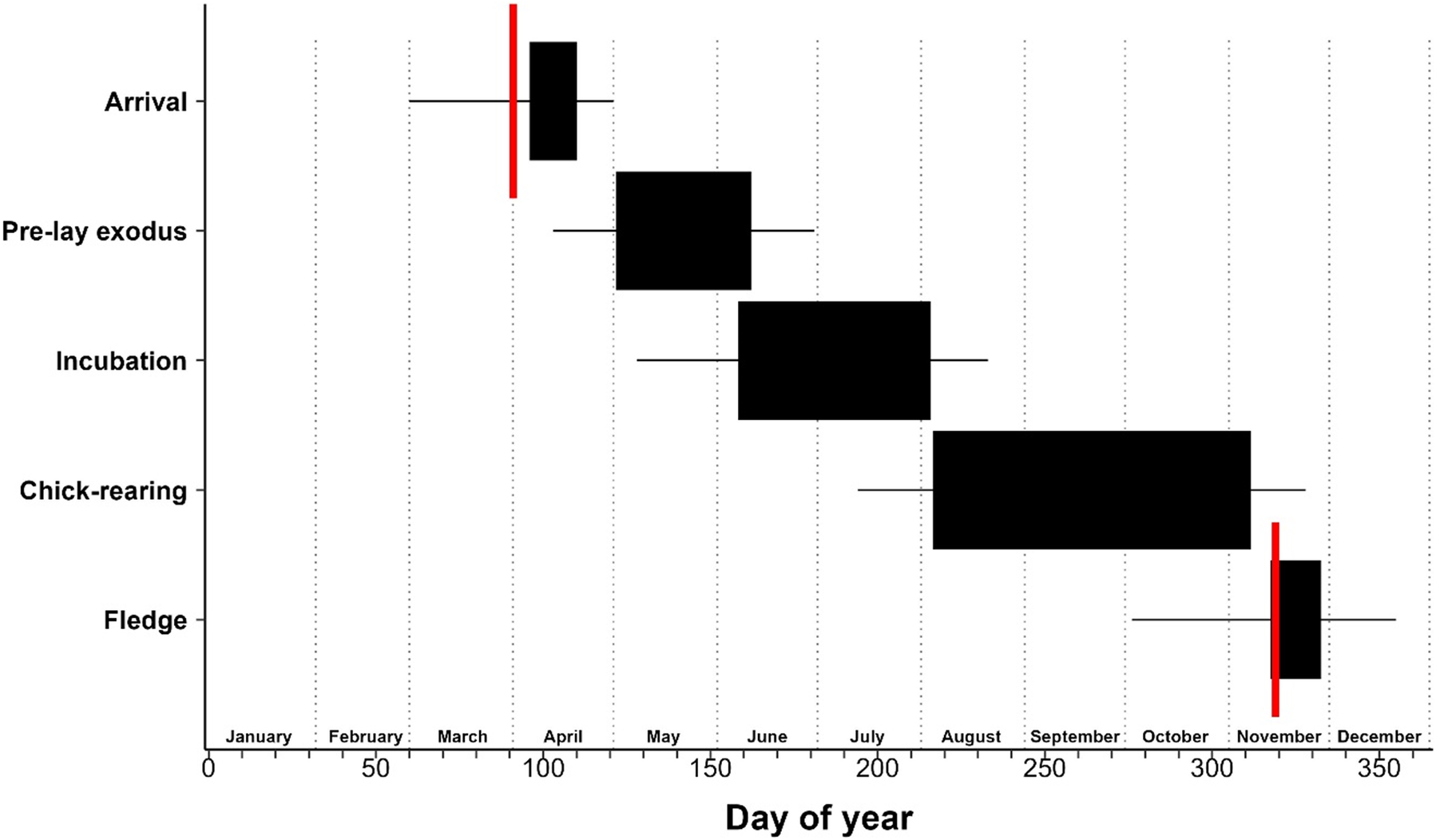
Figure 6. Breeding phenology of the ʻuaʻu on Kaua‘i (black). For arrival and fledge, solid blocks are centred on the mean and bounded by the standard deviation. For the periods of pre-lay exodus, incubation, and chick rearing, solid blocks start at the mean initiation date of that phase and the width of the bar represents the mean duration of the phase. In all cases, lines represent the minimum and maximum observed dates for each phase. Red lines indicate the mean arrival and fledge dates, respectively, for ʻuaʻu on Lānaʻi.
Discussion
This paper has provided detailed information on the breeding phenology and behaviour of two distinct breeding populations of the endangered ʻuaʻu on the islands of Kaua‘i and Lānaʻi. The ʻuaʻu has a spring–summer breeding season (like most Hawaiian seabirds), presumably due to a combination of prey availability and day length (Harrison Reference Harrison1990). This was also found to be the case for another endangered Procellariid – the ‘a‘o – on Kaua’i (Raine et al. Reference Raine, Driskill, Rothe and Vynne2022b). Breeding birds are generally present on Kauaʻi between mid-April and late November and a few weeks earlier for birds on Lānaʻi, and they have an asynchronous breeding season. Considering the very first arrival ever recorded of a breeding adult and the very last recorded fledging date, ʻuaʻu breeding pairs and their chicks can be found on Kaua‘i over a 287-day period each year, from mid-March to mid-December, while birds on Lānaʻi can be found over a 278-day period from early March to early December. Invariably, breeding pairs returned every year to breed (i.e. they did not take a year off).
Previous work did not find a significant difference in timing of breeding phenology between the two islands, but that analysis was limited to fledgling fallout data (Kauaʻi) and fledging dates from a very small sample of burrows (Lānaʻi) and did not have the extensive data sets that we had available over a decade of burrow monitoring. In general, ʻuaʻu breeding on Kauaʻi arrives 12 days later than their counterparts on Lānaʻi, and chicks fledge six days later than they do on Lānaʻi. Considering phenology data now available for this species across its entire Hawaiian breeding range, birds breeding in east Maui arrive a month earlier than those on Lānaʻi and six weeks earlier than birds on Kauaʻi. Progressing from earliest to latest arrival, ʻuaʻu arrive on east Maui, Lānaʻi, Hawaiʻi Island, and Kauaʻi in that order. These differences in the timing of breeding phenology for ʻuaʻu across the island chain presumably reflect and/or reinforce genetic differentiation between subpopulations of the species (Wiley et al. Reference Wiley, Welch, Ostrom, James, Stricker and Fleischer2010). This is also evident in other aspects of their biology and ecology, such as distinctly different vocalisations (Judge Reference Judge2011), different breeding habitats ranging from wet montane forest (Raine et al. Reference Raine, Driskill, Rothe and Vynne2022b; VanZandt et al. Reference VanZandt, Delparte, Hart, Duvall and Penniman2014) to dry volcanic plains (Simons Reference Simons1985), and even the location of foraging grounds, with each island population utilising different short-trip foraging grounds during the breeding season (Raine in prep.). Combined, all these factors contribute to the genetic isolation between the various subpopulations. These differences amplify the need for conservation management at an island level. Furthermore, these differences require careful consideration when attempting to replicate conservation actions on different islands, undertake translocations between islands, or when considering conservation needs at the species level.
Understanding breeding phenology of any species is vital to ensure that management actions are timed to key aspects of the breeding season and are tailored to the nuances between island populations. For example, predator control within colonies should be ramped up in mid-February on both islands prior to the arrival of breeding birds, allowing for the removal of predators before the birds return to their burrows in April. Adults are particularly vulnerable to predators such as cats from the second week of June through July when they are in their burrows incubating for two weeks at a time. Furthermore, the strategy employed by this species, leaving the chick alone in the burrow for multiple days at a time shortly after it hatches, and adults only being present in the burrow for short feeding periods when they return, means that chicks are particularly vulnerable to rat depredation from August onwards until fledging. This species suffers high levels of rat depredation in unmanaged colonies (Raine et al. Reference Raine, Driskill, Vynne, Harvey and Pias2020) and this life history trait helps to explain this level of vulnerability. Conversely, the lack of attendance for extended periods of time at the burrow by adults would serve to reduce adult vulnerability to cat, dog, and pig depredation during the chick-rearing stage. Interestingly, this is different from another endangered Procellariid seabird – the ‘a‘o – on which we have conducted detailed phenology assessments. In that species, adults visit the burrow every evening and stay with the chicks all night (Raine et al. Reference Raine, Driskill, Rothe and Vynne2022b). This may potentially make ‘a‘o chicks less vulnerable to rat depredation but conversely increases the risk to adults of depredation as they are sitting in their burrows throughout the night.
Unlike the ‘a‘o on Kaua‘i and many other seabird species worldwide (e.g. Chevillon et al. Reference Chevillon, Tourmetz, Dubos, Soulaimana-Mattoir, Hollinger and Pinet2022; Deppe et al. Reference Deppe, Rowley, Rowe, Shi, McArthur and Gooday2017; Raine et al. Reference Raine, Holmes, Travers, Cooper and Day2017; Rodríguez and Rodríguez Reference Rodríguez and Rodríguez2009; Telfer et al. Reference Telfer, Sincock, Byrd and Reed1987), light attraction does not affect ʻuaʻu to the same degree, with only small numbers of grounded fledglings found on Kaua‘i annually. However, grounded ʻuaʻu do need to be rescued otherwise they will die of dehydration or starvation or get run over by cars or eaten by cats and dogs (Deppe et al. Reference Deppe, Rowley, Rowe, Shi, McArthur and Gooday2017; Rodríguez et al. Reference Rodríguez, Rodríguez, Pérez, Marrero and Negro2012). Rescue campaigns for grounded fledglings should therefore be aware of not only the dates of the fledging period (for both islands covering a two-month period, from mid-October to mid-December, with a peak in mid-November), but also the timing of fledging. Timing of fledging, while clearly a key component of focusing searches to be most efficient, is often poorly known for seabird species, as was highlighted in a recent study considering fallout for Wedge-tailed Shearwaters Ardenna pacifica in Oahu (Urmston et al. Reference Urmston, Hyrenbach and Swindle2022). There is a clear peak of fledging of ʻuaʻu in the first few hours after dark with a small number fledging throughout the remainder of the night. To maximise searcher efficiency for grounded birds, particularly around hotels, resorts, and businesses, two searches should therefore be undertaken to look for grounded birds. The first should be undertaken 2–3 hours after dark to locate any birds grounded during the peak of fledgling, while the second should be completed by 40 minutes before dawn to locate birds grounded during the rest of the night. If the second search is not undertaken by this time, some of the night’s grounded fledglings may be missed as they will seek crawl spaces and other forms of cover once light is visible on the horizon, making them very difficult to find (Raine et al. Reference Raine, Driskill, Rothe, Rossiter, Gregg and Anderson2024b). It is also worth highlighting the fact that birds fledge from burrows irrespective of moon illumination. Therefore, annual fallout patterns should not be considered representative of annual variation in fledging dates; rather, they are entirely a function of increased light attraction on dark nights with no moon during the time the bird fledged, made worse when coupled with inclement weather conditions.
Understanding the phenology of this species is also important for colony monitoring purposes, as sufficient burrow checks are necessary to accurately assess reproductive success rates and should encompass the entire breeding season. On Kaua‘i and Lānaʻi, a total of eight colony monitoring trips are carried out at intervals throughout the breeding season to ensure that key breeding phases (i.e. arrivals, incubation, chick rearing, and fledging) are all recorded. Checks are undertaken in late February/early March (deployment of monitoring equipment prior to arrival), April (arrival and pre-lay exodus), June (incubation), August (chick rearing), October (early fledging), November (peak fledging), and December (late fledging, removal of all monitoring equipment). We recommend that other projects monitoring this species follow the same protocols (based on their respective phenological inflection points) to ensure sufficient data are collected to accurately calculate reproductive success rates and allow for direct comparisons between management sites.
These data are also useful for projects engaged in translocation or social attraction efforts. Projects such as these can be an important aspect of conservation as they aim to create highly protected colonies inside predator-proof fence enclosures, in many cases aiming to do so in areas that are more accessible to monitoring and management teams. Examples on Kaua‘i include the Nihoku Ecoystem Restoration Project (translocation and social attraction), Honopū (social attraction only), and Pōhākea (social attraction only). Using breeding phenology data will help to optimise the timing of these and other projects. However, the clear differences in phenological timing between Kauaʻi and Lānaʻi from this study, and between other islands as shown in previous studies, are also an important consideration for any project considering translocating birds from one island to another (something that has been discussed to augment small populations on other islands). Project managers would need to consider compatibility in timing of phenology between donor islands and recipient islands, as it is quite possible that birds from different islands would be less likely to breed with each other due to these differences. These differences are in some cases extreme, such as the six-week difference between east Maui birds and those on Kauaʻi. Locating compatible source colonies in terms of phenology is therefore critical as otherwise there is the risk that translocated birds would be completely out of synch with resident birds, which would otherwise be an important cohort to bolster a colony creation project of this nature.
Lastly, having detailed phenology data is an important component to understanding powerline collision risk. Collision monitoring has shown that ʻuaʻu and ʻaʻo are highly susceptible to collisions with powerlines and other similar hazards (Travers Reference Travers, Young and Vanderwerf2022; Travers et al. Reference Travers, Driskill, Stemen, Geelhoed, Golden and Koike2021, Reference Travers, Driskill, Scott, Hanna, Flaska and Bache2023). The visitation data collected during this study clearly highlight the risk to individual breeding birds. Overall, breeding pairs of adult ʻuaʻu arrived and departed from burrows on average 63 times over the course of a season. For birds breeding in areas where colony flyways cross powerlines or other similar hazards, each breeding pair would transit past hazards on both the inbound and outbound flight resulting in 126 potential crossings per year. Hypothetically, if a colony of only 100 burrows has a flyway that passes over high-collision-risk powerlines (due to factors such as topography, prevailing wind direction, and exposure height), there would be an average of 12,600 transits of breeding birds from that colony alone past the hazard in a single year, coupled with many more transits of non-breeders and sub-adults. The high frequency of nocturnal transits for ʻuaʻu is a critical explanatory factor in the high powerline collisions detected in areas with infrastructure (Travers et al. Reference Travers, Driskill, Stemen, Geelhoed, Golden and Koike2021, Reference Travers, Driskill, Scott, Hanna, Flaska and Bache2023). That being said, when compared with the ʻaʻo, the more infrequent burrow visitation rate of ʻuaʻu compared with ʻaʻo (which had 114 visits per pair, equating to 22,800 transits for a 100-burrow colony each season; Raine et al. 2023) means that although their exposure risk to collisions is high it is not as high as for ʻaʻo (if all other factors were equal). This is presumably one explanatory variable for why powerline collisions on Kauaʻi are proportionally higher for ʻaʻo.
While we now have detailed information on the breeding phenology of breeding adults, the colony attendance patterns of non-breeders within the population remain unknown. Future work will concentrate on addressing this group of birds, potentially using acoustic recordings, cameras positioned on known prospector burrows, and the monthly patterns of attendance of prospecting birds at newly created social attraction sites on Kauaʻi. Understanding the peak periods of sub-adult activity on Kauaʻi will help to identify risk for this portion of the breeding population from threats such as powerline strike and depredation by introduced predators.
Acknowledgements
Seabird work in 2021–2023 was undertaken through Archipelago Research & Conservation (on Kauaʻi) and Pūlama Lānaʻi (on Lānaʻi). Seabird monitoring work between 2011 and 2020 was undertaken through the Kauaʻi Endangered Seabird Recovery Project (KESRP), which is a joint project of the Department of Land and Natural Resources (DLNR), Division of Forestry and Wildlife (DOFAW), and the Pacific Co-operative Studies Unit (PCSU) of the Research Corporation of the University of Hawaiʻi. We would like to thank staff from both PCSU and DOFAW for their support during the data collection, particularly D. Duffy (PCSU), S. Mann, C. Mottley, and A. Siddiqi (DOFAW). We would like to thank all the field technicians who have worked in extremely rugged terrain over the years to collect the data, as well as the field crew leaders. On Kauaʻi, predator control operations were undertaken by staff from Hallux Ecosystem Restoration LLC (A. Dutcher, K. Pias, and staff), National Tropical Botanical Gardens (U. Nagendra, C. Nagle, and staff), and the Hawaii Department of Forestry and Wildlife, while on Lānaʻi it was undertaken by Pūlama Lānaʻi. Pūlama Lānaʻi would like to thank C. Pisani, L. Kain, J. Deslippe, and C. Donehower for their hard work conducting predator control operations on Lānaʻi. Funding for work on Kauaʻi was provided from several sources: (i) the Kauaʻi Island Utility Cooperative, (ii) National Fish & Wildlife Foundation (NFWF) via the American Bird Conservancy (ABC), and (iii) multiple State Wildlife Grants provided by U.S. Fish and Wildlife Service (USFWS). Funding for work on Lānaʻi was provided by Pūlama Lānaʻi and NFWF. We extend our thanks to all staff working for these entities who have been involved in processing the funding and facilitating the work, particularly D. Huff, D. Bissell, and C. Yuh (KIUC), S. Hall (NFWF), B. Keitt, and S. McKeon (ABC), M. Bogardus, A. Nadig, L. Nagatini, and K. Matsuoka (USFWS). All work carried out for this paper was undertaken under permits via DLNR and USFWS. The National Tropical Botanical Garden and the State of Hawaii kindly granted access to the various study sites. We would also like to thank Kamaka Gallagher for translating the summary into ʻolelo hawaiʻi.









