INTRODUCTION
Species of the brine shrimp, Artemia, are found in a variety of harsh environments in many parts of the world (Triantaphyllidis et al., Reference Triantaphyllidis, Abatzopoulos and Sorgeloos1998; Van Stappen, Reference Van Stappen, Abatzopoulos, Beardmore, Cleeg and Sorgeloos2002) where they encounter severe hypersalinity, high doses of ultraviolet radiation, very low oxygen tensions and extremes of temperature (Abatzopoulos et al., Reference Abatzopoulos, Beardmore, Clegg and Sorgeloos2002). The temperature range variess widely (5–40°C), the lower limit being consequences of the extreme habitats already discussed such as those in the Tibetan Plateau, Atacama Desert, and Patagonia, in northern and southern Chile, respectively (Gajardo & Beardomore, Reference Gajardo and Beardmore2012). Artemia environments vary considerably in terms of water anionic composition (Triantaphyllidis et al., Reference Triantaphyllidis, Abatzopoulos and Sorgeloos1998), climatological conditions from humid–subhumid (Vanhaecke et al., Reference Vanhaecke, Tackaert, Sorgeloos, Sorgeloos, Bengtson, Decleir and Jaspers1987) to Saharan (Ben Naceur et al., Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2009, Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2012a) and in altitudes from sea level up to 4500 m (Xin et al., Reference Xin, Sun, Zhang, Triantaphyllidis, Van Stappen and Sorgeloos1994). These stressors on individuals can have an impact on the molecular–cellular and physiological characteristics of Artemia (Schuler & Conte, Reference Schuler and Conte2009). However, the brine shrimp Artemia is one of the very few invertebrates that have the striking ability to adapt, live and reproduce in such extreme habitats, regulating the osmotic and ionic concentration of their tissues (Vasudevan, Reference Vasudevan2012).
The brine shrimp Artemia is of considerable economic importance in fish and shellfish larviculture (Bengtson et al., Reference Bengtson, Léger, Sorgeloos, Browne, Sorgeloos and Trotman1991). The quality of the Artemia product differs from strain to strain and from location to location, in terms of hatching and biometric characteristics (Vanhaecke & Sorgeloos, Reference Vanhaecke, Sorgeloos, Persoone, Sorgeloos, Roels and Jaspers1980, Reference Vanhaecke and Sorgeloos1982), as well as for their nutritional value which is not constant but varies among strains and within batches of each strain, causing unreliable outputs in marine larviculture (Léger et al., Reference Léger, Bengtson, Simpson and Sorgeloos1986).
Modern aquaculture is a young industry that has shown impressive growth in the last three decades. A great deal of interest has been generated in developing an artificial larval diet as an alternative to live feed, but the artificial diet is still generally accepted much less than live food (Tandler & Kolkovski, Reference Tandler, Kolkovski, Lavens, Sorgeloos, Jasper and Ollivier1991). Artemia is an excellent live food source in larviculture of crustaceans and fish. Artemia decapsulated cysts (Stael et al., Reference Stael, Sanggontanagit, Van Ballaer, Puwapanich, Tunsutapanich, Lavens, Lavens, Jaspers and Roelants1995), juveniles (Lim et al., Reference Lim, Soh, Dhert and Sorgeloos2001) and adults (Wouters et al., Reference Wouters, Gomez, Lavens and Calderon1999) have the great advantage of satisfying the nutritional requirements of various aquatic species. However, the nauplii are the most widely used organisms for the fry production of marine as well as freshwater fish and crustaceans, due to their convenience as an off-the-shelf food and requiring only 24 hours of incubation from cysts (Sorgeloos, Reference Sorgeloos, Persoone, Sorgeloos, Roets and Jaspers1980; Léger et al., Reference Léger, Bengtson, Simpson and Sorgeloos1986).
Lipids and amino acids are major sources of metabolic energy during the embryonic and pre-feeding larval stages in fish. At hatch, the yolk-sac larvae have high levels of these energy sources, but they are dramatically reduced during the endogenous feeding stage (Evans et al., Reference Evans, Zhu, Parrish, Brown and Shahidi2000). Several studies have shown the important role of essential fatty acids (EFA), such as docosahexaenoic acid (DHA, 22:6n-3), eicosapentaenoic acid (EPA, 20:5n-3), and arachidonic acid (ARA, 20:4n-6) in larval fish nutrition (McEvoy et al., Reference McEvoy, Naess, Bell and Lie1998; Estevez et al., Reference Estevez, McEvoy, Bell and Sargent1999; Sargent et al., Reference Sargent, Bell, McEvoy, Tocher and Estevez1999). The quantitative and qualitative study of EFA requirements is an active research field in marine larviculture in which important progress has been made (Curé et al., Reference Curé, Gajardo, Coutteau, Gajardo and Coutteau1996). Levels of this EFA vary tremendously from strain to strain and even from batch to batch, the causal factor being the fluctuations in biochemical composition of the primary producers available to the adult population (Lavens et al., Reference Lavens, Léger, Sorgeloos, De Pauw, Jaspers, Ackefors and Wilkins1989). Previous studies have focused on the factors affecting the fatty acid profile of cysts and nauplii (Léger et al., Reference Léger, Bengtson, Simpson and Sorgeloos1986; Navarro & Amat, Reference Navarro and Amat1992; Ruiz et al., Reference Ruiz, Medina, Cohen, Amat and Navarro2007a). Specifically, variations in the fatty acid composition of Artemia cysts and nauplii are determined by differences in the fatty acid composition of the food ingested by the parental population (Vos et al., Reference Vos, Léger, Vanhaecke and Sorgeloos1984; Léger et al., Reference Léger, Bengtson, Simpson and Sorgeloos1986; Lavens et al., Reference Lavens, Léger, Sorgeloos, De Pauw, Jaspers, Ackefors and Wilkins1989), the genotype (Navarro & Amat, Reference Navarro and Amat1992) or the selective choice of substrate for feeding (Ruiz et al., Reference Ruiz, Medina, Cohen, Amat and Navarro2007a).
There are two limiting factors that constrain the use of Artemia nauplii in marine aquaculture. The first is related to the shortage of Artemia spp. resources, evidenced by the Artemia cyst crisis of the Great Salt Lake of Utah (Van Stappen, Reference Van Stappen, Lavens and Sorgeloos1997), which was exacerbated by a massive increase in cyst demand corresponding with increases in aquaculture world-wide. The second is fatty acid composition, which determines the nutritional value of different stocks as food for marine fish larvae. In recent years, there has been a worldwide effort to find new Artemia strains and to characterize them with regard to their potential for application in aquaculture. Watanabe et al. (Reference Watanabe, Arakawa, Kitajima, Fukusho and Fujita1978) divided Artemia cyst samples into two categories according to their fatty acid profiles: freshwater-type Artemia, with a high concentration of linolenic acid (ALA, 18:3n-3) and low concentration of eicosapentaenoic acid (EPA, 20:5n-3), which are only suitable for feeding freshwater animals; and marine-type Artemia, with a higher EPA content and generally lower ALA, and which are more suitable for culturing marine species.
The aim of this research was to identify the fatty acid profiles of Artemia cysts sampled in Tunisia in order to obtain information of interest to aquaculture.
MATERIALS AND METHODS
Cysts location and sampling
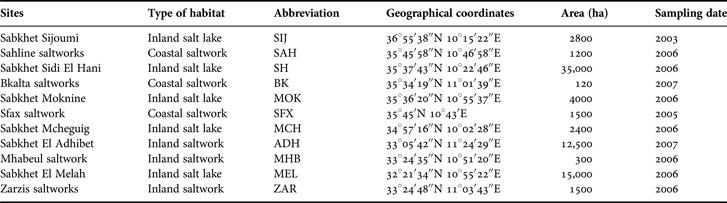
Artemia salina cysts samples were collected directly from the banks of salt ponds of eleven Tunisian salts lakes. They were first kept in saturated brine in order to avoid hydration, and then treated following the protocol described by Sorgeloos et al. (Reference Sorgeloos, Lavens, Léger, Tackaert and Versichele1986). The biotopes and populations from where cysts were sampled are summarized in Table 1. The biotopes were very diverse: most were located inland, but a few were coastal. Sahline saltworks (SAH) and Sabkhet El Adhibet (ADH) populations, described by Ben Naceur et al. (Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2008, Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2010) respectively, are included for the sake of comparison.
Table 1. Sources of Tunisian Artemia cysts studied for their fatty acids composition.

Fatty acid analysis
Prior to lipid extraction, cyst samples were hydrated in distilled water under strong aeration until cysts were observed to be completely spherical. They were then decapsulated with sodium hypochlorite (Sorgeloos et al., Reference Sorgeloos, Lavens, Léger, Tackaert and Versichele1986). Lipid extractions and fatty acid analyses were carried out as in Navarro et al. (Reference Navarro, Amat and Sargent1992a, Reference Navarro, Amat and Sargentb). Total lipids were extracted and stored in chloroform/methanol (ratio 2/1 v/v) with 0.01% butylated hydroxytoluene (BHT) (Sigma Chemical) as an antioxidant. Lipid aliquots were transmethylated overnight after the addition of nonadecaenoic fatty acid (19:0) (99% pure; Sigma Chemical) as an internal standard. Fatty acid methyl esters (FAMEs) were extracted with hexane/diethyl ether (ratio 1/1 v/v) and purified by thin-layer chromatography (silica gel G60, Merck, Darmstadt, Germany) using hexane/diethyl-ether/acetic acid (ratio 85/15/1.5 v/v) as the solvent system. Analyses of FAMEs were performed with a Fisons Instruments GC 8000 (Thermo Electron) gas chromatograph equipped with a fused silica 30 m × 0.25 mm open tubular column (tracer, TR-WAX, film thickness: 0.25 μm; Teknokroma, Barcelona, Spain) and a cold on-column injection system, using helium as carrier and a 50–220°C thermal gradient. Peaks were recorded on a personal computer using the software Chrom-Card for Windows (Fisons CE Instruments, Milan, Italy), and were identified by comparison with known standards. Each fatty acid analysis was done in duplicate except for cysts harvested from Sabkhet Sijoumi (SIJ) and Sabkhet El Adhibet (ADH) where only one sample was subjected to fatty acid analysis.
Statistical analysis
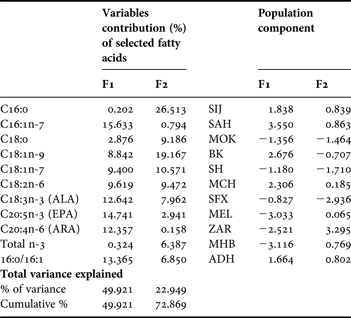
Variables were entered into XLSTAT-Pro 7.5 in order to conduct multivariate principal components analysis (PCA). In this analysis, different variables (relative proportions of selected fatty acids) are displayed on a single plane (principal axis) that accounts for maximum dispersion (variance). Principal component plots are positions of the original variables along the new axes (principal components; PC). Points in the component plot are the variables, and the coordinates of each variable are its factor loadings. Variables at the end of each axis have high loadings on only that factor. Variables near the intersection of the axes are associated with neither factor. The graphical representation (factor score plot) of scores of cases shows the relationships among populations, and is also useful for identifying outliers and unusual cases. The nine most common fatty acids used as variables, for statistical differentiation between population, are the palmitic (16:0), stearic (18:0), palmitoleic (16:1n-7), cis-vaccenic (18:1n-7), oleic (18:1n-9), linoleic (18:2n-6), linolenic (18:3n-3), eicosapentaenoic (20:5n-3) and arachidonic (20:4n-6), in addition to the total n-3 fatty acids and C16:0/C16:1 ratio.
RESULTS
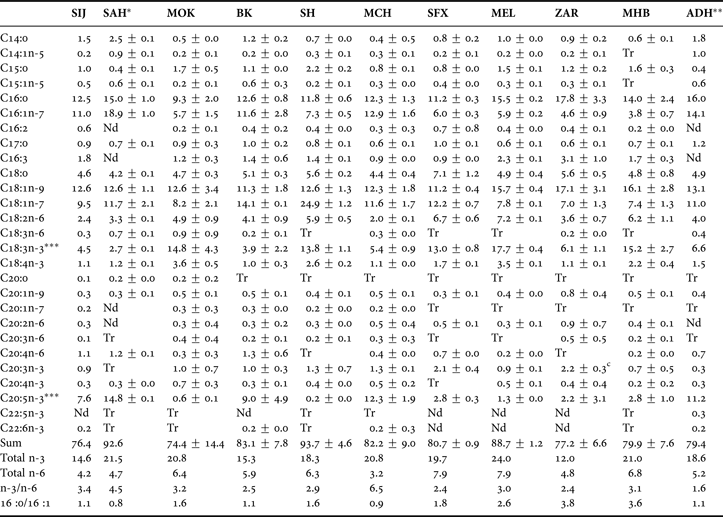
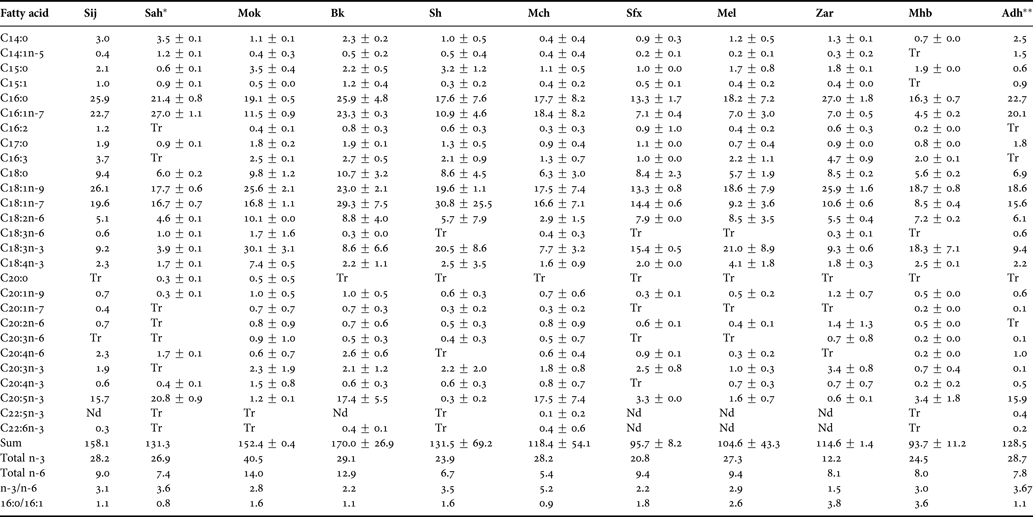
Fatty acid profiles of cysts from the Tunisian Artemia populations investigated are shown in Tables 2 and 3. In all cases, more than 70% of the peaks were identified, and the total sum of the identified FA vary between 95.6 and 158.1 mg.g−1 of dry weight, for Sfax (SFX) and Sijoumi (SIJ) respectively. Palmitic (16:0), palmitoleic (16:1n-7), stearic (18:0), cis-vaccenic (18:1n-7), oleic (18:1n-9), linoleic (18:2n-6), linolenic (18:3n-3) and eicosapentaenoic (20:5n-3) were the major fatty acids. Based on the Artemia classification reported by Watanabe et al. (Reference Watanabe, Arakawa, Kitajima, Fukusho and Fujita1978), Artemia salina populations from Sijoumi (SIJ), Sahline (SAH), Bekalta (BK), Mcheguig (MCH) and El Adhibet (ADH) could be ascribed to marine-type Artemia (high EPA, low ALA and C16:0/C16:1 ratio ≤1.1), whereas the population from Moknine (MOK), Sidi El Hani (SH), Sfax (SFX), El Melah (MEL), Zarzis (ZAR) and Mhabeul (MHB) could be categorized as freshwater-type exhibiting a higher quantity of C18:3n-3 than C20:5n-3, and C16:0/C16:1 ratio higher than 1.6. On the other hand, docosahexaenoic acid (22:6n-3) was absent or found in trace (<0.2%). Proportions of palmitoleic acid (16:1n-7) were higher in marine-type populations. In general, arachidonic acid (20:4n-6) was found in higher quantity in all marine-type cysts (except in those harvested from Mcheguig), than in freshwater-type cysts samples (except in those samples from Sfax).
Table 2. Mean± SD of fatty acid composition (% of total fatty acids) of total lipids from Tunisian populations of Artemia cysts. Data are mean of 2 replicates except for cysts harvested from SIJ and ADH where only one sample was subjected to fatty acid analysis.

Tr, trace amount (<0.2%); Nd, not detected; *, Ben Naceur et al. (Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2008); **, Ben Naceur et al. (Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2010); ***, Ben Naceur et al. (Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2012b).
Table 3. Mean ± SD of fatty acid composition (mg.g−1 DW) of total lipids from Tunisian populations of Artemia cysts. Data are mean of 2 replicates except for cysts harvested from SIJ and ADH where only one sample was subjected to fatty acid analysis.

Tr, trace amount (<0.2%); Nd, not detected; *, Ben Naceur et al. (Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2008); **, Ben Naceur et al. (Reference Ben Naceur, Ben Rejeb Jenhani and Romdhane2010).
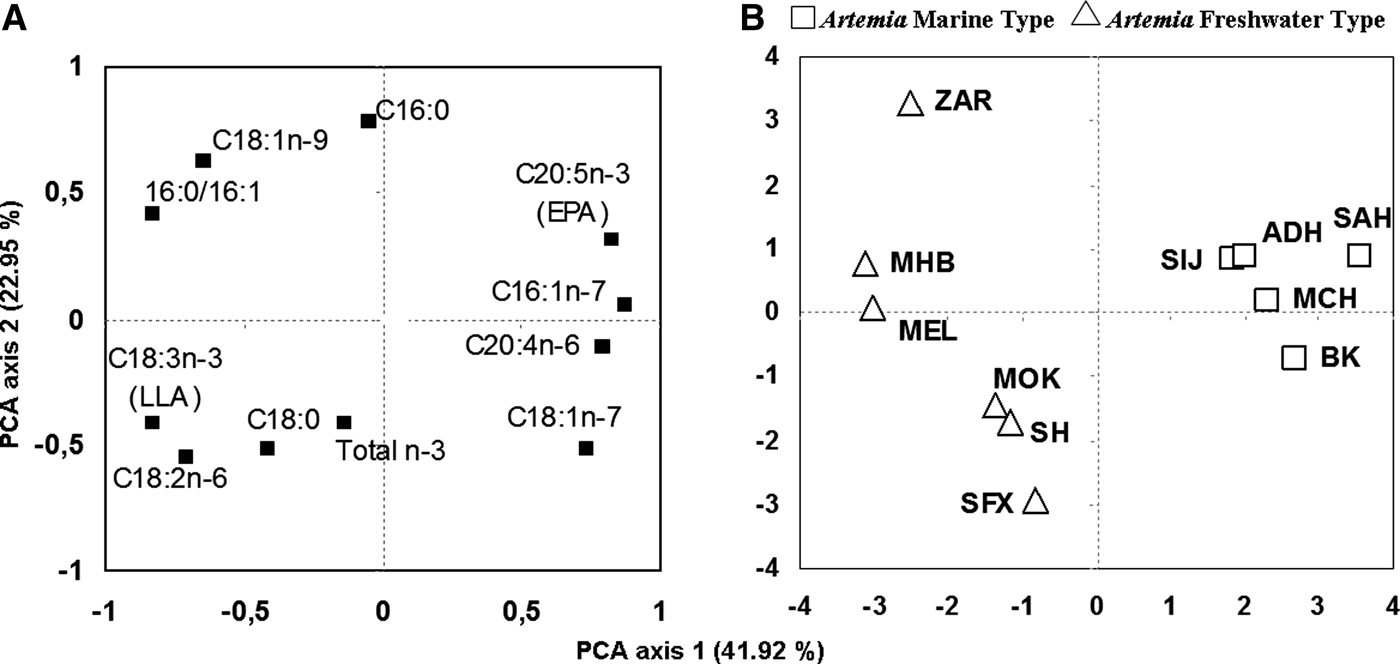
Principal component analysis revealed two main directions of variation. Variables were most correlated with factor 1 (horizontal axis) rather than with factor 2 (vertical axis), with axis 1 explaining 41.92% and axis 2 explaining an additional 22.95% of the total variance. The component plot (Figure 1A) obtained from a PCA illustrates the variables that were responsible for separation along the two PCs calculated. The two PCs of this analysis accounted for 64.87% of the variation in the data set. Variables defining marine-type (C16:1n-7, C20:5n-3 and C20:4n-6) or freshwater-type (C18:3n-3 and C16:0/C16:1 ratio) are associated with component 1. In fact, relatively to the first component (PCA axis 1), palmitoleic acid (16:1n-7), linolenic acid (18:3n-3), eicosapentaenoic acid (20:5n-3), arachidonic acid (20:4n-6) and C16:0/C16:1 ratio are the most important fatty acids variable between cysts samples differentiation with a total contribution of 68.73%. Whereas, for the second component (PCA axis 2), palmitic acid (16:0), cis-vaccenic acid (18:1n-7) and oleic acid (18:1n-9), are the most important variables with a total contribution of 56.25% (Table 4). Negative loadings of linolinic acids (ALA) and C16:0/C16:1 ratio were observed relatively to the principal component 1, whereas palmitoleic acid, eicosapentainoic acid (EPA) and arachidonic acid (ARA) show positive loadings. When the fatty acids data were projected onto the PCs generated, a factor score plot was obtained (Figure 1B) in which the populations were grouped according to variable (fatty acid) loadings. Marine-type populations are located on the right hand side of component 1, and freshwater-type populations on the left hand side. In fact, the ACP divided studied populations into two groups regarding the principal component 1 (Table 4); the first group with a positive populations component including marine-type populations (SIJ, SAH, BK, MCH and ADH), and the second group with a negative populations component including freshwater-type populations (MOK, SH, SFX, MEL, ZAR and MHB). Note that the freshwater-type populations were more dispersed than marine-type populations, which can be explained by the difference observed in the quantity of the fatty acids defining freshwater-type such as C18:1n-9 and C18:2n-6, and C16:0/C16:1 ratio.

Fig. 1. (A) Component plot and (B) factor score plot, of principal component analysis (PCA) of selected fatty acids (% of total fatty acids) from total lipids of Tunisian Artemia cysts.
Table 4. Contribution and portion of each parameter (selected fatty acids in %) used to characterize decapsulated cysts of Tunisian Artemia populations.

DISCUSSION
Since Seale (Reference Seale1933) and Rollefsen (Reference Rollefsen1939) reported the high nutritional value of freshly hatched nauplii of Artemia as food for fish fry, the use of brine shrimp Artemia in aquaculture has increased exponentially. Nevertheless, nutritional deficiencies of the freshly hatched Artemia nauplii have been reported by some workers such as Dannevig & Hansen (Reference Dannevig and Hansen1952), who considered that Artemia diet was not adequate for the culture of larval Clupea, or Morris (Reference Morris1956), who pointed out that fish larvae did not thrive as well on Artemia nauplii which have used up their yolk as they do on freshly hatched nauplii. In fact, in the late 1970s, when many fish and shrimp hatcheries started to be commercial, switching from one source of Artemia to another provoked unexpected problems. Japanese, American and European researchers studied these problems and soon confirmed variations in nutritional value when using different geographical sources of Artemia for fish and shrimp species (see Merchie, Reference Merchie, Lavens and Sorgeloos1996). Studies in Japan and the multidisciplinary ‘International Study on Artemia’ revealed that the concentration of the essential fatty acids (eicosapentaenoic acid, EPA) in Artemia nauplii was determining its nutritional value for larvae of various marine fish and crustaceans (Léger et al., Reference Léger, Bengtson, Simpson and Sorgeloos1986). Therefore, Artemia nauplii have been offered as food source for more than 85% of the marine animals cultivated so far (Kinne, Reference Kinne and Kinne1977). Marine fish, particularly at early stages of development, require highly unsaturated fatty acids (HUFA) of the (n-3) family (Léger et al., Reference Léger, Gatesoupe, Metailler, Luquet and Fremont1979). In fact, as marine fish have a limited capability to elongate and desaturate linolenic acid (18:3n-3) to (n-3) HUFA, they rely on dietary sources of (n-3) HUFA to satisfy their requirements for eicosapentaenoic and docosahexaenoic acid (Watanabe, Reference Watanabe1993). For this fact, the nutritional value of nauplii seems to be determined by their content of (n-3) HUFA.
It is known that the fatty acid composition of Artemia nauplii can vary among strains and also from one batch to another within the same strain (Léger et al., Reference Léger, Bengtson, Simpson and Sorgeloos1986). Several authors reported that the fatty acid profile of Artemia reflect the fatty acid profile of their food resulting from the variations in the composition of the microalgae available in their natural habitat (Léger et al., Reference Léger, Bengtson, Simpson and Sorgeloos1986; Lavens et al., Reference Lavens, Léger, Sorgeloos, De Pauw, Jaspers, Ackefors and Wilkins1989; Navarro & Amat, Reference Navarro and Amat1992). However, Navarro & Amat (Reference Navarro and Amat1992) brought attention to a possible genotypic influence on the fatty acid profile of Artemia, given the presence and proportion of some fatty acids in cysts, irrespective of dietary levels available to parental populations. Most recently, Ruiz et al. (Reference Ruiz, Amat and Navarro2007b), after examination of the fatty acids profile of two Artemia species, A. persimilis and A. franciscana cultured in coexistence at mesocosm scale, revealed that interspecific differences in fatty acid composition are greater than intraspecific variability, demonstrating that aside from a high phenotypic effect of diet on the fatty acid composition of the animals, a species-specific genotypic effect should not be discarded. In the present study we reported the fatty acid profile of eleven Artemia salina populations from Tunisia. The results revealed that, such as for other Artemia populations, palmitic (16:0), stearic (18:0), palmitoleic (16:1n-7), cis-vaccenic (18:1n-7), oleic (18:1n-9), linoleic (18:2n-6), linolenic (18:3n-3) and/or eicosapentaenoic (20:5n-3) acids were the most important fatty acids on Artemia decapsulated cysts (see Navarro et al., Reference Navarro, Amat and Sargent1992a; Abatzopoulos et al., Reference Abatzopoulos, Baxevanis, Triantaphyllidis, Criel, Pador, Van Stappen and Sorgeloos2006; Ruiz et al., Reference Ruiz, Medina, Cohen, Amat and Navarro2007a). In addition, the low rate of the arachidonic acid and/or the lack of the docosahexaenoic acid observed in Tunisian Artemia populations were also reported for other Artemia strains from different geographical origin (see Navarro et al., Reference Navarro, Amat and Sargent1992a; Kara et al., Reference Kara, Bengraine, Derbal, Chaoui and Amarouayache2004; Camargo et al., Reference Camargo, Duran, Rada, Hernandez, Linero, Muelle and Sorgeloos2005; Abatzopoulos et al., Reference Abatzopoulos, Baxevanis, Triantaphyllidis, Criel, Pador, Van Stappen and Sorgeloos2006; Ruiz et al., Reference Ruiz, Medina, Cohen, Amat and Navarro2007a).
Artemia stocks from different geographical origins were divided into two main categories: ‘marine type Artemia’ with a high content in eicosapentaenoic acid (EPA); and ‘freshwater type Artemia’ with a high concentration of linolenic acid (ALA). Moreover, Navarro et al. (Reference Navarro, Amat and Sargent1993) stated that the ratio C16:0/C16:1 was lower in marine-type than in freshwater-type cysts. Although, these authors considered in this ratio the sum of C16:1n-9 and C16:1n-7, our results support this assertion, because the C16:0/C16:1 ratio was lower (0.8–1.1) in all marine-type populations than in freshwater-type (1.6–3.8). It can thus be concluded that the C16:0/C16:1 ratio is a good biomarker that characterizes samples in terms of marine or freshwater-type, especially for Artemia cysts which exhibit approximately the same level of ALA and EPA, and, hence, would be classified as mixed type, such as reported by Ruiz et al. (Reference Ruiz, Medina, Cohen, Amat and Navarro2007a). Furthermore, Watanabe (Reference Watanabe, Espinosa de los Monteros and Labarta1987) reported that ARA was associated with marine-type Artemia. In our case, all marine-type populations described in this work exhibited higher values of ARA than did freshwater types, except for MCH population (marine-type Artemia; inland site) and SFX population (freshwater-type Artemia; costal site). This result is in contrast with the results of Navarro et al. (Reference Navarro, Amat and Sargent1992a), where this fatty acid was present regardless of the inland (freshwater-type) or coastal (marine-type) origin of populations. Otherwise, PCA revealed that palmitoleic acid (16:1n-7), linolenic acid (18:3n-3), eicosapentaenoic acid (20:5n-3), arachidonic acid (20:4n-6) and C16:0/C16:1 ratio are the most important fatty acids that contribute to the discrimination between freshwater and marine-type Artemia with a total contribution of 68.73%, according to the first component, explaining 41.92% of the total variance. While, according to the second component (explaining 22.95% of the total variance), palmitic (16:0), cis-vaccenic (18:1n-7) and oleic (18:1n-9) represents the major fatty acids permitting the differentiation between strains from the same Artemia type, specially for freshwater-type Artemia, with a total contribution of 56.25%.
Considering the potential use of the Tunisian Artemia population in aquaculture, our results revealed that Sijoumi, Sahline, Bekalta, Mcheguig and El Adhibet populations exhibit higher values of EPA, ranging from 15.7 to 20.8 mg.g−1 dry weight. These values were similar to or even higher than those recorded for other Artemia strains: 0.3–2.4 mg.g−1 dry weight for San Francisco Bay and 0.3–8.6 mg.g−1 dry weight for Colombian strains (Camargo et al., Reference Camargo, Duran, Rada, Hernandez, Linero, Muelle and Sorgeloos2005), 1.8–7.2 mg.g−1 dry weight for Artemia urmiana (Abatzopoulos et al., Reference Abatzopoulos, Baxevanis, Triantaphyllidis, Criel, Pador, Van Stappen and Sorgeloos2006), 21.6–43.0 mg.g−1 dry weight for Artemia tibetiana (Van Stappen et al., Reference Van Stappen, Sui, Xin and Sorgeloos2003) and 2.4–10.96 mg.g−1 dry weight for Spanish strains (Navarro et al., Reference Navarro, Amat and Sargent1992a). Merchie (Reference Merchie, Lavens and Sorgeloos1996) revealed that commercial provisions of Artemia cysts containing high EPA levels are limited and, consequently, these cysts are very expensive. The relationship between fatty acid composition of Artemia nauplii used for aquaculture purposes and the successful culture of several fish and crustacean species is well studied, in particular the levels of EPA and DHA, as well as their ratio (DHA/EPA) (Sui et al., Reference Sui, Wille, Cheng and Sorgeloos2007). These fatty acids play a crucial role in aquaculture applications for the formation of biological membranes, growth, stress resistance and pigmentation in cultured marine organisms (Mourente et al., Reference Mourente, Rodriguez, Tocher and Sargent1993). However, as in other natural Artemia populations, the ratio DHA/EPA was found in a low level in these Tunisian studied populations. Moreover, apart from these two essential fatty acids, it has been shown that arachidonic acid (ARA) also plays a significant role on the fish larval growth and pigmentation in several marine fish (Koven et al., Reference Koven, Barr, Lutzky, Ben-Atia, Harel, Behrens, Weiss and Tandler2000) since it provides precursors for eicosanoid production (Castell et al., Reference Castell, Bell, Tocher and Sargent1994). However, the requirement for ARA in fish seems to depend on the fish species and larval development, and needs to be dosed with extreme care since its effect seems to depend on the DHA concentration (Koven et al., Reference Koven, Barr, Lutzky, Ben-Atia, Harel, Behrens, Weiss and Tandler2000). The comparison between ARA contents obtained in the studied Tunisian population (from <0.2 to 1.5 mg.g−1 dry weight) and those reported for other Artemia strain revealed that this fatty acid was present in more or less similar quantity (Navarro et al., Reference Navarro, Amat and Sargent1992a; Camargo et al., Reference Camargo, Duran, Rada, Hernandez, Linero, Muelle and Sorgeloos2005; Ruiz et al., Reference Ruiz, Medina, Cohen, Amat and Navarro2007a). Therefore, to ensure these quantities in EPA (for freshwater-type Artemia cysts), ARA and DHA, special enrichment formulations had to be developed (Sorgeloos et al., Reference Léger, Bengtson, Simpson and Sorgeloos1986).
In conclusion, Tunisian Artemia cysts show that, as for the other Artemia populations, palmitic (16:0), palmitoleic (16:1n-7), stearic (18:0), cis-vaccenic (18:1n-7), oleic (18:1n-9), linoleic (18:2n-6), linolenic (18:3n-3) and eicosapentaenoic (20:5n-3) were the major fatty acids, and that Artemia salina populations from Sijoumi, Sahline, Bekalta, Mcheguig and El Adhibet could be ascribed to marine-type Artemia, whereas the population from Moknine, Sidi El Hani, Sfax, El Melah, Zarzis and Mhabeul could be categorized as freshwater-type. PCA revealed that palmitoleic acid (16:1n-7), linolenic acid (18:3n-3), eicosapentaenoic acid (20:5n-3), arachidonic acid (20:4n-6) and C16:0/C16:1 ratio are the most important fatty acids which contribute to the discrimination between freshwater and marine-type Artemia, and that palmitic (16:0), cis-vaccenic (18:1n-7) and oleic (18:1n-9) represents the major fatty acids permitting the differentiation between strains from the same Artemia type, specially for freshwater-type Artemia. Finally, further research on marine and freshwater-type Artemia is needed to clarify the role of environmental factors and genetic characteristics which can affect the fatty acid profile.
ACKNOWLEDGEMENT
The authors thank Professor Navarro Juan-Carlos from Instituto de Acuicultura de Torre de la Sal (Spain) who gave many useful suggestions and made FAMEs injection.