INTRODUCTION
Soil salinization and alkalization frequently co-occur, which has caused severe problems in some areas (Kawanabe and Zhu, Reference Kawanabe and Zhu1991). In saline and sodic soils, Na+, Ca2+, Mg2+ and K+ are the main cations of soluble mineral salts and Cl−, SO42−, HCO3−, CO32− and NO3− are the main corresponding anions (Läuchli and Lüttge, Reference Läuchli, Lüttge and Tanji2002). In recent years, many studies attached importance to the effects of alkaline salt stress on plants growth. They mainly compared the effects of neutral (such as NaCl and Na2SO4) and alkaline sodium salts (ASS) (such as NaHCO3 and Na2CO3) on plant growth and indicated that alkaline salt stress had caused more harmful effects on plants than neutral salt stress, and there were two distinct stresses which they called salt stress and alkali stress, respectively (Shi and Yin, Reference Shi and Yin1993; Yang et al., Reference Yang, Chong, Li, Kim, Shi and Wang2007). Compared to salt stress, plants usually accumulate more sodium ions and organic solutes such as proline, soluble sugar (SS) and betaine to take part in osmotic adjustment in alkali stress (Li et al., Reference Li, Liu, Zhang, Lin and Mu2009; Yang et al., Reference Yang, Shi and Wang2008a). Unlike salt stress, the accumulation of organic acid (OA) is the special physiological mechanisms of plants responding to alkali stress (Chen et al., Reference Chen, Cui, Sun, Guo, Yang, Jin, Fang and Shi2009; Shi et al., Reference Shi, Yin, Yang and Zhao2002; Zhang and Mu, Reference Zhang and Mu2009).
Potassium is an essential nutrient and abundant cation in plant cells, and plays major biochemical and biophysical roles in plant growth (Szczerba et al., Reference Szczerba, Britto and Kronzucker2009). Potassium is also important in alleviating detrimental effects of abiotic stress in plants (Cakmak, Reference Cakmak2005). Some reports revealed that low levels of exogenous potassium application were beneficial for salt-stressed plants and could improve the salt tolerance of plants (Akram et al., Reference Akram, Ashraf and Akram2009; Ashraf et al., Reference Ashraf, Rahmatullah, Bhatti, Afzal, Sarwar, Maqsood and Kanwal2010; Kaya et al., Reference Kaya, Tuna, Ashraf and Altunlu2007; Neid and Biesboer, Reference Neid and Biesboer2005; Shirazi et al., Reference Shirazi, Ashraf, Khan and Naqvi2005; Zheng et al., Reference Zheng, Jia, Ning, Xu, Li and Jiang2008). However, the alleviation of K+ on salt-stressed plants only happened when K+ concentration was low. When potassium contents increased to the same amount as sodium level in saline soils, it would bring severe damage to the plants. The damage of 100 mM K+ on plants is higher than that of Na+ in the study of neutral salts (Benlloch-González et al., Reference Benlloch-González, Fournier, Ramos and Benlloch2005; Ramos et al., Reference Ramos, Lo´pez and Benlloch2004), and K+ was inhibitorier than Na+ independently of the accompanying anion (Sosa et al., Reference Sosa, Llanes, Reinoso, Reginato and Luna2005).
In alkaline soils, most studies care about sodium ions and high pH effects on plants, and seldom consider potassium impacts. If K+ content increases to a high level in alkaline soils, what will happen to plant growth, positive or negative? Whether is it another stress condition for plants? Some studies indicate the severe effects of alkali stress with sodium ions, and the corresponding physiological responses characteristic of plants (Li et al., Reference Li, Liu, Zhang, Lin and Mu2009; Yang et al., Reference Yang, Chong, Li, Kim, Shi and Wang2007, Reference Yang, Shi and Wang2008a, Reference Yang, Wang, Li, Shi and Wang2008b; Zhang and Mu, Reference Zhang and Mu2009). If APS is another stress, what is the plant growth and physiological response? In order to answer these questions, mixed ASS (NaHCO3:Na2CO3 = 9:1) and APS (KHCO3:K2CO3 = 9:1) were used to treat 10-day-old wheat seedlings. We compared the influences of two alkaline salts on wheat seedlings growth, photosynthesis, ions absorption and organic solutes synthesis to explore the physiological responses mechanisms of wheat to these alkaline stresses. According to this experiment, we can provide scientific proof for a better management in alkaline soil planting.
MATERIALS AND METHODS
Plant material and stress conditions
The experiment was conducted at Northeast Normal University (Changchun city in China). The material was wheat (Triticum aestivum) cv. Jimai 3 (growing period was 82 days and a 1000-seed weight was 37 g), developed by Jilin Agricultural University. Homogeneous seeds were sown in 15-cm diameter plastic pots containing washed sand. All pots were placed outdoors and protected from rain. Each pot contained 15 seedlings, and was watered with Hoagland's nutrient solution daily before treatment. The Hoagland solution consisted of 5.00 mmol L−1 Ca2+, 2.00 mmol L−1 Mg2+, 6.04 mmol L−1 K+, 22.2 μmol L−1 (EDTA)-Fe2+, 6.72 μmol L−1 Mn2+, 3.16 μmol L−1 Cu2+, 0.765 μmol L−1 Zn2+, 2.10 mmol L−1 SO42−, 1.00 mmol L−1 H2PO4−, 46.3 μmol L−1 H3BO3, 0.556 μmol L−1 H2MoO4 and 15.04 mmol L−1 NO3− (Yang et al., Reference Yang, Shi and Wang2008a).
Two alkaline salts with Na+ (NaHCO3:Na2CO3) and another two alkaline salts with K+ (KHCO3:K2CO3) were mixed respectively in a 9:1 molar ratio, and used in the ASS stress group and APS stress group, respectively. Three concentration treatments were applied: 40, 80, 120 mM. The pH of treatment solutions was 9.10, 9.16, 9.17 for ASS, and 9.23, 9.18, 9.13 for APS, respectively. These treatment concentrations referred to the total salt concentrations of NaHCO3 + Na2CO3 or KHCO3 + K2CO3. Therefore, in the ASS stress solution of 40 mM, a mixture of 36 mM NaHCO3 and 4 mM Na2CO3 would result in total ions concentrations of Na+, HCO3− and CO32− of 44, 36 and 4 mM, respectively.
Stress treatments
When the seedlings were 10-day-old, 21 pots with uniformly seedlings were selected and randomly divided into seven sets, with three pots per set. Each pot was a single replicate with three replicates per set. One set was control treatment and the pots were maintained by watering with nutrient solution; the other sets were treated with stress treatments. All pots were watered thoroughly with 250 mL of treatment solutions applied in two proportions at 16:30–17:30 daily. The duration of treatment was nine days, which was determined by the onset of unusual growth in seedlings in the highest alkalinity.
Gas exchange characteristics
Net photosynthetic (P N) and transpiration (E) rates, stomatal conductance (g s) and intercellular CO2 concentration (C i) of leaves were determined on a fully expanded youngest leaf of each plant at 9:30–11:00 at the end of stress treatments, using a portable open flow gas exchange system LI-6400 (LI-COR, USA). The photosynthetically active radiation (PAR) was 1200 μmol m−2 s−1. Measurements were repeated five times for each blade, a total of six blades per pot, and the averages were recorded.
Harvest and pretreatment
All plants were harvested in the morning after the final treatment, and were washed with tap water followed by the distilled water. We recorded the shoots weight after they were oven-dried at 105 °C for 15 min, and then dried at 70 °C to a constant weight. After smashing, 100 mg shoots sample was treated with 10 mL deionized water at 100 °C for 1 h, and the extract was used to determine the contents of inorganic ions, SS and OA. Another 100 mg dry shoot sample per pot was treated with 10 mL of 3% (w/v) aqueous sulfosalicylic acid and the extractant was used to determine the proline content.
Determination of inorganic ions
An atomic absorption spectrophotometer (TAS-990, Purkinje General, Beijing) was used to determine contents of Na+, K+, free Ca2+ and free Mg2+. The contents of Cl−, NO3−, SO42− and H2PO4− were determined by ion chromatography (DX-300 ion chromatographic system; AS4A-SC ion-exchange column, CD M-II electrical conductivity detector, mobile phase: Na2CO3/NaHCO3 = 1.7/1.8 mM; DIONEX, Sunnyvale, USA).
Determination of organic solutes components
The organic acid components contents were determined by ion chromatography (DX-300 ion chromatographic system; ICE-AS6 ion-exclusion column, CDM-II electrical conductivity detector, AMMS-ICE II suppressor, mobile phase: 0.4 mM heptafluorobutyric acid; the flow rate was 1.0 mL/min; the column temperature was set at 20 °C, and the injection volume was 50 μL; DIONEX, Sunnyvale, USA). All sample solutions were filtered through a 0.22 μm filter before using. The content SS and proline were estimated spectrophotometrically using anthrone (Dubois et al., Reference Dubois, Gilles, Hamilton, Rebers and Smith1956) and ninhydrin (Zhu et al., Reference Zhu, Deng and Zuo1983), respectively.
Statistical data analysis
A one-way analysis of variance (ANOVA) was performed using the statistical program SPSS 13.0 (SPSS Inc, Chicago, IL, USA). The means and calculated standard errors are reported. Different salt levels were compared by least-significant difference multiple comparison (p < 0.05).
RESULTS
Biomass and gas exchange characteristic
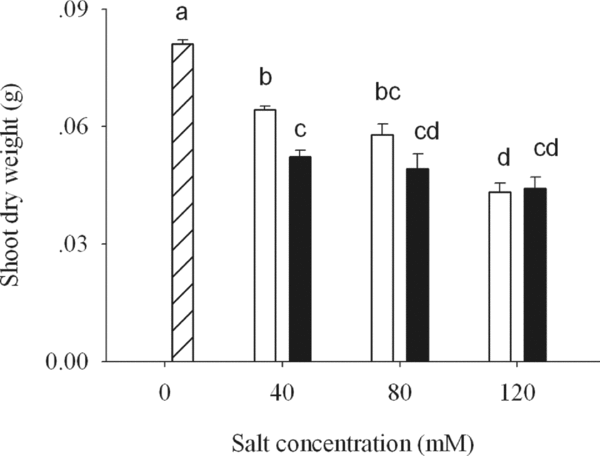
Compared to control treatments, there was a significant decrease in dry weight of wheat shoots under both stresses (p < 0.05; Figure 1), more for APS than for ASS at 40 mM. With increasing alkalinity, the decrease was gradual and marked in ASS (p < 0.05), but was sharp at 40 mM in APS then remained unchanged (Figure 1).

Figure 1. Shoot dry weight of wheat under CK (control) (![]() ), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
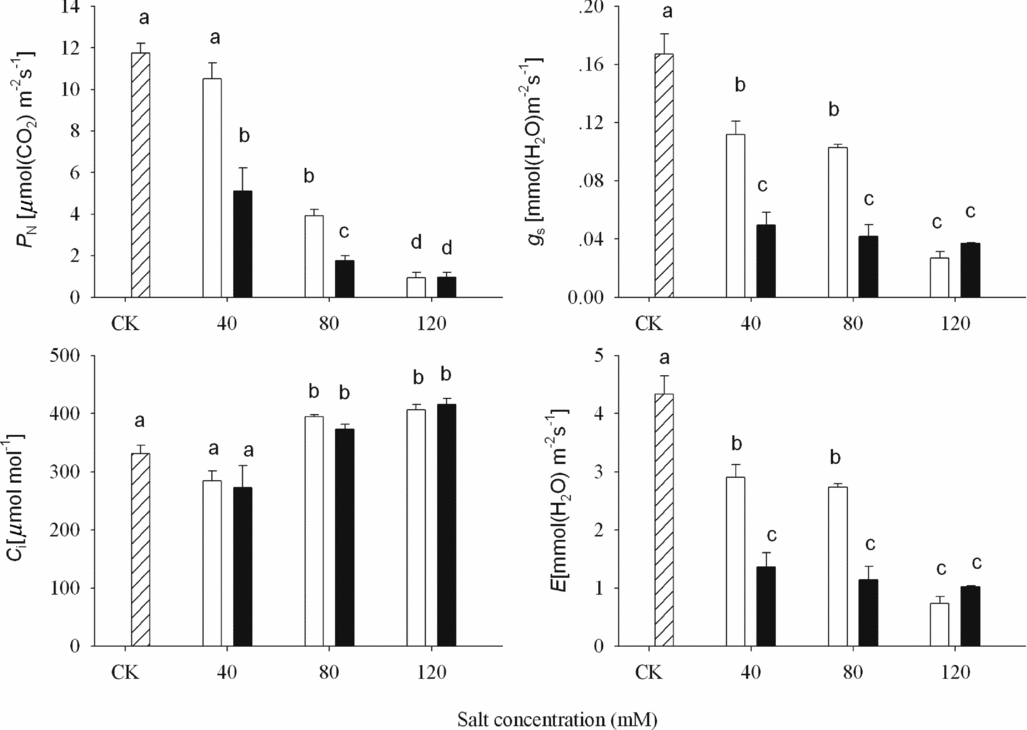
Among the gas exchange characteristic, P N in wheat shoot decreased significantly and gradually with increasing alkalinity in both alkali stresses (p < 0.05; Figure 2). Both g s and E decreased gradually and significantly in ASS treatment, but reduced markedly at 40 mM then remained unchanged in APS treatment. The tendency was similar with shoot dry weight, which showed a ‘ladder’ in ASS and a ‘L’ in APS treatment with the increasing alkalinity. The values of P N, g s and E in APS were lower than those in ASS except 120 mM (p < 0.05; Figure 2). C i increased significantly at higher concentration in both alkali stresses (p < 0.05).

Figure 2. Gas exchange characteristics of wheat under CK (control) (![]() ), alkaline sodium salt (■) and alkaline potassium salt (□) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
), alkaline sodium salt (■) and alkaline potassium salt (□) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
Absorption of inorganic ions
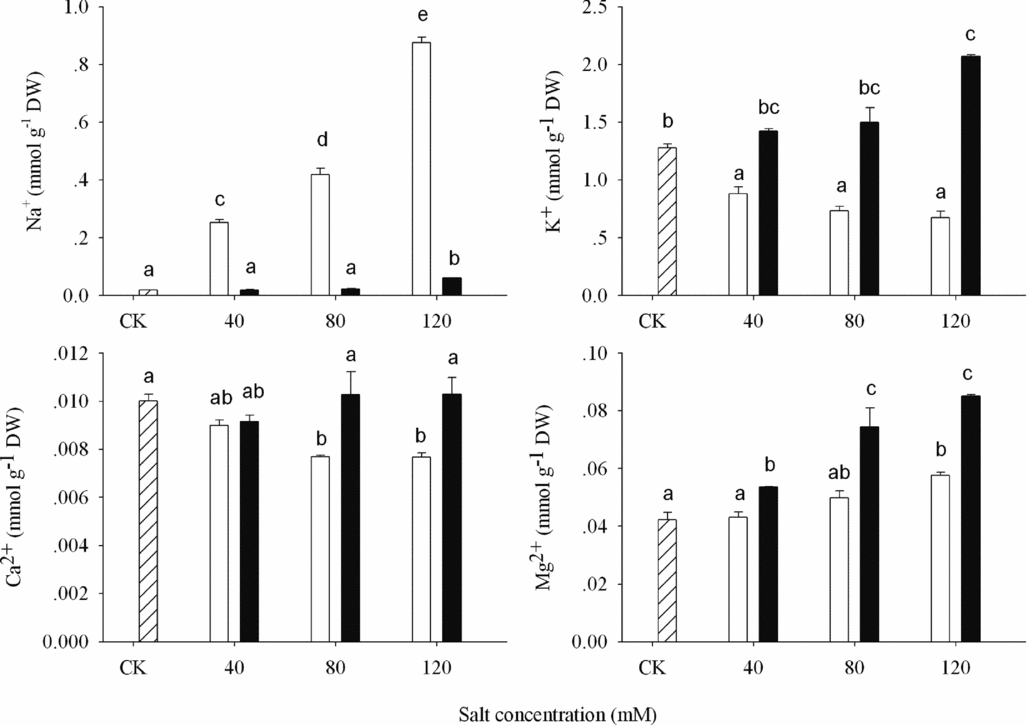
The content of cations (Na+, K+, free Ca2+, free Mg2+) and anions (Cl−, NO3−, SO42−, H2PO4−) were measured in shoots of wheat seedlings at different alkaline stresses (Figures 3 and 4). Compared to the ASS treatment to controls, Na+ content in shoots increased 11.4, 21.3, 45.5-fold, and K+ decreased 31.0, 42.7, 47.1% at 40, 80 and 120 mM, significantly and separately (P < 0.05; Figure 3). In APS treatment, K+ contents increased 11.6, 17.4, 62.2% and Na+ increased 3.8, 22.6%, 2.23-fold separately under 40, 80, and 120 mM potassium ions stress. Free Ca2+ contents reduced and free Mg2+ accumulated in ASS treatment. There was no change happened in free Ca2+ content in APS treatment. Free Mg2+ accumulation was higher in APS than in ASS treatment.

Figure 3. Cations concentration (Na+, K+, Ca2+ and Mg2+) in shoot of wheat under CK (control) (![]() ), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).

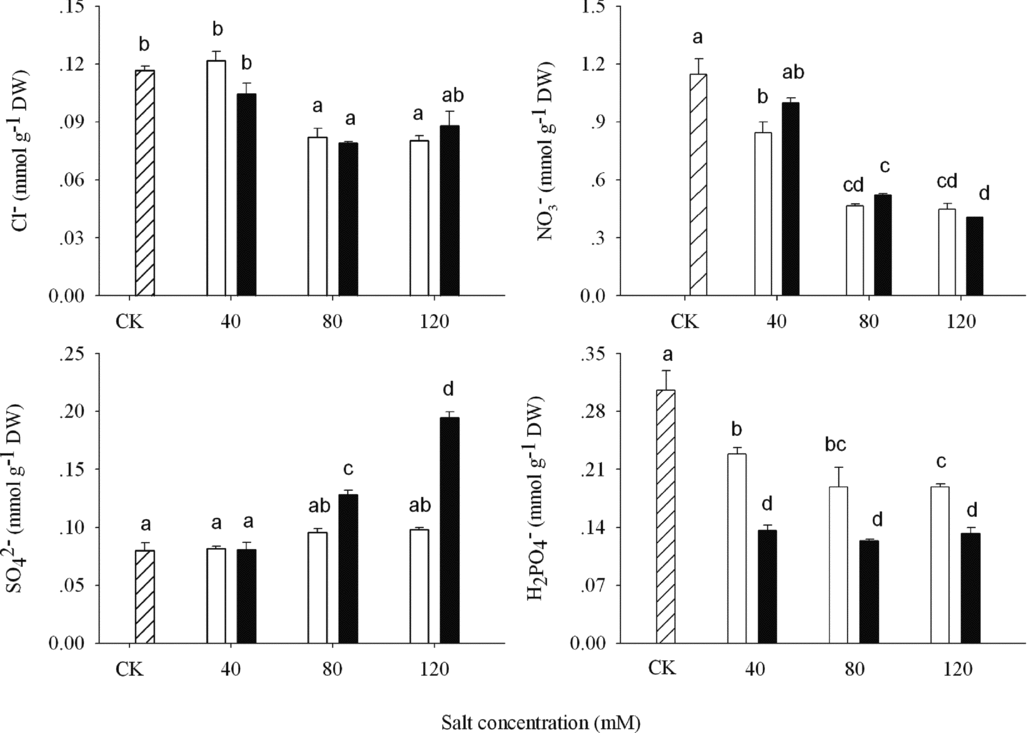
Figure 4. Anions concentration (Cl−, NO3−, SO42− and H2PO4−) in shoot of wheat under CK (control) (![]() ), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
In ASS and APS treatments, the contents of Cl− and NO3− decreased significantly when alkalinity was above 80 mM then remained unchanged (Figure 4). The content of SO42− had no obvious change under ASS treatment but increased under APS treatment (p < 0.05; Figure 4). The H2PO4− contents decreased at 40 mM then remained unchanged with the increasing alkalinity, more for APS than for ASS.
Synthesis of organic solutes
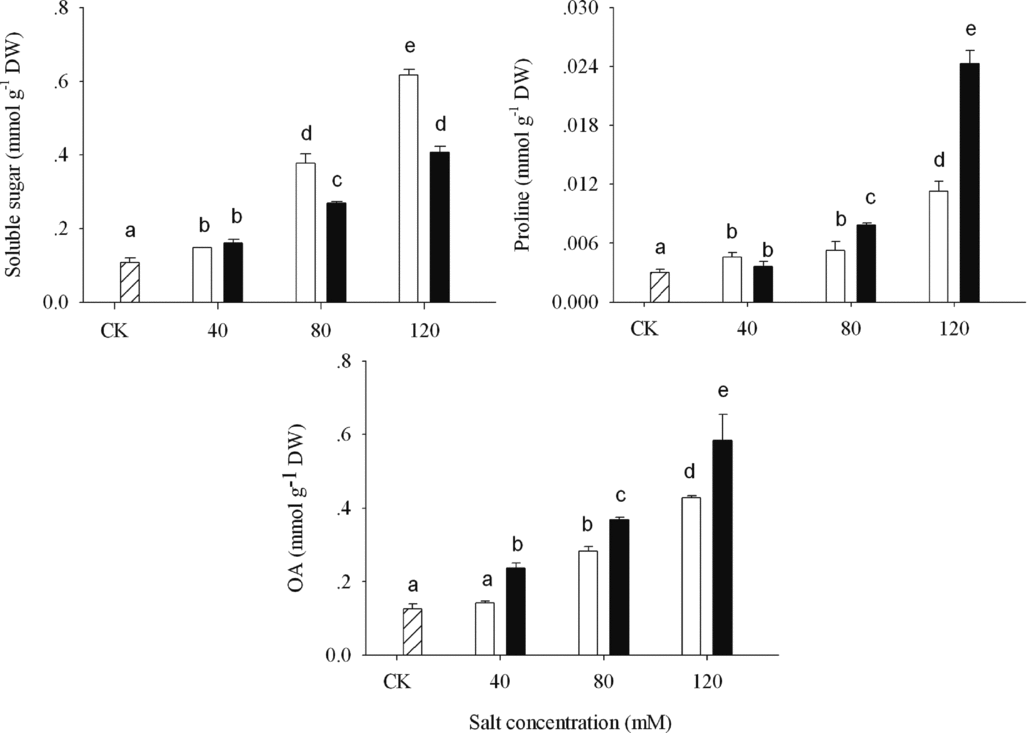
The organic solutes, such as SS, OA and proline, all of them accumulated in ASS and APS treatments (Figure 5). When the alkalinity was above 80 mM, wheat seedlings shoots had more proline and OA synthesis and less SS accumulation in ASS than in APS treatments (p < 0.05).

Figure 5. Organic solutes concentration (soluble sugar, proline and total organic acid) in shoot of wheat under CK (control) (![]() ), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
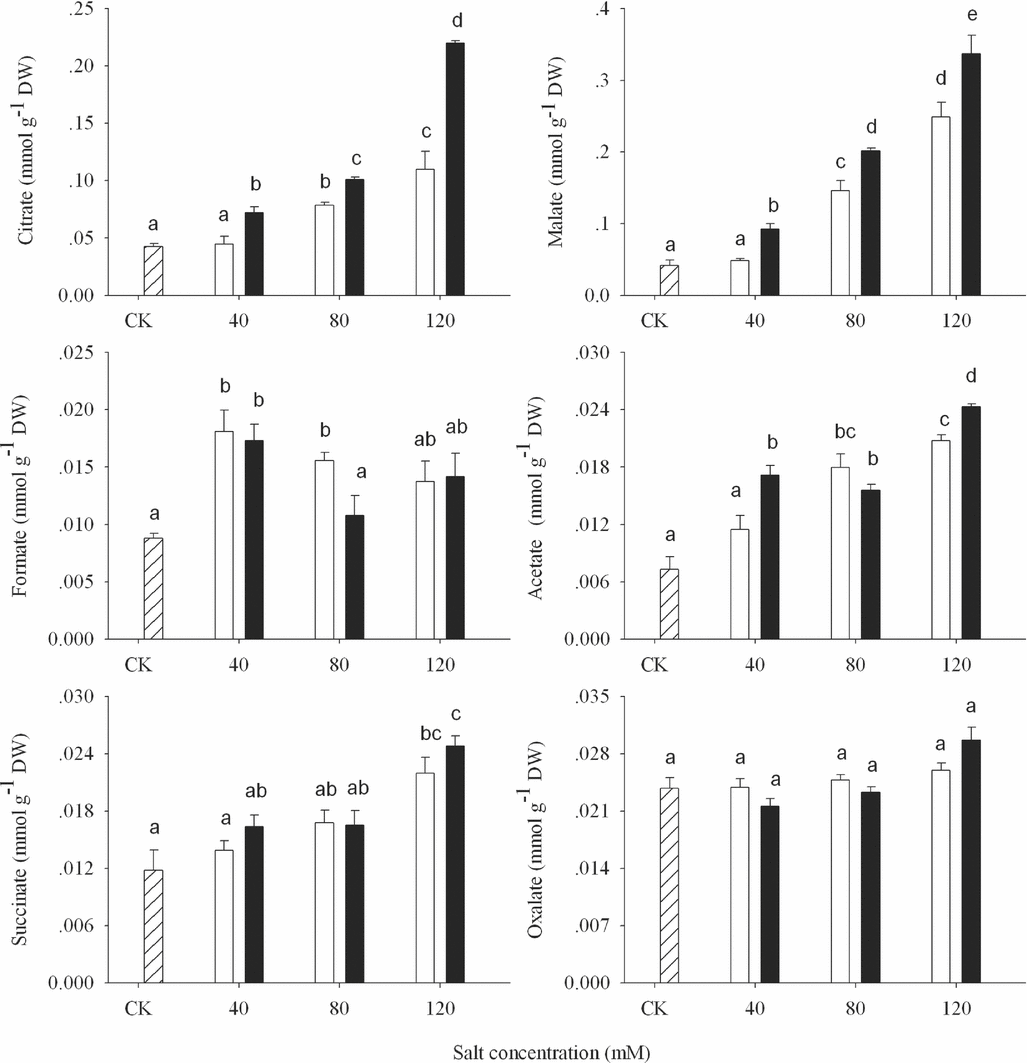
Organic acids are almost entirely dissociated from their protons and exist as OA anions (Ma et al., Reference Ma, Ryan and Delhaize2001). In this experiment, the citrate, malate, formate, acetate, succinate and oxalate were detected in wheat shoots (Figure 6). Among the OA components, the tendency of citrate and malate was similar to total OA contents, which was synthesized more in APS than in ASS (p < 0.05; Figure 6). Although the content of acetate and succinate increased significantly (p < 0.05), there was no significant difference between ASS and APS treatments. A small variation of formate content was found among the different alkalinity in both alkaline treatments when the alkalinity was over 40 mM. There was no significant change in oxalate content in both alkaline stresses (p > 0.05).

Figure 6. Organic acid components (citrate, malate, formate, acetate, succinate and oxalate) in shoot of wheat under CK (control) (![]() ), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
), alkaline sodium salt (□) and alkaline potassium salt (■) treatments. The 10-day-old seedlings were subjected to alkaline sodium salt stress (NaHCO3:Na2CO3 = 9:1) and alkali potassium salt stress (KHCO3:K2CO3 = 9:1) for nine days. The values are means (± S.E.) of three replicates. Salt concentrations were 40, 80 and 120 mmol L−1. Different letters indicate significant difference among treatments (p < 0.05).
DISCUSSIONS
Plant growth depends on the supply of inorganic nutrients. Nevertheless, extreme nutrient conditions could cause the toxicity or deficiency to a varying extent for different plant species (Maathuis and Amtmann, Reference Maathuis and Amtmann1999). K+ is essential to all plant life, and it is the major cationic inorganic nutrient in most terrestrial plants. When plants grow in saline soils, one of the key elements in salinity tolerance is the capacity to maintain a high cytosolic K+/Na+ (Ashraf et al., Reference Ashraf, Rahmatullah, Bhatti, Afzal, Sarwar, Maqsood and Kanwal2010). Previous studies revealed that the application of low extra potassium level was beneficial for salt-stressed plants. For example, alleviation of NaCl stress symptoms through simultaneously applied elevated (from 6 to 21 mM) KNO3 was found in the salt-induced wheat cultivars (Zheng et al., Reference Zheng, Jia, Ning, Xu, Li and Jiang2008). Foliar application 1.5% K2SO4 could significantly improve the growth and photosynthetics of the salt-stressed sunflowers (Akram et al., Reference Akram, Ashraf and Akram2009). Ten mM KCl induced salinity tolerance in wheat (Shirazi et al., Reference Shirazi, Ashraf, Khan and Naqvi2005) and 5 mM KNO3 addition improved salt tolerance of the melon (Kaya et al., Reference Kaya, Tuna, Ashraf and Altunlu2007). However, too much potassium in soils or growth substrate may have adverse influence on plants. Comparing the effects of 100 mM sodium salts (NaCl) to potassium salts (KCl) on Cynara cardunculus (Benlloch-González et al., Reference Benlloch-González, Fournier, Ramos and Benlloch2005) and Atriplex nummularia (Ramos et al., Reference Ramos, Lo´pez and Benlloch2004), the damage of K+ was higher than that of Na+ in the study of neutral salts. The study of osmotic and specific ion effects on the germination of Prosopis strombulifera indicated that K+ was more inhibitory than Na+ independently of the accompanying anion (Sosa et al., Reference Sosa, Llanes, Reinoso, Reginato and Luna2005).
There was a similar result in our study that high K+ level had negative effect on wheat growth. Photosynthetic capacity was related to salt-stress intensity, and plants showed lower values of P N when they grew on more severe stress. With the increasing alkalinity from 40 to 120 mM, photosynthetic rate decreased 10.5, 66.7 and 92.0% in ASS treatment, and 56.5, 84.9 and 92.0% in APS treatment in comparison to controls (Figure 2). Photosynthetic rate was inhibited more severely in APS than ASS treatment at 40 and 80 mM, which showed that wheat seedlings suffered more harmful effect and had lower tolerance to APS than ASS treatments. The tendency of gs and E was similar with PN. These reductions in stomatal conductance and transpiration rates represented adaptive mechanisms to cope with excessive salt, rather than merely a negative consequence of it (Clark et al., Reference Clark, Newton and Barker1999). Inadequate photosynthesis caused the reduction of plants growth rate in stress conditions. Both alkali stress treatments decreased the biomass of wheat shoots. The decrement of APS treatment was higher than ASS treatment. However, the K+ level remained unchanged after 40 mM in APS treatment. This may be related to the short treatment time. The marked reduction of photosynthesis with increasing K+ concentration showed the physiological change immediately, but the morphology change would be delayed.
The marked reduction of growth and photosynthesis indicated that K+ was more toxic than Na+ at the same concentrations, consistent with previously reported in plants thriving in salty environments (Egan and Ungar, Reference Egan and Ungar1998, Ramos et al., Reference Ramos, Lo´pez and Benlloch2004). The two alkaline salts treatments had similar pH (9.10–9.17 and 9.13–9.23), so the result of APS having more severe effects than ASS on wheat growth was not caused by pH but by K+.
ASS treatments induced large absorption of Na+, which was 45.5-fold at 120 mM compared with controls. At the same alkalinity, APS treatment accumulated K+, which only was 62.2% higher than controls. The fewer increase of K+ contents led to higher damage, which may be related to osmotic adjustment activity. Ramos et al. (Reference Ramos, Lo´pez and Benlloch2004) showed that Na+ contributed more efficiently than K+ to perform this function, although it had been proposed that both K+ and Na+ were involved in osmotic adjustment of plants in response to high soil salinity.
The K+ increased less, but it had actual higher concentration than Na+. In saline soils, Na+ that entered root cells in the outer part of the root was likely pumped back out again via plasma membrane Na+/H+ antiporters encoded by the gene SOS1 (Munns et al., Reference Munns, James and Lauchli2006). High sodic salt levels induced the expression of an amiloride-resistant Na+/H+ antiporter that could account for the remarkable tolerance to NaCl (Tartari and Forlani, Reference Tartari and Forlani2008). Therefore, high levels of Na+ and a lack of H+ in the outer part of wheat seedlings root in alkaline stress resulted in high Na+ and H+ concentration gradients that existed between intracellular and extracellular of root, which made it easier for Na+/H+ antiporters to export H+ and import excess Na+.
Similar with the transport of Na+ in ASS treatment, the addition of high K+ level and lacking of H+ in the outer part of wheat seedlings root promoted the passive uptake of K+ in APS treatment. There were other important K+ channels that were different from Na+ transport. High external K+ level made plants start the low-affinity transport system for K+ predominantly functions (generally above 1 mM), which was thermodynamically passive (Szczerba et al., Reference Szczerba, Britto and Kronzucker2009). It existed on biological membranes, for uptake of K+ from the growth medium (Blumwald et al., Reference Blumwald, Aharon and Apse2000). High-affinity K+ transporter family members may be an expression of a K+/Na+ symporter at low [Na+]ext (Szczerba et al., Reference Szczerba, Britto and Kronzucker2009). A consequence of both the passive uptake of K+ and its active entry of net positive charge required the active removal of protons to maintain electrical neutrality (Gerendás and Schurr, Reference Gerendás and Schurr1999; Rodriguez-Navarro, Reference Rodriguez-Navarro2000). Thus, the APS condition needed wheat seedlings expending extra energy to transport K+, which disturbed the normal growth and reduced the energy for photosynthesis and soluble solutes synthesis.
In ASS treatment, the saline ions Na+ inhibited the absorption of K+ and free Ca2+. However, K+ in APS treatment had no significant effect on other cations’ accumulation (Figure 3). Both alkali stresses promoting free Mg2+ accumulation indicated that free Mg2+ might be the special physiological responses of wheat to the alkaline salts. With regard to the function and metabolize mechanisms of free Mg2+, we need to do further research.
H2PO4− concentrations in alkali stress were always decreased significantly, which might be closely related to the deposition of phosphate caused by the high pH of alkali stress (Yang et al., Reference Yang, Chong, Li, Kim, Shi and Wang2007). So, alkaline salts condition caused phosphorus deficiency happening. The decreasing phosphate out of wheat seedling roots led to the low contents of H2PO4− intracellular of cell (Figure 4), more for APS than for ASS treatments. The reduction in H2PO4− uptake was due to the alkaline salts specifically; however, the oversupply of K+ would aggravate the reduction. The anions H2PO4− absorption relied on H+-solute symporters; this activity weakened because of the lower H+ concentrations out of wheat seedlings root. K+ transported into wheat root that did not rely on K+/H+ antiporters entirely, resulted in lower H+ concentration in APS than in ASS treatment, which led to lower H2PO4− content. There was no difference between ASS and APS treatments in Cl− or NO3− uptake, indicating that H2PO4− anion was the key anions responding to alkali stress. Its changes affected the growth, photosynthesis and other physiological metabolisms directly. Photosynthesis rates were correlated with P content in leaf tissue of plants (Johnson, Reference Johnson1984). Orthophosphate had an important role in photosynthetic metabolism. Phosphorus limitation or deficiency could decrease net CO2 exchange (Fredeen et al., Reference Fredeen, Raab, Rao and Terry1990; Terry and Ulrich, Reference Terry and Ulrich1973), diminish protein synthesis that worked as photosynthetic components, which were as some of the most abundant proteins in the plant (Eaton, Reference Eaton1950), and limit the growth (Lapointe, Reference Lapointe1987). The decrement of H2PO4− may be related to the change of net photosynthetic, transpiration rates and shoots biomass. The more reduction was in H2PO4− contents, the more decrement was in growth and photosynthesis. Further work would be needed to verify the relationship between reduced phosphate uptake and reducing photosynthesis.
In saline conditions, the osmotic adjustment, which occurs through the accumulation of inorganic components (such as Na+) in plants, is less energy and carbon demanding than adjustment by organic solutes (Munns et al., Reference Munns, James and Lauchli2006). In order to lower the toxic antion of Na+ in the cytoplasm, plants generally compartmentalize Na+ into vacuoles, and synthesize the compatible organic solutes in the cytoplasm to prevent dehydration (Munns, Reference Munns2002; Parida and Das, Reference Parida and Das2005). In our study, wheat seedlings in both alkaline stresses accumulated SS, proline and OA to take part in osmotic adjustment (Figure 5). The SS accumulation was consistent with the result of Kerepesi and Galiba (Reference Kerepesi and Galiba2000), who reported that salt-tolerant wheat varieties could accumulate more SS as a useful indicator of salt tolerance. However, APS induced wheat seedlings spending more energy on K+ transport, and weaken the power export for the big solutes synthesis of SS.
Proline is another important and compatible organic solute for osmotic adjustment. Proline acts as a signalling/regulatory molecule able to activate multiple responses that are components of adaptation to abiotic stress including salt stress (Maggaio et al., Reference Maggaio, Miyazaki, Veronese, Fujita, Ibeas, Damsz, Narasimhan, Joly and Bressan2002). Proline can balance the accumulation of Na+ and Cl− as a result of salinity (Meloni et al., Reference Meloni, Gulotta and Martinez2008). Wheat seedlings accumulated much more proline in both alkaline stresses, more for APS than ASS treatments. Nageswara Rao et al. (Reference Nageswara Rao, Krishnasastry and Udayakumar1981) studied the role of potassium in proline metabolism and indicated that species differed in the proline biosynthetic pathway and in finger millet potassium had a role in proline biosynthesis. A positive relationship was found between potassium and proline, and K+ could help convert arginine into proline (Nageswara Rao et al., Reference Nageswara Rao, Krishnasastry and Udayakumar1981).
OA is another osmoprotectants and participates in osmotic adjustment, liking SS and proline. The citrate and malate were also the main components of OA in wheat seedlings in both alkaline stresses, the change of which was consistent with the total OA contents. Excessive potassium caused severe reduction in photosynthetic CO2 fixation and impairment in partitioning and utilization of photosynthates. Such disturbances resulted in excess of photosynthetically produced electrons and thus stimulation of reactive oxygen species (ROS) production by intensified transfer of electron to O2 (Cakmak, Reference Cakmak2005). OAs here involved not only in osmotic adjustment, but also in antioxidant response. Mitochondrial ROS mediated the expression of the alternative oxidase (AOX), and AOX accumulation resulted in an increase in cellular citrate concentration, which suggested that citrate and/or other tricarboxylic acid (TCA) cycle intermediates might be important signal metabolites (Gray et al., Reference Gray, Maxwell, Villarimo and Mclntosh2004). In addition, evidence also existed for the direct scavenging of ROS by organic acids (Nath et al., Reference Nath, Ngo, Hebbel, Croatt, Zhou and Nutter1995; Varma et al., Reference Varma, Devamanoharan and Morris1995). Thus, there appeared to be cross-talk between mitochondrial carbon metabolism and ROS generation in the signalling process for the induction of AOX1 (Gray et al., Reference Gray, Maxwell, Villarimo and Mclntosh2004).
CONCLUSION
The effects of potassium salts in soil on plants growth were related to the K+ concentration. In alkaline soils, high K+ content had negative effects on wheat seedlings growth. APS was another severe salt stress, the intensity of which was higher than that of ASS. The decrements of growth and photosynthesis in APS treatment were higher than in ASS treatment. Wheat seedlings showed similar physiological response mechanisms to alkaline stress. Both inorganic ions and organic solutes took part in the osmotic adjustment. The differences were that APS depended on K+, but ASS on Na+.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31100403, 31172259) and the Key Laboratory of Vegetation Ecology, Ministry of Education (grant no. 20130813).