Introduction
Honey bees (Apis mellifera ligustica) provide important pollination services that ensure food security and improve the nutritional value of human diets, thus they have a high economic value (Vanbergen, Reference Vanbergen2013). Additionally, honey bees play a vital role in the balance between agricultural economic development and regional ecology as social insects and pollinators of flowering plants (Zhang et al., Reference Zhang, Chen, Li, Ma, Yu, Wang, Liu and Xu2018). In order to maintain normal growth, development, and reproduction, honey bees must constantly obtain pollen from the nature as the material basis of life activities (Manning, Reference Manning2001). In their natural state, honey bees obtain fats, proteins, sterols, vitamins, and minerals from pollen. However, the lipid concentration and fatty acid composition of pollen differ markedly among various pollen types; additionally, pollen is only utilized seasonally in some countries and regions. In modern agriculture, beehives are frequently placed in large monoculture areas, where bees forage on a single pollen type (Naug, Reference Naug2009). This could lead to malnutrition owing to low pollen quantity, quality, or diversity; malnutrition can be exacerbated in agricultural monocultures (Stoate et al., Reference Stoate, Boatman, Borralho, Carvalho, Snoo and Eden2001; Kremen et al., Reference Kremen, Williams and Thorp2002; Hendrickx, Reference Hendrickx2007) and greenhouses (Kalev et al., Reference Kalev, Dag and Shafir2002). Insects only have trace amounts of polyunsaturated fatty acids (PUFAs) and they lack the necessary enzymes to synthesize them (Shen et al., Reference Shen, Lai, Feng, Parnell, Wan, Wang, Li, Ordovas and Kang2010). Fatty acids are the main components of membranes and play an important role in their function (Hulbert and Abbott, Reference Hulbert and Abbott2012). They are also essential for reproduction and development and are sources of energy and fat body development during the winter (Manning, Reference Manning2001). PUFAs can enhance the phagocytosis and killing ability of animal macrophages, promote the natural killer cells response, and stimulate the non-specific immune function of animals to protect animal health (Li et al., Reference Li, Chen, Qin, Yu, Chen, Ding and Li2015). PUFAs play important roles in improving the survival and antioxidant capacity of an organism as well as in promoting anti-stress and preventing disease (Wu and Chen, Reference Wu and Chen2012; Jin et al., Reference Jin, Lu, Yuan, Li, Qiu, Sun, Ma, Ding and Zhou2017).
Arachidonic acid (ARA), an omega-6 (n-6) PUFA, is an important component of membranes, as it helps maintain membrane mobility and prevent inflammation. ARA, as the precursor of a series of bioactive substances such as prostaglandins, thromboxanes, and leukotrienes (Piomelli, Reference Piomelli1993), plays a series of vital roles in the insect immune defense response and has a variety of cell defense functions (Tallima and Ridi, Reference Tallima and Ridi2018). Both ARA and eicosanoids are implicated in lipid hydrolysis (Fain et al., Reference Fain, Leffler and Bahouth2000), glucose transportation (Nugent et al., Reference Nugent, Prins, Whitehead, Wentworth, Chatterjee and O'Rahilly2001), and other functions in adipose tissue. ARA and its metabolites act as primary messengers, participate in multiple physiological and pathological processes in the body, and play an important role in the process of parasitic infection of host cells (Aguilar et al., Reference Aguilar, Racotta, Goytortúa, Wille, Sorgeloos, Civera and Palacios2012). ARA can increase the expression of c-fos and egr-1 and promote bodily growth and development by inducing cell growth (Bessonart et al., Reference Bessonart, Izquierdo, Salhi, Hernández-Cruz, González and Fernández-Palacios1999; Koven et al., Reference Koven, Barr, Lutzky, Ben-Atia, Weiss and Harel2001). ARA can alter the physical stability of the cell membrane, signaling pathways, and the pattern of lipid mediators, which subsequently affect immune responses (Norambuena et al., Reference Norambuena, Morais, Estévez, Bell, Tocher, Navarro, Cerdà and Duncan2013). Some studies showed that appropriate ARA levels in diets were helpful in maintaining growth performance and physiological processes and in improving the antioxidant status and immune response of organisms (Gabler et al., Reference Gabler, Spencer, Webel and Spurlock2008; Tian et al., Reference Tian, Ji, Oku and Zhou2014). Luo et al. (Reference Luo, Tan, Li and Yin2012) found that dietary ARA contributes to improved growth and survival rates. Atalah et al. (Reference Atalah, Hernández-Cruz and Benítez-Santana2011) showed that ARA levels in the diet of gilthead sea bream (Sparus aurata) larvae affected their tissue fatty acid profile. It has been confirmed that in fish (Khozin et al., Reference Khozin, Cohen, Pimenta, Nechev and Zilberg2006), such as Crassostrea gigas (Delaporte et al., Reference Delaporte, Soudant, Moal, Giudicelli, Lambert and Séguineau2006) and Crassostrea corteziensis (Hurtado et al., Reference Hurtado, Reza, Ibarra, Wille, Sorgeloos, Soudant and Palacios2009), dietary ARA can regulate the immune and antibacterial functions of the organism.
Malnutrition leads to weight loss, a reduced life span, and possibly damages the growth of the larvae and the queen (Zaytoon et al., Reference Zaytoon, Matsuka and Sasaki1988; Schmidt et al., Reference Schmidt, Schmidt, Rao, Wang and Xu1995; Brodschneider and Crailsheim, Reference Brodschneider and Crailsheim2010), thereby becoming one of the main causes behind the decline of bee populations. At present, there is an increasing number of studies on the nutrition and physiology of honey bees, but no studies have been performed on the potential modulatory influence of ARA on the non-specific immune system of honey bees. Moreover, few studies have examined the fatty acid composition of bees following a dietary supplementation with ARA. In order to systematically evaluate the nutritional influences of ARA on honey bees; we examined the effects of dietary ARA on the growth, longevity, fatty acid profiles, non-specific and adaptive immune functions, and relative expression levels of immune-related genes of bees. The results of this study could provide a scientific basis for the formulation of feeding standards for A. m. ligustica.
Materials and methods
Experimental design and experimental diets
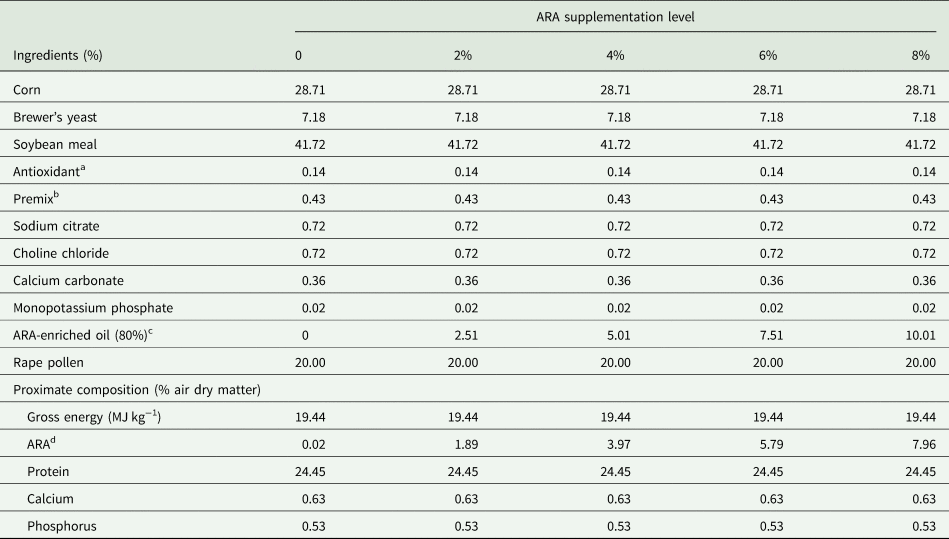
The experiment was performed at Shandong Agricultural University (Tai'an, China). Twenty-five bee colonies headed by sister queens from the same breeding line were randomly allocated into five groups with five colonies each. The control group (group A) was fed a basal diet in which no ARA was added. The four experimental groups (B, C, D, and E) were fed diets supplemented with different ARA (ARA content: 80% of total fatty acids in the form of ARA-methyl ester; Jiangsu TianKai Biotechnology, Nanjing, China) levels (2, 4, 6, and 8%, respectively). The compositions of the basal diets are shown in table 1.
Table 1. Basal diet formulation and compositions (%)

a Antioxidant were used to prevent the powder from deteriorating.
b The premix provided per kg of diet vitamin VA 5000 IU, VB1 7.5 mg, VB2 6.8 mg, VC 303.2 mg, VE 240 mg, VD 2000 IU, VB6 13 mg, niacin 24 mg, folic acid 20 mg, inositol 500 mg.
c An oily substance containing 80% ARA.
d The values of ARA were measured, and the other nutrient levels were calculated.
Feeding trials
The ingredients of each diet were first homogenized and then oil and sucrose were added to the mixture and mixed thoroughly. A certain amount of water was added to moisten the diets. All diets were kneaded until the homogenous dough was formed and were then placed in the middle of the hive on top of the uppermost frames in each of the 25 colonies. During the experimental period, the colonies were examined every 3 days and their diets were renewed to make sure they had soft and moist food. The experimental period was 50 days, including 12 days of preliminary feeding period and 38 days of official test period.
Sample collection
To obtain the newly emerged worker bees, the queen of each colony was confined on an empty comb for 5 h using a queen spawning controller (Changge Jihong Beekeeping Equipment Co., He'nan, China). The laid-comb was then transferred to the super and the queen was kept in the lower chamber. A queen excluder was used to separate the queen from the super (Wang et al., Reference Wang, Ma, Zhang, Cui, Wang and Xu2016). The sealed brood comb was put into a worker bee controller equipment (Changge Jihong Beekeeping Equipment Co.) which can separate the newly emerged worker bees from other bees. New bees begin to emerge 21 days after the queen lays her eggs. Two hundred newly emerged worker bees in worker bee controller equipment per colony were labeled with paint on their thorax within 5 h after the emergence of the worker bees. The painted worker bees are released from the controller. One hundred and seventy painted worker bees were collected from each colony in 7 and 14 days of age (85 per day) and were stored at −80°C immediately for the enzyme activity and genes expressions determination.
Emerged weight of worker bees
Twenty newly emerged worker bees (5 h-old) per colony were obtained for measuring the body weight. Each worker bee was placed in a 2 ml plastic centrifuge and weighed individually using an electronic scale (precision: 0.1 mg). The experiment was repeated three times and the averages were used for statistical analysis.
Fatty acid analysis
Thirty 14-day-old worker bees per colony were used for the fatty acid analysis. (Each sample was a mixing pool of ten bees, three samples per colony.) The complete gastrointestinal tracts of worker bees were removed by pulling out the terminal abdominal segment with forceps. The lipid content of bees was determined by chloroform-methanol extraction method (Wang et al., Reference Wang, Chen and Cui1993). The bees were dried overnight at 105°C to determine moisture content, pulverized in an electric blender, and added to a 10 ml screw-cap volumetric glass tube. Lipids for fatty acid analysis were extracted by a 2 ml benzene and petroleum ether (1:1 v/v) mixture for 24 h. Then, 2 ml of 0.4 mol l−1 potassium hydroxide in a methanol solution was added and set aside for 15 min after shaking up. The ddH2O was slowly added into a glass tube along the tube wall and the glass tube was gently turned until the liquid's level rose to the bottle's mouth. Then, the supernatant was sucked into the centrifuge tube, was centrifuged at 12,000 rpm for 5 min, and put in a sample bottle for analysis. The analysis of fatty acid methyl esters was performed using a gas chromatograph with a mass detector (GC-MS) of the ion trap type (Agilent technologies 7890/5975, USA), equipped with an HP-5 capillary column (30 m, 0.32 mm i.d., 0.25 mm film thickness). The carrier gas was helium, at a flow rate of 1 ml min−1. The sample volume of 1 μl was injected at 280°C (split ratio 1:20). Column temperature was initially 80°C for 1 min, then gradually increased to 180°C at 20°C min−1, and finally increased to 320°C at 5°C min−1. For GC-MS detection, an electron ionization system was used with ionization energy of 70 eV. Ion source and ion trap temperatures were set at 180 and 280°C, respectively.
Phenoloxidase, antiprotease, and lysozyme activity
Each sample pool of 10 worker bees was homogenized with phosphate-buffered saline (PBS) at a ratio of 1:9 (w/v) for 180 s. Then, the mixture was centrifuged at 12,000 rpm for 15 min at 4°C and the supernatant was transferred into a new centrifuge tube. PO, antiprotease, and lysozyme assays were performed by insect enzyme-linked immunosorbent assay (MLBIO Biotechnology, Shanghai, China), according to the kit's instructions. The experiment was repeated three times and the averages were used for statistical analysis.
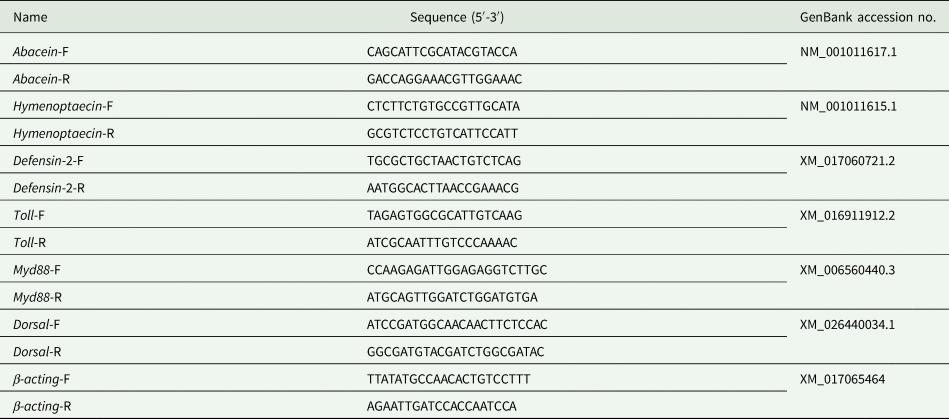
qRT-PCR analysis
Total RNA was extracted from 7- and 14-day-old worker bees by the Total RNA Kit II (OMEGA, Norcross, Georgia, USA). The purity and quality of the RNA were determined by using an ultramicro spectrophotometer (DS-11; DeNovix Inc., Wilmington, USA). The Transcriptor First Strand cDNA Synthesis Kit (Roche, Japan) was used to synthesize first-strand cDNA, which was stored at −20°C. The design and synthesis of all the qRT-PCR primers were completed by Sangon Biotechnological Company (Shanghai, China). All sequences of the PCR primers are listed in table 2. The relative expression levels of target genes were detected by TransStart Top Green qPCR SuperMix (TransGen Biotech, Beijing, China) in the 7500 Real-time System (ABI, USA). The reactions were performed in a final volume of 20:0.4 μl for each primer (10 μmol l−1), 2 μl cDNA (50 ng μl−1), 0.4 μl passive reference dye, 10 μl Taq DNA polymerase (5 U μl−1, TransGen Biotech), and 6.8 μl nuclease-free water. The reaction conditions were as follows: (1) pre-denaturation for 30 s at 94°C; (2) 40 cycles of amplification (denaturation for 5 s at 94°C, annealing and extension for 34 s at 60°C). Eight bees per colony were used for qRT-PCR. Three technical replicates were performed for each sample. The β-actin gene (Gene Bank accession no. XM_017065464) was used as the reference gene. Relative expression levels of genes were calculated using the 2−ΔΔCt method.
Table 2. The sequences of gene primers for qRT-PCR

Challenge test
On day 30 after the beginning of the feeding trials, 30 newly emerged worker bees (less than 5 h of age) were captured from each of 25 colonies and were placed in a wooden cage (10 cm × 7 cm × 8 cm). Each cage was provided with a 50% (w/v) sucrose solution, clean water, and the corresponding diet with the worker bees’ original colony. The experiment still included five diet treatments with 0, 2, 4, 6, and 8% ARA, respectively (5 cages per treatment, 30 bees per cage). The bee cages were kept in an incubator (32 ± 1°C, 57 ± 10% relative humidity, and darkness). On days 7 and 14, all worker bees were injected dorsally with 1 μl of 108 viable Escherichia coli bacteria diluted in PBS (Heike et al., Reference Heike, Klara, Tautz, Hildburg and Muriel2013). For injection, we used disposable calibrated (1–5 μl) glass capillaries with fine tips. The injections were administered between the second and third abdominal tergites. As a negative control, worker bees were injected dorsally with 1 μl of sterile saline. After infection, the dead bees were removed from each cage and a census of the remaining bees was performed every 24 h for 30 days. The data were used to plot a survival curve.
Statistical analysis
Statistical analysis was performed using the SAS 9.1 software (SAS, USA). The data values in each group were normally distributed or approximately normally distributed, and the variances were homogeneous using Levene's test. So, differences among groups data were analyzed using one-way ANOVA. A P-value <0.05 was considered statistically significant. The data are presented as the mean ± SEM.
Results
Emerged weight of worker bees
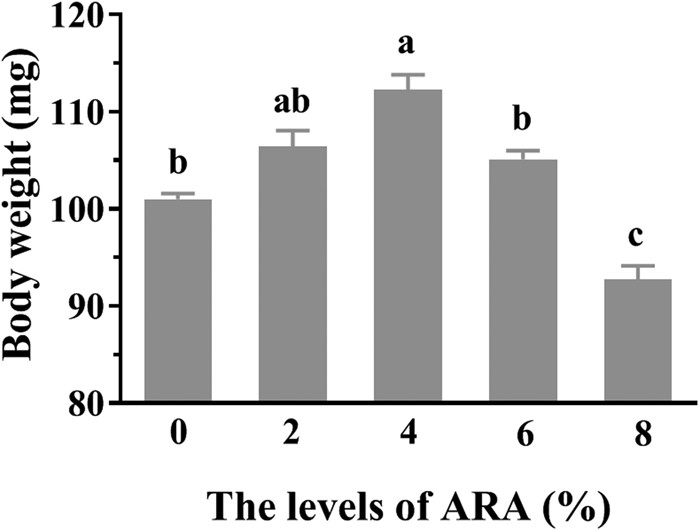
The average wet weight of emerged worker bees during the experiment is shown in fig. 1. Diets containing different ARA concentrations during early spring had a significant effect on the wet weight of emerged worker bees (P < 0.05) (fig. 1). The average wet weight of emerged worker bees was the highest in the 4% ARA group (112.24 mg). Non-significant differences were detected between the 4 and 2% ARA groups (P > 0.05) (fig. 1). In contrast, the 8% ARA group had the lowest weight (92.73 mg). Furthermore, there were no significant differences between the 6% ARA group and the control group (0% ARA) (P > 0.05) (fig. 1).

Figure 1. The effects of different dietary ARA levels on emergence weight of worker bees. Vertical bars represent the mean ± SEM (n = 300) and letters represent significant differences (P < 0.05).
Analysis of fatty acid composition in the bodies of 14-day-old worker bees
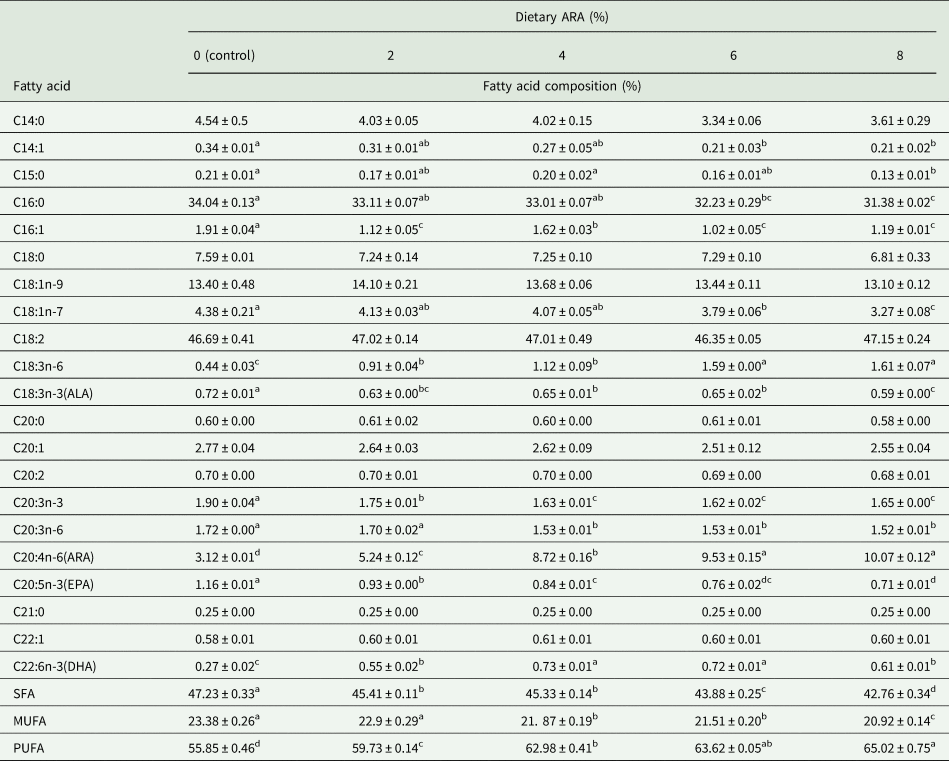
Table 3 displays the analysis of the fatty acid composition in the bodies of 14-day-old worker bees. The diets containing different ARA concentrations had a significant influence on the fatty acid composition in the bodies of 14-day-old worker bees; the proportion of ARA increased significantly, reaching 10.07% in the high level groups, as compared to 3.12% in the control group (P < 0.05) (table 3). Along with the increase in ARA, there were significant decreases in the proportion of saturated (SFA) and monounsaturated fatty acids (MUFA), especially C15:0 and C16:0, compared with the control group (P < 0.05) (table 3). A significant increase in PUFAs, especially ARA and docosahexanoic acid (DHA), was observed in ARA-supplemented groups (P < 0.05), while the opposite was true for α-linolenic acid, γ-linolenic acid, and eicosapentanoic acid (EPA) (P < 0.05) (table 3).
Table 3. Effects of ARA levels on fatty acid composition of 14-day-old worker bees

SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; ALA, α-linolenic acid; ARA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
In the same row, values with different small letter superscripts mean significant difference (P < 0.05), while with the same or no letter superscripts mean no significant difference (P > 0.05).
Note: The contents of some fatty acids that are minor, trace amount, or not detected were not listed in the table.
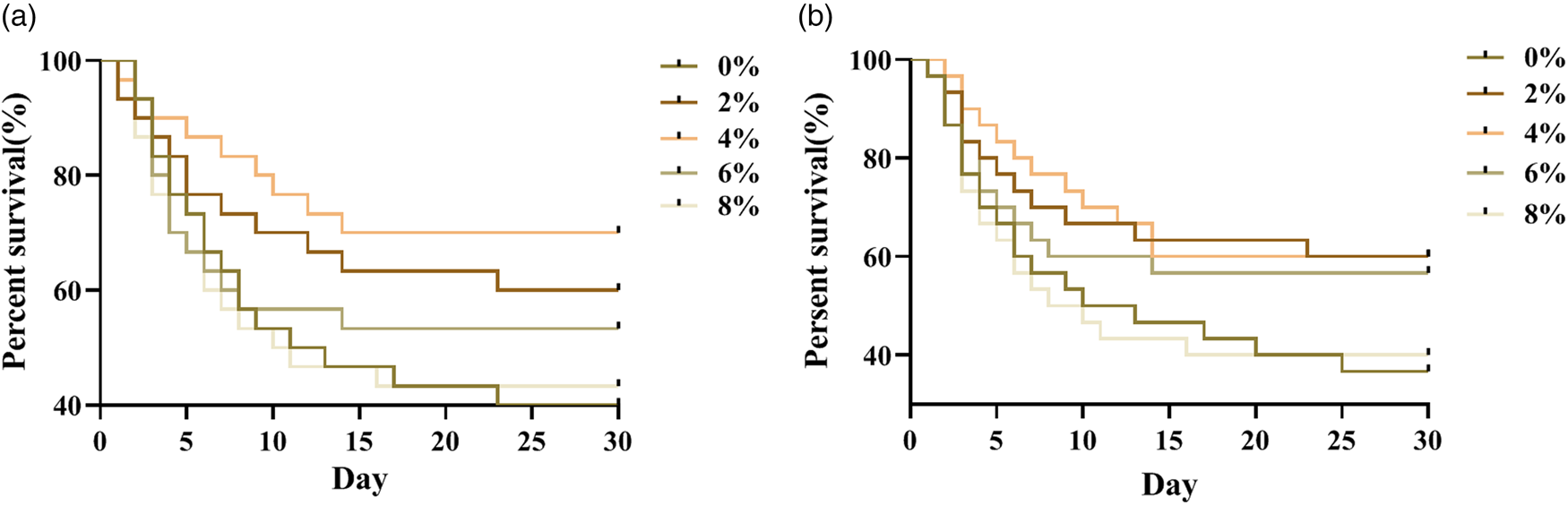
Survival rate of honeybees infected with E. coli
Worker bees were infected with E. coli by injection, respectively, at 7 and 14 days old. The deaths of bees were recorded every day to plot the survival curve (fig. 2). The results showed the dietary ARA levels have significant effects on the survival rate of bees infected with E. coli (P < 0.05). Adding 2, 4 and 6% ARA could effectively increase the survival rate of bees infected with E. coli at 7 or 14 days of age. Of those, 4% addition level worked best. This suggested that ARA have the potential to help bees fight infection. It was speculated that ARA may affect the immune function of bees. Therefore, the effects of ARA on the immune-enzyme activity and the expression of immune-related genes in bees were examined in the following experiment.

Figure 2. The effects of different dietary ARA levels on survival rate of worker bees infected with E. coli bacteria for 30 days. (a) The worker bees were injected dorsally with 1 μl of 108 viable Escherichia coli bacteria diluted in phosphate-buffered saline (PBS) at 7 days old. (b) The worker bees were injected dorsally with 1 μl of 108 viable Escherichia coli bacteria diluted in phosphate-buffered saline (PBS) at 14 days old (n = 150).
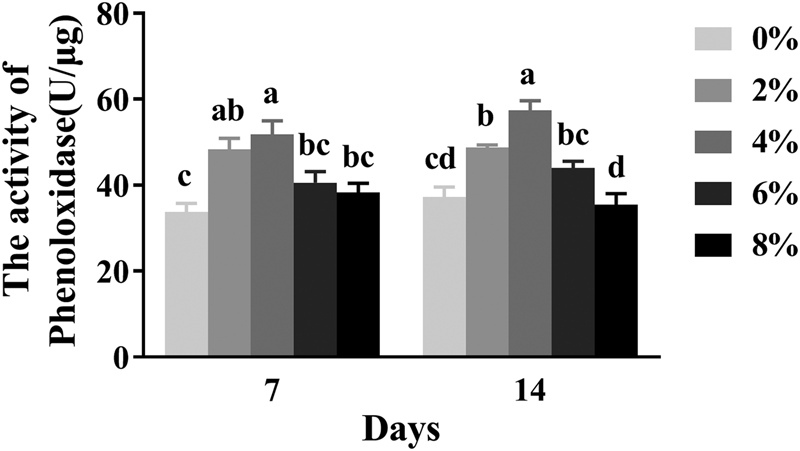
Phenoloxidase activity
The activity of phenoloxidase (PO) in 7- and 14-day-old worker bees is shown in fig. 3. Diets containing different ARA concentrations had a significant effect on the activity of PO in 7- and 14-day-old worker bees (P < 0.05). The PO activity of 7- and 14-day-old worker bees appeared to increase at first and then decreased, compared with the control group, with increasing ARA levels. The PO activity of 7- and 14-day-old worker bees in the 4% ARA group was the highest, while the PO activity in the 2% ARA group was significantly higher than that of the control group (0% ARA) (P < 0.05). However, the PO activity of 14-day-old worker bees in the 6 and 8% ARA groups was not significantly different from that of the control group (P > 0.05) (fig. 3).

Figure 3. The effects of different dietary ARA levels on polyphenol oxidase (PO) activity of worker bees. Vertical bars represent the mean ± SEM (n = 40) and letters represent significant differences (P < 0.05).
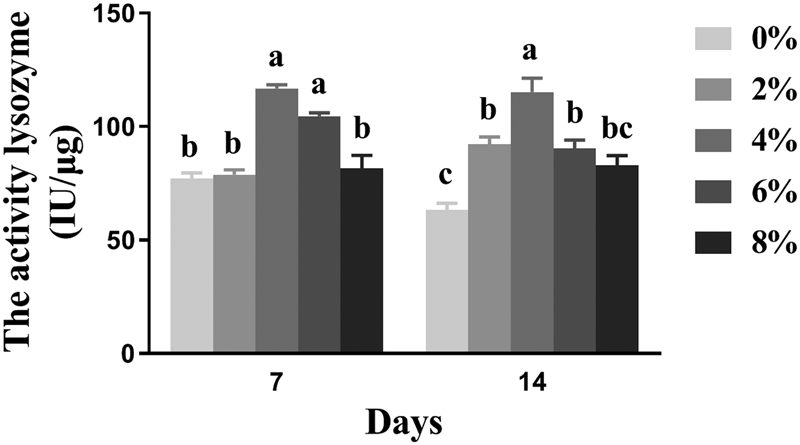
Lysozyme activity
The influence of different ARA levels on the lysozyme activity of worker bees is shown in fig. 4. A significant increase in the lysozyme activity of 7-day-old worker bees was observed in groups fed the 2 and 4% ARA diets (P < 0.05); the group fed the 4% ARA diet had the highest lysozyme activity among all experimental groups (fig. 4). No significant changes were observed in the lysozyme activity of the control, 6% ARA, and 8% ARA groups (P > 0.05) (fig. 4). The lysozyme activity of 14-day-old worker bees in the 2 and 4% ARA groups was significantly higher than that of the control group (P < 0.05); the group fed the 4% ARA diet had the highest lysozyme activity (fig. 4). There were no significant differences (P > 0.05) in the lysozyme activity of 14-day-old worker bees between the 6% ARA and control groups. The 8% ARA group had the lowest lysozyme activity (fig. 4).

Figure 4. The effects of different dietary ARA levels on lysozyme activity of worker bees. Vertical bars represent the mean ± SEM (n = 40) and letters represent significant differences (P < 0.05).
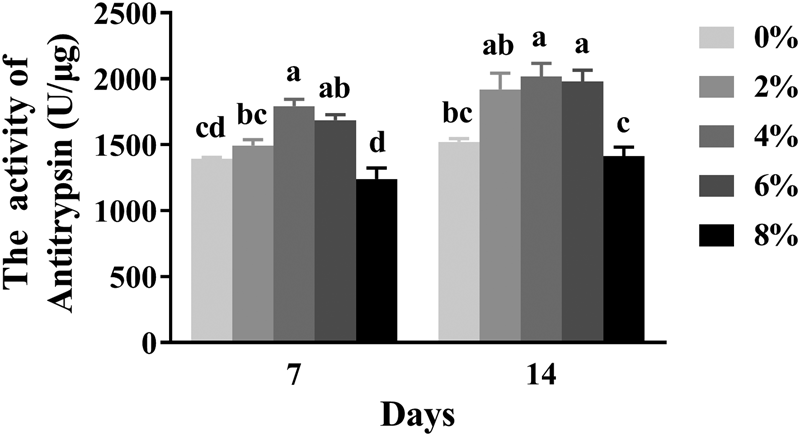
Antitrypsin activity
The effects of different dietary ARA levels on the antitrypsin activity of 7- and 14-day-old worker bees are shown in fig. 5. The antitrypsin activity of 7- and 14-day-old worker bees was influenced significantly by different dietary ARA levels (P < 0.05). The antitrypsin activity of 7-day-old worker bees was significantly higher in the 4 and 6% ARA groups than in the groups fed other diets (P < 0.05). The lowest antitrypsin activity was detected in the 8% ARA group (fig. 5). The antitrypsin activity of 14-day-old worker bees increased significantly in the 2, 4, and 6% ARA groups (P < 0.05). No significant differences were observed in the activity of antitrypsin between the 8% ARA and control groups (P > 0.05) (fig. 5).

Figure 5. The effects of different dietary ARA levels on antitrypsin activity of worker bees. Vertical bars represent the mean ± SEM (n = 40) and letters represent significant differences (P < 0.05).
Genes expression profiles of immune-related signaling molecules
Different dietary ARA levels had significant influences on the mRNA expressions of antimicrobial peptides (AMPs) and Toll signaling pathway-related genes (fig. 6). The hymenoptaecin mRNA expression increased at first and then decreased compared with the control group, with increasing dietary ARA levels. The maximum mRNA expression in 7- and 14-day-old worker bees was observed in the 4% ARA group (P < 0.05). The same gene expression trend was obtained for the defensin-2, toll, myd88, and dorsal genes. The lowest gene expression in 7- and 14-day-old worker bees was observed in the 8% ARA group (P < 0.05). No significant differences were observed in the mRNA expression of abaecin, hymenoptaecin, defensin-2, toll, and myd88 of 7-day-old worker bees between the 2% ARA and the control group (P > 0.05). The mRNA expression of toll and myd88 in 14-day-old worker bees did not differ significantly between the 2 and 4% ARA groups (P > 0.05); in both of these groups, the mRNA expression of the aforementioned genes was significantly higher than in the control group (P < 0.05).

Figure 6. The effects of diets with different ARA concentrations on the relative mRNA expressions of immune-related genes. (a) The mRNA expressions of antimicrobial peptides (AMP) genes in worker bees at 7 days old. (b) The mRNA expressions of Toll signaling pathway-related genes in worker bees at 7 days old. (c) The mRNA expressions of antimicrobial peptides (AMP) genes in worker bees at 14 days old. (d) The mRNA expressions of Toll signaling pathway-related genes in worker bees at 14 days old. Vertical bars represent the mean ± SEM (n = 40) and letters represent significant differences (P < 0.05).
Discussion
It was found that Drosophila lack the necessary enzymes to synthesize long-chain PUFAs (Shen et al., Reference Shen, Lai, Feng, Parnell, Wan, Wang, Li, Ordovas and Kang2010) which is the reason for long-chain PUFAs occurring in very low concentrations in insects (Stanley-Samuelson and Pedibhotla, Reference Stanley-Samuelson and Pedibhotla1996). Arien et al. (Reference Arien, Dag, Zarchin, Masci and Shafir2015) found low concentrations of eicosatrienoic acid (C20:3n-3 cis) in bee brains and bodies. This finding supports the hypothesis that C20 PUFAs are present in insects, but in very low concentrations, sometimes just around detection level (Stanley-Samuelson and Pedibhotla, Reference Stanley-Samuelson and Pedibhotla1996; Jeffries et al., Reference Jeffries, Dempsey, Behari, Anderson and Merkler2014). We detected 3.12–10.07% ARA in bees’ body depending on the different ARA levels in diets that suggested ARA has necessary functions for bee health. Nutriology call these fatty acids, which are necessary for the body's growth and development but cannot be synthesized by themselves and can only be obtained from the diet, essential fatty acids. A lack of essential fatty acids, such as ARA, in food can lead to the death of insect larvae, molting failure, abnormal development of adult insects, and reduced fecundity (Ma et al., Reference Ma, Wang, Hang, Wang, Yang and Xu2015). The content of ARA in the natural food of bees varies greatly. The average contents of ARA in rape pollen, lotus pollen, and tea pollen are 0.12, 0.14, and 0.25 mg/g, respectively (Yang, Reference Yang2014). Some species of pollens are deficient in ARA. This suggested that supplemental ARA in bees’ diet artificially would be necessary. However, there are few studies examining the effect of ARA on the growth performance of bees, while there are many studies on mammals and aquatic species. Bessonart et al. (Reference Bessonart, Izquierdo, Salhi, Hernández-Cruz, González and Fernández-Palacios1999) showed that a diet deficient or abundant in ARA inhibits the growth and development of gilthead sea bream. Yuan et al. (Reference Yuan, Li, Mai, Xu, Zhang and Ai2015) showed that the growth rate of larval half-smooth tongue sole fed a diet with 1.42% ARA was significantly higher than that of other groups. In the present study, the average weight of emergent worker bees increased significantly when ARA was added in 2 and 4% in the diet; however, the 8% ARA group had the lowest weight. These results suggest that the appropriate ARA level could be vital for bees to maintain a normal growth, while insufficient or excessive dietary ARA levels may inhibit their development. These results corroborate those obtained in previous studies on various fish species (Willey et al., Reference Willey, Bengtson and Harel2003; Xu et al., Reference Xu, Ai, Mai, Xu, Wang, Ma, Zhang, Wang and Liufu2010; Luo et al., Reference Luo, Tan, Li and Yin2012). Some other studies, however, have suggested that different ARA levels have no significant influence on the growth and survival of fish species, such as the black sea bass (Centropristis striata) (Rezek et al., Reference Rezek, Watanabe, Harel and Seaton2010), the summer flounder (Paralichthys dentatus) (Willey et al., Reference Willey, Bengtson and Harel2003), and the gilthead sea bream (Fountoulaki et al., Reference Fountoulaki, Alexis, Nengas and Venou2003). These differences could be attributed to the species, the study methods, or the different growth stages.
To examine the effect of exogenous ARA intake on the fatty acid composition of bees’ bodies, we analyzed the fatty acid composition of bees’ bodies. A series of studies have shown that the fatty acid composition of the body and tissues is influenced by dietary fatty acid composition and PUFA is conserved preferentially at the expense of MUFA and SFA (Block et al., Reference Block, Harris and Pottala2008). The n-3 and n-6 PUFAs, DHA, and EPA are preferentially retained when certain fatty acids are added to the diet, while some fatty acids, such as SFA and MUFA, are selectively metabolized (Luo et al., Reference Luo, Li, Bai and Gong2008; Sharma et al., Reference Sharma, Kumar, Sinha, Ranjan, Kithsiri and Venkateshwarlu2010). In the present study, ARA-rich diets led to changes in the fatty acid composition of the bee body and as the dietary ARA concentration increased, there was an accompanying ARA increase in the bee body, corroborating the results of studies on fish (Villalta et al., Reference Villalta, Estévez and Bransden2005). However, SFA and MUFA had different trends in the experimental groups, possibly because the preservation of PUFA in bees was prioritized, while SFA and MUFA were used preferentially. Meanwhile, with the increase of the dietary ARA concentrations, the EPA contents of the bees’ bodies generally decreased in a linear manner, which is similar to reports on gilthead sea bream (Anholt, Reference Anholt2004), Senegalese sole (Solea selegalensis) (Villalta et al., Reference Villalta, Estévez and Bransden2005), and Japanese sea bass (Lateolabrax japonicas) (Xu et al., Reference Xu, Ai, Mai, Xu, Wang, Ma, Zhang, Wang and Liufu2010).
At present, an increasing number of studies show that ARA plays a vital role in the immune system and the regulation of the inflammation reaction such as the modulation of T-cell signaling (Calder, Reference Calder2003), while ARA-derived metabolites, notably PGE2, PGI2, and PGF2a, readily help in resolving inflammation and wound healing via regulating the production of angiogenic factors (Pozzi and Zent, Reference Pozzi and Zent2009). The oxidative metabolism of ARA can produce some bioactive substances, such as eicosanoids, whose main biological function seems to be the limitation of inflammatory responses and the regulation of the differentiation and proliferation of several immune cells, while it also has a number of different cell defense functions (Stanley, Reference Stanley2011). Many studies showed that ARA has an important role in the growth, differentiation, migration, and proliferation of immune cells in the thymus (James et al., Reference James, Gibson and Cleland2000). Jacobi et al. (Reference Jacobi, Moeser, Corl, Harrell and Bilksager2012) suggested that piglets fed diets with 5% ARA are more resilient to damaged tissues than piglets fed with 0 or 0.5% ARA.
Honey bees, like all insects, lack a typical adaptive immune system. Instead, they have evolved mechanisms to detect and limit microbial infection, such as cooperative social behavior, physical barriers, and the innate immune system (Randolt et al., Reference Randolt, Gimple, Geissend-Rfer, Reinders, Prusko and Mueller2008). The innate immune reaction was mediated by four signaling pathways, mainly Toll, Imd, Janus kinase/signal transducer and activator of transcription, and c-Jun N-terminal kinase (Lamiable and Imler, Reference Lamiable and Imler2014). The Toll and Imd pathways play an important role in the regulation of transcription of target genes coding for AMPs (Lemaitre and Hoffmann, Reference Lemaitre and Hoffmann2007; Avadhanula et al., Reference Avadhanula, Weasner, Hardy, Kumar and Hardy2009; Costa et al., Reference Costa, Jan, Sarnow and Schneider2009). The Toll signaling pathway is chiefly involved in the defense against Gram-positive bacteria, fungi, and viruses. To some extent, the expression level of the toll receptor gene can reflect whether a foreign body invasion signal can be transmitted in a timely manner and produce a corresponding immune response. The toll receptor gene has been studied in bees. The honey bee genome encodes major members of insect Toll and AMP pathways, mainly including abaecin, hymenoptaecin, defensin-2, toll, myd88, and dorsal (Brutscher et al., Reference Brutscher, Daughenbaugh and Flenniken2015).
In the present study, the expression of abaecin, hymenoptaecin, defensin-2, toll, myd88, and dorsal was significantly regulated by the dietary ARA level. The maximum mRNA expression of abaecin, hymenoptaecin, defensin-2, myd88, and toll was observed in the 4% ARA group, while it decreased significantly in the 6 and 8% ARA groups. While there are few studies on the influences of dietary ARA on the expression of AMP and Toll signaling pathway-related genes, studies on fish and mammals show that these genes could be regulated by dietary fatty acids (Gabler et al., Reference Gabler, Spencer, Webel and Spurlock2008; Zuo et al., Reference Zuo, Ai, Mai, Xu, Wang, Xu, Liufu and Zhang2012). Ding et al. (Reference Ding, Zhou, Kong, Zhang and Ye2017) suggested that the mRNA expression of myd88 and TLR3/toll first increased and then decreased, with increasing dietary ARA levels.
Lysozymes are important defense molecules of the non-specific immune system and can hydrolyze the glycoside bonds on the cell walls of Gram-positive bacteria such as Mlysodeikticus, Bacillus megaterium, and Staphylococcus aureus, so as to dissolve the bacteria. Meanwhile, lysozymes play an important role in parasitic, bacterial, and viral infection resistance, while their presence allows us to evaluate the immune status of an animal's body (Bedick et al., Reference Bedick, Tunaz, Nor Aliza, Putnam, Ellis and Stanley2001). In this study, the lysozyme activity of worker bees was significantly affected by different dietary ARA levels. Worker bees in the 2 and 4% ARA groups had a significantly higher lysozyme activity than those in groups fed the other diets; the lowest lysozyme activity was observed in the 8% ARA group, suggesting that excessive ARA has an inhibitive effect on the immune response, which has also been found in studies on fish (Bell and Sargent, Reference Bell and Sargent2003; Hurtado et al., Reference Hurtado, Reza, Ibarra, Wille, Sorgeloos, Soudant and Palacios2009; Shahkar et al., Reference Shahkar, Yun, Lee, Kim, Kim, Lee and Bai2016). Ding et al. (Reference Ding, Zhou, Kong, Zhang and Ye2017) showed that an appropriate dietary ARA level improves the serum lysozyme activity in prawns, while excess ARA levels have the opposite effect (Ding et al., Reference Ding, Zhou, Kong, Zhang and Ye2017).
PO in insects, a kind of oxidase-containing copper ion binding site, is synthesized in the hemolymph as an inactive zymogen called pro-phenol oxidase and is one of the main non-specific immune responses in arthropods (Kan et al., Reference Kan, Kim, Kwon, Park, Roh and Lee2008). PO catalyzes the formation of quinones, which can non-specifically cross-link neighboring molecules to form melanin at sites of injury or the entire surface of invading microorganisms. PO plays an important role in cuticular sclerotization and in the defense of insects and crustaceans against pathogens and parasites (Kumar et al., Reference Kumar, Christophides, Cantera, Charles, Han and Meister2003). In this study, the PO activity of bees was significantly affected by different dietary ARA levels. When worker bees were fed the 4% ARA diet, their PO activity was the highest, while the PO activity in the 2% ARA group was significantly higher than that in the control group.
In conclusion, dietary ARA concentrations significantly influenced the weight, antibacterial competence, fatty acid composition, PO activity, lysozyme activity, antitrypsin activity, and some immune-related gene expression of worker bees. The optimal dietary ARA concentration for worker bees was 4%. However, the non-specific immunity of worker bees in the 8% ARA group decreased significantly compared with other groups; this result suggests that excess ARA levels in the diet may influence the health status of bees.
Acknowledgements
This research was supported by the earmarked fund for the China Agriculture Research System (No. CARS-44), the Efficient Ecological Agriculture Innovation Project of the Taishan Industry Leading Talent Program (LJNY202003), and the Natural Science Foundation of Shandong Province (ZR2020QC191).