Introduction
Schistosomiasis is a major neglected tropical disease impacting the lives of over 250 million people in 78 countries and 779 million are at risk of infection (McManus et al., Reference McManus, Dunne, Sacko, Utzinger, Vennervald and Zhou2018). The treatment of choice is praziquantel (PZQ), a drug discovered in the 1970s (Andrews et al., Reference Andrews, Thomas, Pohlke and Seubert1983; Chai, Reference Chai2013). However, a major limitation of PZQ is its lack of efficacy against the immature, migrating stages of the parasite (Xiao et al., Reference Xiao, Keiser, Chen, Tanner and Utzinger2010, Reference Xiao, Sun and Chen2018). In addition, reliance on this single compound to treat all Schistosoma species is a continuing concern, especially as increases in the use of PZQ in current mass drug administration (MDA) control programmes may result in the development of resistance to the drug (Hotez and Fenwick, Reference Hotez and Fenwick2009; Lo et al., Reference Lo, Addiss, Hotez, King, Stothard, Evans, Colley, Lin, Coulibaly, Bustinduy, Raso, Bendavid, Bogoch, Fenwick, Savioli, Molyneux, Utzinger and Andrews2017). Therefore, research is required to increase the longevity or efficacy of PZQ.
Although the exact mechanism of action of PZQ remains unclear, its action on calcium homoeostasis of mature Schistosoma parasites is apparent (Day et al., Reference Day, Bennett and Pax1992; Doenhoff et al., Reference Doenhoff, Cioli and Utzinger2008; Xiao et al., Reference Xiao, Sun and Chen2018). The contraction and paralysis observed in PZQ-treated schistosomes are explained by membrane depolarization and the influx of extracellular calcium into the worms, which causes vacuolation and disintegration of the parasite surface (Doenhoff et al., Reference Doenhoff, Cioli and Utzinger2008; Xiao et al., Reference Xiao, Sun and Chen2018). Although the mode of action of PZQ is thought to be multi-faceted (Pica-Mattoccia et al., Reference Pica-Mattoccia, Doenhoff, Valle, Basso, Troiani, Liberti, Festucci, Guidi and Cioli2009), a massive influx of calcium ions into the cells possibly via the pore-forming α1 subunit of a calcium channel is a major feature of PZQ sensitivity (Greenberg, Reference Greenberg2005).
Previously, we reported the significant upregulation of genes related to calcium signalling pathways and possible drug resistance in schistosome adults following in vivo exposure to PZQ in mouse models of schistosomiasis (You et al., Reference You, McManus, Hu, Smout, Brindley and Gobert2013). Ca2+/calmodulin-dependent protein kinase II (CaMKII) was one of the key calcium regulatory genes shown to be upregulated in response to PZQ treatment (You et al., Reference You, McManus, Hu, Smout, Brindley and Gobert2013). Furthermore, the inhibition of CaMKII function in Schistosoma worms significantly improved the efficacy of PZQ, as shown by a considerable reduction in worm motility (You et al., Reference You, McManus, Hu, Smout, Brindley and Gobert2013). Extending this pivotal study, we explore here the use of selective and non-selective CaMK/kinase inhibitors to improve the efficacy of PZQ against juvenile and adult schistosomes in vitro and in vivo.
Materials and methods
Ethics statement
The conducts and procedures involving animal experiments were approved by the Animal Ethics Committee of the QIMR Berghofer Medical Research Institute (project number A0104-016), which adheres to the Australian code of practice for the care and use of animals for scientific purposes, as well as the Queensland Animal Care and Protection Act 2001; Queensland Animal Care and Protection Regulation 2002. This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Puerto Rican strain of S. mansoni was maintained in ARC out-bred Swiss female mice and Biomphalaria glabrata snails at the QIMR Berghofer Medical Research Institute from stocks originating from the National Institute of Allergy and Infectious Disease Schistosomiasis Resource Centre, Biomedical Research Institute (Rockville, Maryland, USA). General methods followed those previously published (Jones et al., Reference Jones, McManus, Sivadorai, Glanfield, Moertel, Belli and Gobert2007; You et al., Reference You, Zhang, Moertel, McManus and Gobert2009).
Preliminary CaMK11 inhibitor assays with mechanically transformed S. mansoni schistosomula
Schistosoma mansoni cercariae were obtained by shedding infected B. glabrata under a bright light. Cercariae were transformed mechanically to schistosomula (Milligan and Jolly, Reference Milligan and Jolly2011) and cultured in Basch's medium (Basch, Reference Basch1981), with media changes every 3 days, depending on the age of schistosomula required. Schistosomula were dispensed into a 96-well flat bottom plate (100 schistosomula/well in 200 μL of Basch's medium) and CaMK inhibitors with or without PZQ were added to each well to the required concentration.
The effects of 15 commercially available selective and non-selective CaMK inhibitors (Table 1) were assessed on 2- and 7-day-old schistosomula with or without the presence of PZQ.
Table 1. CaMK inhibitors used for initial in vitro screening of schistosomula
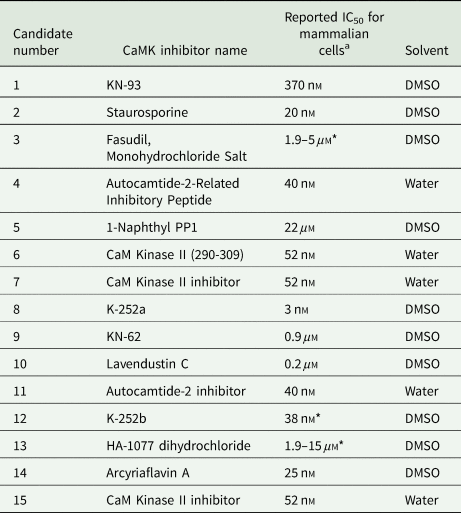
a Reported IC50 as per manufacturer.
*Activity against various kinases.
All inhibitors were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, California, USA), or Selleckchem (Houston, Texas, USA). Inhibitory concentrations used were based on the IC50s reported by the manufacturer for each inhibitor for mammalian cells. Selective and non-selective CaMK inhibitors were tested at published mammalian 1 × IC50 and 10 × IC50 alone or in combination with the two PZQ concentrations over variable periods of exposure (4, 24 and 48 h). All the inhibitors were diluted in water or DMSO as indicated by the chemical suppliers (Table 1). PZQ was dissolved in DMSO.
Based on PZQ dosages provided by recent in vitro drug screening assays using newly transformed schistosomula (de Moraes et al., Reference de Moraes, Nascimento, Yamaguchi, Kato and Nakano2012; Marxer et al., Reference Marxer, Ingram and Keiser2012; Xiao et al., Reference Xiao, Sun and Chen2018), a range of PZQ concentrations, 0.24–500 μ m, was tested during an initial screen for anti-schistosomal activity. From these in vitro assays, LD5 and LD25 of PZQ for 2-day schistosomula were chosen as the upper and lower concentrations, respectively, P1 (1 μ m) and P20 (20 μ m), for further studies.
Each treatment was performed in triplicate (separate wells), and the experiments were repeated three times; 24 h after treatment, the schistosomula were stained and fixed with 1:5 Trypan blue in 1% paraformaldehyde (in PBS) (Allison and Ridolpho, Reference Allison and Ridolpho1980) for 20–30 min. The parasites were washed until the well contents were clear, and the dead/damaged and unaffected schistosomula were counted under an inverted microscope at 100 magnification to calculate the percentage of dead/damaged schistosomula in each treatment group compared with the untreated control (1% DMSO in Basch's medium). A minimum of 100 parasites were counted in each treatment well.
Based on the results of the preliminary in vitro assays, two kinase inhibitors, Staurosporine (STSP) and 1Naphthyl PP1 (1NAPP1) were chosen for further studies since they exhibited the most promising inhibitory effects against schistosomula.
Quantification of the biochemical inhibition of CaMK II activity by selected inhibitors
A soluble worm antigen preparation (SWAP) was extracted from adult S. mansoni worms (You et al., Reference You, Zhang, Jones, Gobert, Mulvenna, Rees, Spanevello, Blair, Duke, Brehm and McManus2010) and its protein concentration quantified using the Bradford assay (Bio-Rad, Hercules, California, USA). The CaMKII activity of SWAP (0.028 μg μL−1) with different concentrations of STSP (0.0025–1.28 μ m) or 1NAPP1 (2.75–1100 μ m) was quantified using CycLex CaM Kinase II Assay Kits (CycLex Co., Ltd. Nagano, Japan), according to the manufacturer's guidelines.
Effects of CaMK inhibition on adult parasite motility
Adult S. mansoni worms were obtained by perfusion of infected mice (6–9 weeks old female Swiss), 6 weeks post cercarial challenge. Male and female adult worms were separated and cultured overnight in RPMI medium supplemented with 20% (v/v) Fetal Bovine Serum (FBS), and 1% (v/v) penicillin and streptomycin. The motility of adult worms before or after treatment was assessed using the xCELLigence system (Roche Inc., Basel, Switzerland) established in our laboratory as described (You et al., Reference You, McManus, Hu, Smout, Brindley and Gobert2013). Briefly, a single female or male adult worm was transferred individually into each well of a 96-well xCELLigence plate (ACEA Biosciences, San Diego, California, USA). RTCA controller software (Roche Inc.) was used to determine how the information was gathered from the xCELLigence plate. Each worm was cultured in 180 μL of completed medium per well and motility was monitored every 15 s for 3 h to obtain a baseline motility reading (to identify healthy parasites) prior to the addition of an inhibitor. STSP (0.2 μ m) and 1NAPP1 (220 μ m), with or without an IC50 of PZQ [as previously reported (Smout et al., Reference Smout, Kotze, McCarthy and Loukas2010)], were added to wells (six wells per treatment) and motility observed using the xCELLigence system over 24 h. Experiments performed with previously reported PZQ IC50 (188 ng mL−1) dose reduced worm motility below 50% (Smout et al., Reference Smout, Kotze, McCarthy and Loukas2010), as compared to untreated controls. Similar reductions in motility were observed in groups treated with STSP and 1NAPP1 alone. A variation was evident between the effective concentration (EC50) for male and female adult worms. As a result, PZQ at a concentration of 37.6 ng mL−1 (0.12 μ m) (1/5 × IC50 for S. mansoni) for male worms and 94 ng mL−1 (0.3 μ m) (1/2 × IC50 for S. mansoni) for female worms, were used for subsequent experiments. STSP and 1NAPP1 were used at 5 × IC50 concentrations, 0.1 and 110 μ m respectively, for both male and female worms. Dead worms (heat killed) were included as immobile controls and exhibited 0% motility. Positive control worms were incubated in the presence of the DMSO concentration equivalent to that used for the highest drug concentration and represented 100% worm motility. Statistical analyses were undertaken using GraphPrism 5.0 (You et al., Reference You, McManus, Hu, Smout, Brindley and Gobert2013).
Biochemical activity of CaMKII in adult male and female worms exposed to 1NAPPI and STSP with or without PZQ
After 24 h of inhibitor drug treatment, and following the motility assay, protein was extracted from male and female worms using 1% Triton X-100 in Tris-buffered saline supplemented with 1% (v/v) complete protease inhibitor cocktail (Tran et al., Reference Tran, Freitas, Cooper, Gaze, Gatton, Jones, Lovas, Pearce and Loukas2010). CaMKII activity of the worm protein extract was measured in different treatment groups using the CycLex CaM Kinase II Assay Kit (CycLex Co.).
Mammalian cell cytotoxicity
Neonatal foreskin fibroblast (NFF), human hepatocellular carcinoma (HepG2), human hepatoma (Huh7) and mouse hepatocellular carcinoma (AML12) cell lines (from ATCC) were cultured in complete media as described (Boyle et al., Reference Boyle, D'Souza, Pierce, Adams, Cantor, Johns, Maslovskaya, Gordon, Reddell and Parsons2014) and were tested for the toxicity of varying concentrations of STSP (2000–0.002 μ m) and 1NAPP1 (220–1.72 μ m), with or without PZQ, using the sulforhodamine B (SRB) assay (Boyle et al., Reference Boyle, D'Souza, Pierce, Adams, Cantor, Johns, Maslovskaya, Gordon, Reddell and Parsons2014).
Initial in vivo treatment with PZQ to determine sub-therapeutic dosage against adult parasites
Three different PZQ dose regimens were used in 6–9-week-old female Swiss mice infected with 140 S. mansoni cercariae at 5 weeks post infection to determine a sub-therapeutic dose. The dosage threshold for the clearance of a patent S. mansoni infection from mice is considered as the therapeutic dose. PZQ was dissolved in 2.5% (v/v) cremophor EL (Sigma, St Louis, Missouri, USA) and given by oral gavage to three groups of mice (n = 3) at a dose of 250 mg kg−1 day−1 for 1 day; 250 mg kg−1 day−1 for 3 days (total dose 750 mg kg−1) and an escalating dose from 150–350 mg kg−1 over 5 days (total dose 1250 mg kg−1) (Chuah et al., Reference Chuah, Jones, McManus, Nawaratna, Burke, Owen, Ramm and Gobert2016). All the mice were perfused 49 days post-infection. Total male and female adult worm numbers were counted, and mouse livers were collected and processed for egg counts (Zhang et al., Reference Zhang, Li, Duke, Jones, Kuang, Zhang, Blair, Li and McManus2011; Chuah et al., Reference Chuah, Jones, McManus, Nawaratna, Burke, Owen, Ramm and Gobert2016).
Dose escalation study of inhibitors
Optimum 1NAPP1 and STSP doses for mice were calculated using data obtained from in vitro assays, mammalian cytotoxicity assays and previously published data (Hill et al., Reference Hill, Tillery, Rose and Posey1994; Abe et al., Reference Abe, Kubota, Otani, Furukawa, Watanabe, Kumai, Akiyama, Akinaga and Kitajima2001; Soskis et al., Reference Soskis, Ho, Bloodgood, Robichaux, Malik, Ataman, Rubin, Zieg, Zhang, Shokat, Sharma, Cowan and Greenberg2012; Robichaux et al., Reference Robichaux, Chenaux, Ho, Soskis, Dravis, Kwan, Sestan, Greenberg, Henkemeyer and Cowan2014). The full dosage for 1NAPP1 was 0.4 mg kg−1 (approximate plasma concentration-22 μ m) given by tail vein intravenous (i.v) injection according to a previous study (Mizukami et al., Reference Mizukami, Sato, Camps, Ji, Rueckle, Swinnen, Tsuboi, Takeda and Ichijo2014). 1NAPP1 (1 mg) was first dissolved in 525 μL DMSO, which was then diluted 10 times with 0.9% (v/v) saline (600 μ m) (Mizukami et al., Reference Mizukami, Sato, Camps, Ji, Rueckle, Swinnen, Tsuboi, Takeda and Ichijo2014). The full dose of STSP was 10 mg kg−1 (Hill et al., Reference Hill, Tillery, Rose and Posey1994) given as a single dose by oral gavage. The concentration in the plasma of these mice was expected to reach ~40 μ m given the bioavailability of 13% with similar compounds in a 30 g mouse (Hill et al., Reference Hill, Tillery, Rose and Posey1994).
Dose escalation studies were carried out using 6–9-week-old female Swiss mice for the two inhibitors selected for further characterization. Mice were given 1/100th of the expected final dose and observed for a period of 7 days for any weight loss or development of any abnormal veterinary features. A second dose was given at the 1/10th of the expected final dose after 7 days to the same mice and monitored as above. A third dose was given at the standard expected dose and monitored as above. Tested at day 3 and day 7 post treatment, both the alanine transaminase (ALT) and aspartate transaminase (AST) colour endpoint assays (BioScientific, Max Discovery™, Austin, Texas, USA) were used to determine changes in liver enzymes as indicators of hepatotoxicity.
Combination of PZQ and CaMK inhibitors in in vivo trials
Three animal trials were conducted as follows. Six-week-old female outbred Swiss mice were used for in vivo experiments to determine the efficacy of the inhibitor and PZQ treatment of S. mansoni-infected animals. Trials 1 and 2 were carried out to test the efficacy of the selected inhibitors with/without PZQ and PZQ only. Each trial had three treatment groups depending on the time point of treatment post-cercarial challenge chosen. The time points for drug treatment were either on day 2 post-infection, day 7 post-infection or week 5 post-infection, corresponding to the targeting of day 2 or day 7 schistosomula or adult parasites. There were six groups in trial 1 and 2 including the control (no treatment), PZQ only, STSP only, 1NAPP1 only, PZQ/STSP and PZQ/1NAPP1. There were five mice/group in trial 1 and eight mice/group in trial 2. Mice were infected with 140 cercariae/mouse in trial 1 and 100 cercariae/mouse in trial 2. In trial 3, all mice (10 mice/group) were infected with 80 cercariae/mouse and the effect of three different STSP doses was tested in combination with PZQ given at 5 weeks post-infection.
1NAPP1 was reconstituted as described above and given 0.4 mg kg−1 day−1 i.v per mouse in trial 1 (plasma concentration, ~22 μ m, which is closer to the IC50, because of the requirement for intravenous administration and to prevent possible toxicity) (Soskis et al., Reference Soskis, Ho, Bloodgood, Robichaux, Malik, Ataman, Rubin, Zieg, Zhang, Shokat, Sharma, Cowan and Greenberg2012; Robichaux et al., Reference Robichaux, Chenaux, Ho, Soskis, Dravis, Kwan, Sestan, Greenberg, Henkemeyer and Cowan2014) and increased to 0.6 mg kg−1 day−1 in trial 2 (plasma concentration, ~33 μ m) to achieve the required plasma concentrations.
STSP was given at the same dose of 10 mg kg−1 day−1 as a single oral gavage (approximate plasma concentration 40 μ m) in both trial 1 and trial 2. In trial 3, different doses of STSP (2.5, 5 and 10 mg kg−1 day−1 as a single dose) were given in combination with PZQ to each group.
PZQ was given orally at a dose of 250 mg kg−1 dissolved in 2.5% (v/v) cremophor EL (Chuah et al., Reference Chuah, Jones, McManus, Nawaratna, Burke, Owen, Ramm and Gobert2016). The control group was given 2.5% (v/v) cremophor EL orally and 10% DMSO in 0.9% (v/v saline) i.v. In the groups treated with STSP/PZQ by oral gavage, there was a 45 min interval between the two doses to reduce potential interactions between the drugs or solvents in the stomach.
Feces were collected from individual mice at 6 weeks post-infection in all groups before worm perfusion at 7 weeks post-infection. Feces was fixed in 10% formalin and processed for egg counting as described previously (Zhang et al., Reference Zhang, Li, Duke, Jones, Kuang, Zhang, Blair, Li and McManus2011). Total and female adult worm numbers were counted and then fixed in 10% (v/v) buffered formalin. Mouse livers were collected and processed for egg counting (Zhang et al., Reference Zhang, Li, Duke, Jones, Kuang, Zhang, Blair, Li and McManus2011; Chuah et al., Reference Chuah, Jones, McManus, Nawaratna, Burke, Owen, Ramm and Gobert2016).
All data were analysed using GraphPad Prism 7.02. The group means were compared between each other using the Kruskal–Wallis test.
Real-time PCR of selected calcium signalling genes
Total RNA was isolated (Hoffmann et al., Reference Hoffmann, Johnston and Dunne2002) from adult male and female S. mansoni worms, respectively, treated with STSP and 1NAPP1 with or without PZQ. All samples were DNase treated (Promega, Madison, Wisconsin, USA) prior to cDNA synthesis (Gobert et al., Reference Gobert, McManus, Nawaratna, Moertel, Mulvenna and Jones2009) using Quantitect Reverse Transcription kits (Qiagen Inc.). The expression levels of selected key genes involved in the calcium-mediated signalling pathways (Table S2) were tested on the worm cDNA samples using real-time PCR (Moertel et al., Reference Moertel, Gobert and McManus2008). Tubulin α (Araujo-Montoya et al., Reference Araujo-Montoya, Rofatto, Tararam, Farias, Oliveira, Verjovski-Almeida, Wilson and Leite2011) was used as the reference gene. All other primers were designed using Primer3 web version 4.0.0 online software. qPCR was performed using SYBR Green master mix (Thermo Fisher Scientific ) on a Corbett Rotor Gene 6000 (Corbett Life Sciences). Rotor-Gene 6000 Series software (version1.7) and GraphPad Prism (version 7.02) were used to analyse the data, using the standard curve method as described (Gobert et al., Reference Gobert, McManus, Nawaratna, Moertel, Mulvenna and Jones2009).
Results
CaMKII inhibitor assays with mechanically transformed S. mansoni schistosomula
The numbers of live/dead schistosomula were counted (dead parasites absorb the blue stain) using the trypan blue exclusion staining method as described (Pinto et al., Reference Pinto, Soares and Mittmann2011) to determine the impact of CaMK inhibitors on schistosomula viability.
Figure 1 shows a summary of responses of 2-day-old (Fig. 1A) and 7-day-old (Fig. 1B) schistosomula to all the selected CaMK inhibitors 24 h post-treatment. Schistosomula treated with 1NAPP1 (candidate 5) demonstrated ⩾50% death rates in the 2- and 7-day-old groups with or without PZQ. However, there was no significant difference in the response of day 2 and day 7 schistosomula to NAPP1 except when combined with the higher dose of PZQ. STSP (candidate 2) combined with PZQ resulted in ⩾50% death rates with 2-day-old schistosomula only.

Fig. 1. In vitro lethality effect of CaMK11 inhibitors, with and without praziquantel on S. mansoni juvenile parasites. (A) In vitro 2-day-old schistosomula. (B) In vitro 7-day-old schistosomula. The x-axis lists the CaMK inhibitors (see Table 1 for candidate number and corresponding compounds), used at low concentration (a concentration corresponding to those of published mammalian IC50 levels), indicated by the inhibitor number alone, or at high concentration (10x mammalian IC50) designated by an ‘H’, or no inhibitors = 0. Columns refer to PZQ concentrations P0 = no PZQ, P1 = 1 μ m (0.312 μgmL−1), P20 = 20 μ m (6.25 μgmL−1). The bottom dotted line shows the average numbers of dead parasites observed in non-treated controls (without PZQ or CaMK inhibitor). The top dotted line shows the average maximum % death achieved by PZQ alone without any CaMK inhibitor. Note in panel B that both dotted lines are at similar levels. Bars indicate s.e.m. n = 3.
Further testing of STSP with 7-day-old schistosomula, at 4, 24 and 48 h after treatment confirmed the findings presented in Fig. 1B (Fig. 2C, D, G, H, K and L). With 2-day-old schistosomula (Fig. 2A, B, E, F, I and J), an effect with STSP was evident in the 24 h post treatment group, reaching 50% death rate at a concentration of 0.02 μ m in combination with 20 μ m PZQ. The published IC50 for STSP for mammalian cells is also the same as the LD50 for the 2-day-old schistosomula observed here (Fig. 2E). At 48 h there was a further increase in the death rates of schistosomula. The STSP and 20 μ m PZQ combination group always showed higher death rates followed by the STSP and 1 μ m PZQ group. Increasing STSP concentration to 0.4 μ m brought the death rates closer to the PZQ/STSP combined groups (Fig. 2E).

Fig. 2. In vitro effect of STSP and 1NAPP1 on 2-day (A, B, E, F, I, J) and 7-day (C, D, G, H, K, L) old S. mansoni schistosomula at 4 h (A–D), 24 h (E–H) and 48 h (I–L) post treatment. The x-axis shows CaMK inhibitor concentrations, where 0 shows PZQ only treatment. P0 = CaMK inhibitor alone without PZQ, P1 = CaMK inhibitor with 1 μ m (0.312 μgmL−1) PZQ, P20 = CaMK inhibitor with 20 μ m (6.25 μgmL−1 PZQ). n = 3. STSP = Staurosporine, 1 NAAP1 = 1-Naphthyl PP1.
1NAPP1 showed detrimental effects on 7-day-old schistosomula alone or in combination with PZQ, starting from a concentration of 22 μ m (published mammalian IC50) (Fig. 2D, H and L). Similar effects were evident on 2-day-old schistosomula (Fig. 2B, F and J) except for the combination group with 20 μ m PZQ, where PZQ alone showed a 20–40% death rate over the 24–48 h period. This trend was also observed in the STSP-treated groups due to the moderate efficacy of PZQ against schistosomula up to 3 days (Fig. 2E and I). 1NAPP1 achieved a 100% death rate at a concentration of 220 μ m (10 × IC50) with or without PZQ in both day 2- and day 7-old schistosomula.
Quantification of the reduction of CaMKII activity by the selected inhibitors
STSP at a concentration of 0.04 μ m was able to inhibit circa 50% of the CaMKII activity in S. mansoni SWAP, whereas 1NAPP1 reached 50% inhibition at around a concentration of 660 μ m. STSP had a lower IC50 than 1NAPP1 when tested in vitro (Fig. S1).
Motility of S. mansoni male and female worms following drug treatment
To ensure the worms used for drug treatment were alive, motility of all the worms was measured by xCELLigence for 3 h before the addition of any drug. There were no significant differences (P value >0.05) in motility among the groups for either females or males prior to the introduction of the drugs. The range of motility (% relative to untreated worms) was 70–92% for females and 85–110% for males (Fig. 3A and B) at the commencement of the experiment. Female worms treated with STSP and 1NAPP1 alone regained 100% motility after 48 h incubation (Fig. 3C). Motility of females treated with the PZQ/STSP and PZQ/1NAPP1 combinations decreased to 40% and 35%, respectively; however, there was no significant decline compared with female worms treated with PZQ only (P > 0.05). The motility of male S. mansoni was reduced significantly (P ⩽ 0.05) after 48 h treatment with either the PZQ/STSP (31%) or PZQ/1NAPP1 (25%) combination compared with PZQ-treated males (Fig. 3D).

Fig. 3. Effect of CaMK inhibitors on the motility of adult male and female S. mansoni worms. Motility (%) is presented on the left y-axis while the corresponding P value (comparing motility of worms treated with PZQ and PZQ/STSP) is presented on the right y-axis. Motility (%) of female (A) and male (B) S. mansoni worms 3 h prior to treatment are shown in the left panel. Error bars (s.e.m.) are shown at time points 0, 1, 2 and 3 h. Motility (%) of female (C) and male (D) S. mansoni worms, after being exposed for 48 h to STSP and 1NAPP1 with or without PZQ are shown in the right panel. Error bars (s.e.m.) are shown in (C) and (D) every 8 h after treatment; n = 6. PZQ = Praziquantel; STSP = Staurosporine; 1NAPP1 = 1-Naphthyl PP1.
Testing CaMK II activity in adult male and female S. mansoni exposed to 1NAPPI and STSP with or without PZQ
Schistosoma mansoni males treated with STSP alone or in combination with PZQ had significantly reduced CaMKII activity compared with those treated with PZQ alone, measured using the CycLex CaM Kinase II Assay Kit. Males treated with STSP showed a significant reduction (77%, P = 0.02) in CaMKII activity compared with those treated with 1NAPP1 (Fig. 4A). This outcome supported the results of the in vitro assays performed with SWAP (Fig. S1) and showed that STSP was a better inhibitor of CaMKII both in vitro and in vivo. STSP was superior to 1NAPP1 in suppressing CaMKII activity both in males and females, although the response with both inhibitors was less pronounced in female worms. This was representative of the increased reduction in motility in males than in females, as shown in the xCELLigence analysis (Fig. 3). PZQ treatment did not seem to affect CaMKII function as CaMK activity was similar to that of the untreated control group.

Fig. 4. CaMK expression and activity assays in male (M) and female (F) S. mansoni worms treated with various combinations of STSP, 1NAPP1 and PZQ compared to untreated parasites (control). (A) CaMK II enzyme activity in S. mansoni male (M) and female (F) worms treated with various combinations of STSP, 1NAPP1 and PZQ expressed as folds relative to control. Control = 1. (B) Quantitative real-time PCR analysis of CaMKII gene expression levels in different treated groups compared to untreated male and female worms (control). Error bars represent s.e.m. N = 3. STSP = Staurosporine, 1NAPP1 = 1-Naphthyl PP1.
Reciprocal changes were observed in CaMKII gene copy numbers, calculated using real-time PCR, showing significantly higher levels of transcription in males (except in the PZQ-only and STSP-only treatment groups) than in females (P = 0.006–0.287), likely as a result of possible attempts to upregulate CaMKII production.
Real-time PCR
Overall, transcription of the RYR 1, PHK1 and PKC4 genes was significantly lower in female worms, including the untreated controls (Fig. 5), which was also evident in CaMKII expression levels (Fig. 4B). However, the same trend was not evident in the transcription of IP33K2 for most of the groups. RYR1 and PKC4 expression was significantly reduced (P ⩽ 0.05) following treatment with PZQ + STSP in female worms compared with the PZQ-treated group. A similar trend occurred in the expression of PKC4, PHK1 and IP33K2 in male worms treated with PZQ + STSP (P ⩽ 0.05) showing the effect of CaMKII inhibition on related genes in the calcium signalling pathway.

Fig. 5. Quantitative real-time PCR analysis of the expression of selected gene in the calcium signalling pathway in S. mansoni male (M) and female (F) worms treated with various combinations of STSP, 1NAPP1 and PZQ compared to untreated male and female worms (control M and control F). Bars indicate s.e.m.; n = 3. (A) RYR1; (B) PHK1; (C) PKC4; (D) IP33K2.
Mammalian cell cytotoxicity with CamK inhibitors and PZQ
A summary of LD50s calculated following the cytotoxicity assays is presented in Table 2. Detailed dose-response curves are shown in Supplementary Figs S2–S4. AML, Huh7 and NFF showed variable toxicity to 1NAPP1 and STSP. STSP was more toxic to AML (LD50 = 0.001 μ m) and NFF (LD50 = 0.821 μ m) than 1NAPP1 (LD50 = 52.7 and >100 μ m) but less toxic to Huh7.
Table 2. LD 50s calculated by mammalian cell cytotoxicity assays

Dose escalation study
Sub-therapeutic dose level of PZQ 250 mg kg−1 was selected to be combined with CaMK inhibitors in the trials as there was a 43% reduction in adult worm numbers obtained from mice treated with PZQ (250 mg kg−1 dose) when compared to worms perfused from untreated controls; liver egg burdens showed a similar trend but the reduction in numbers (60%) was slightly higher (Fig. S5).
The reported normal range in female Swiss mice for ALT activity is 24–193 IU L−1 and for AST activity is 46–244 IU L−1 (Serfilippi et al., Reference Serfilippi, Pallman and Russell2003). The liver enzymes tested were within the reported normal range for both day 3 and day 7 post-treatment, for all treatment groups, showing no hepatotoxicity with the doses tested. However, with STSP the day 3 enzyme levels were marginally elevated compared to day 7 (Fig. S6).
In vivo trials
In trial 1, mice treated with PZQ alone on the second day post-infection (Fig. 6A, B and C) did not result, at perfusion, in any statistically significant reduction in S. mansoni worm or egg counts compared to the untreated mice. However, treatment with STSP alone resulted in significant reductions in worm numbers (33%, P ⩽ 0.05), liver eggs (46%, P ⩽ 0.01) and fecal eggs (83%, P ⩽ 0.05) compared with untreated control mice. The combined PZQ/STSP group had a significant reduction in liver egg counts compared to the PZQ group. The second trial did not replicate the findings except for the PZQ/STSP combined group which showed significant fecal egg reduction (85%, P ⩽ 0.05) compared to the control group (Fig. 7C).

Fig. 6. Mouse trial 1: in vivo effects of PZQ, STSP, 1NAPP1 and inhibitor/PZQ combinations in a S. mansoni mouse model. Treatments were provided either 2 days (A, B, C), 7 days (D, E, F) or 5 weeks (G, H, I) post cercarial challenge and parasitological parameters were taken from sacrificed animals 6 weeks post cercarial challenge. The numbers of worms, liver eggs and fecal eggs are presented. STSP = Staurosporine, 1NAPP1 = 1-Naphthyl PP1. Bars indicate s.e.m.; n = 5. P values *⩽0.05, **⩽0.01, ***⩽0.001.

Fig. 7. Mouse trial 2: in vivo effects of PZQ, STSP, 1NAPP1 and inhibitor/PZQ combinations in a S. mansoni mouse model. Treatments were provided either 2 days (A, B, C), 7 days (D, E, F) or 5 weeks (G, H, I) post cercarial challenge and parasitological parameters were taken from sacrificed animals 6 weeks post cercarial challenge. The numbers of worms, liver eggs and fecal eggs are presented. STSP = Staurosporine, 1 NAAP1 = 1-Naphthyl PP1. Bars indicate s.e.m.; n = 8. P values *⩽0.05, **⩽0.01, ***⩽0.001.
During the PZQ insusceptible period (group treated on the seventh day post-infection) mice treated with the PZQ/1NAPP1 combination resulted in significant reductions in total worms in both trial 1 and trial 2 (Figs 6D and 7D) and liver eggs reduction in trial 1. The group treated with STSP alone showed a significant liver egg count reduction in trial 1 (Fig. 6E). However, treatment of mice with PZQ alone also, unexpectedly, showed a significant reduction in total worm numbers in trial 2 and liver egg reductions in both trial 1 and trial 2. In the group treated at week 5 post-infection (mature adult worms present), there was a significant reduction in the liver egg burden compared to the control in all groups except for those treated with 1NAPP1 and STSP alone in trial 1 (Fig. 6H). Similar results were evident in trial 2 with the STSP-treated group also showing a significant egg reduction (Fig. 7H). In trial 2, all groups showed significant reductions in fecal egg burdens when compared to the control (Fig. 7I) except for the group treated with 1NAPP1 alone, a trend not evident in trial 1. Of note, the fecal egg count in the STSP-treated group was significantly lower than the PZQ group (94%, P ⩽ 0.05) (Fig. 7I), indicating a potential role for STSP in inhibiting the fecundity of S. mansoni. Although not significant, the groups treated with a combination of PZQ and CaMK inhibitor had low numbers of fecal eggs when compared to the group treated with PZQ alone in both trial 1 and 2. Total worm numbers and liver eggs were significantly reduced in the PZQ/STSP combination group in both trials compared to controls. There was a significant reduction in total worm numbers in the groups treated with the PZQ/CaMK inhibitor combination (69%, 86%) and PZQ alone (77%) in trial 1 compared to controls; this was only replicated in the PZQ/STSP group (87%) in trial 2.
In vivo mouse trial using combinations of PZQ and STSP treatments
Trial 3 tested the effect of three different doses of STSP combined with PZQ, at 5 weeks post infection, in groups of 10 mice infected with 80 cercariae per mouse. All the treatment groups showed significant reductions in worm and egg counts compared with the untreated control group (Fig. 8). The group treated with the highest dose of STSP had the highest reduction in worm and egg counts compared with the control group and had significantly lower (58%) liver egg counts compared with the PZQ group.

Fig. 8. Mouse trial 3: in vivo effects of three varying concentrations of STSP (2.5, 5 and 10 mg kg−1) in conjunction with PZQ (250 mg kg−1) in a S. mansoni mouse model. Treatments were provided on 5 weeks post-cercarial challenge and parasitological parameters were taken from sacrificed animals 6 weeks post-cercarial challenge. The numbers of worms, liver eggs and fecal eggs are presented. STSP = Staurosporine. Bars indicate s.e.m.; n = 10. P values *⩽0.05, **⩽0.01, ***⩽0.001, ****⩽0.0001.
Discussion
PZQ action in schistosomes is dependent on parasite age, with some stages being refractory to treatment. This limits the effectiveness of a single day treatment regime. PZQ has limited efficacy against very young schistosomula [<3 days post cercarial infection (pci)] and older schistosomes (28 day pci), where 10X to 30X the dose of PZQ is needed to kill compared to the adult stage (Pica-Mattoccia and Cioli, Reference Pica-Mattoccia and Cioli2004; Xiao et al., Reference Xiao, Keiser, Chen, Tanner and Utzinger2010); PZQ is inactive against 3–21-day worms, and fully active against the sexually mature blood flukes (Xiao et al., Reference Xiao, Keiser, Chen, Tanner and Utzinger2010). Therefore, we investigated 2-day-old schistosomula in order to focus on a developmental stage with a limited sensitivity period and 7-day-old schistosomula, which are insensitive to PZQ, to test the effects of 15 different CaMKII (selective and non-selective) inhibitors on parasite survival. Given the considerable numbers of parasites required to achieve statistical power and, consequently, the extremely large number of animals needed to provide such worm numbers, with potential animal ethics concerns, mechanically produced schistosomula have generally been used as a high throughput tool for phenotypic pre-screening in drug efficacy studies (de Moraes et al., Reference de Moraes, Nascimento, Yamaguchi, Kato and Nakano2012; Tekwu et al., Reference Tekwu, Anyan, Boamah, Baffour-Awuah, Keyetat Tekwu, Penlap Beng, Nyarko and Bosompem2016), a trait followed here.
Mechanically transformed schistosomula are commonly used for drug screening assays. The concentration of 1 μ m PZQ used in this study is comparable to those used in previous studies (1.5 μ m S. haematobium; 0.7 μ m S. mansoni schistosomula) (Marxer et al., Reference Marxer, Ingram and Keiser2012). In another study (de Moraes et al., Reference de Moraes, Nascimento, Yamaguchi, Kato and Nakano2012), PZQ at 20 μ m was used to treat S. mansoni schistosomula, justifying drug dosages given in the current study, furthermore the refractory nature of in vitro schistosomula to PZQ was also noted. In a recent review by Xiao et al., the use of in vitro parasites and PZQ dosages between 3 and 30 μ m is reported (Xiao et al., Reference Xiao, Sun and Chen2018).
A range of commercially available CaMKII (selective and non-selective) inhibitors were tested on PZQ-sensitive (2-day-old) and PZQ-insensitive (7-day-old) schistosomula to select the most effective inhibitors. In in vitro assays, we were able to select STSP and 1NAPP1, which exhibited the highest level of killing of schistosomula at 2- and 7-day-old tested, in the presence or absence of PZQ. Although not selective, 1NAPPI was a stronger CaMK inhibitor for both day 2 and day 7 schistosomula, but STSP better reduced both CaMK activity in S. mansoni adult protein extracts (SWAP) and the motility of live male worms in vitro. However, the motility of male worms was significantly decreased following treatment with STSP combined with PZQ compared with those exposed to either of these compounds. This result is supported by our previous study (You et al., Reference You, McManus, Hu, Smout, Brindley and Gobert2013) when CamKII transcription was reduced by RNAi with 50–69% in adult S. japonicum with the result that the subsequent effect of an IC50 dosage of PZQ was exacerbated, exhibiting decreased motility from 47–61% to 23–27% in adult worms. It has been reported that PZQ disrupts Ca2+ homoeostasis in adult schistosomes by an unknown mechanism (Cioli and Pica-Mattoccia, Reference Cioli and Pica-Mattoccia2003). Our previous investigations further indicate that CamKII moderates the effects of PZQ through stabilizing Ca2+ fluxes within schistosome muscle and tegument. One hypothesis might be that PZQ action involves an increase of intracellular Ca2+ with CamKII acting to maintain Ca2+ homoeostasis as potentially central to the mode of action of PZQ (You et al., Reference You, McManus and Gobert2015; Nawaratna et al., Reference Nawaratna, You, Jones, McManus and Gobert2018).
In addition, as was evident from the in vitro assays, STSP and 1NAPP1 had better treatment efficacy on 2-day-old schistosomula in the first mouse trial compared with controls. Seven-day-old schistosomula showed less response in vivo to either of the inhibitors, as expected, when compared with initial in vitro assays. However, PZQ/1NAPP1 treatment was a more effective combination against 7-day-old schistosomula, which was confirmed in the second mouse trial. Therefore, 1NAPP1 might represent a superior adjunct therapy to be used with PZQ during the PZQ-insusceptible period. Adult worms showed a better response consistently through all three mouse drug trials to STSP alone or to PZQ/STSP and the PZQ/1NAPP1 combination in vivo compared with untreated controls. However, increasing the 1NAPP1 dose by 1.5 times in trial 2 did not improve the results.
In trial 1, there was a significant worm and egg reduction in mice treated at 5 weeks post infection with the PZQ/STSP or PZQ/NAPPI combination, due to the rapid killing of worms. This was reflected in the significantly decreased motility of adult S. mansoni in vitro after being treated with PZQ/PZQ/STSP or PZQ/NAPPI compared with PZQ-treated worms.
STSP, which is a microbial alkaloid isolated from Streptomyces staurosporeus (Park et al., Reference Park, Abdel-Azeem, Al-Sanea, Yoo, Tae and Lee2013), has been investigated as an anticancer therapy in clinical trials (Eder et al., Reference Eder, Garcia-Carbonero, Clark, Supko, Puchalski, Ryan, Deluca, Wozniak, Campbell, Rothermel and LoRusso2004; Monnerat et al., Reference Monnerat, Henriksson, Le Chevalier, Novello, Berthaud, Faivre and Raymond2004). STSP has been administered at an oral dose of 5–10 mg kg−1 day−1 (Abe et al., Reference Abe, Kubota, Otani, Furukawa, Watanabe, Kumai, Akiyama, Akinaga and Kitajima2001), intraperitoneally or subcutaneously (Hill et al., Reference Hill, Tillery, Rose and Posey1994) in mice. The inability to mount a similar response in killing parasites in vivo could be due to many reasons. STSP was given orally to mice with or without PZQ. Although an oral route is less intrusive than the intravenous route, pharmacokinetic interactions in the stomach, variability in absorption and first pass metabolism are some of the major factors that might have affected the final effective plasma concentration of the drug. Gastric emptying in mice shows an exponential decay with 50% emptying around 30 min (Schwarz et al., Reference Schwarz, Kaspar, Seelig and Kunnecke2002); therefore, we spaced the PZQ and STSP doses 45 min apart to prevent possible drug and solvent interactions in the stomach but, on the other hand, to reach high plasma concentrations around the same time. PZQ absorption in humans is known to be 80% of the oral dose with a plasma half-life of 1–2 h (Andrews et al., Reference Andrews, Thomas, Pohlke and Seubert1983; Chai, Reference Chai2013). Both STSP and PZQ have limited bioavailability (13% and 5%, respectively) (Hill et al., Reference Hill, Tillery, Rose and Posey1994; Abla et al., Reference Abla, Keiser, Vargas, Reimers, Haas and Spangenberg2017). Although adult worms may be initially exposed to a drug concentration similar to that received by oral administration in the mesenteric veins before reaching the liver, the drug exposure of schistosomula, which reside in the lungs, will be affected due to drug clearance before the worms reach the lungs. The discrepancies between the in vitro and in vivo assays could also be explained by the use of mechanically transformed schistosomula rather than naturally transformed schistosomula in vivo.
In trials 1 and 2, the treatment at week 5 post-infection, with the highest published doses of STSP (10 mg kg−1) combined with PZQ (250 mg kg−1), reached the maximum effect in terms of reductions in worm burden and egg counts compared with the control group. Reduced STSP doses used in trial 3 (2.5 and 5 mg kg−1) on adult worm infections, however, yielded lower worm and egg reductions. Mice treated with PZQ/STSP10 (STSP 10 mg kg−1 with PZQ 250 mg kg−1) on week 5 post-infection showed a similar worm reduction, but significantly lower liver egg counts (58%) compared with the PZQ group, indicating the additive effect of STSP in inhibiting the fecundity or egg production of adult parasites.
We have shown that the inhibition of CaMK can be used as a possible adjunct therapy to PZQ in treating juvenile and adult schistosome infections. The in vivo experiments, however, did not show the effects in a similar magnitude to the in vitro results. The experiments could be repeated with different solvents to improve the efficacy of drug delivery. According to the in vitro results, the STSP and 1NAPP1 dose could be further reduced to establish whether similar results would be obtained using the schistosomula stage, which would help to minimize any possible drug side effects. The more selective CaMK inhibitors K-252a (inhibitor 8) and Autocamtide-2 inhibitor (inhibitor 11) could be possible additional candidates for future combination treatment trials. A wider range of inhibitor concentrations/doses could be tested in future follow-up studies with separate control group for each solvent used. To confirm the functional effects of CaMK inhibitors on calcium homoeostasis in worms, the development of a live worm staining technique to detect calcium influx could be used in future studies to yield more phenotypic data to explore the impact of combined drug treatments on schistosomes.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020001250.
Acknowledgements
We gratefully acknowledge the assistance given by Mary Duke in animal experiments. The support of the QIMRB animal facility is also appreciated.
Financial support
This research was funded by the National Health and Medical Research Council (NHMRC) of Australia. DPM is an NHMRC Senior Principal Research Fellow and Senior Scientist at QIMRB.
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
The conducts and procedures involving animal experiments were approved by the Animal Ethics Committee of the QIMR Berghofer Medical Research Institute (project number A0104-016), which adheres to the Australian code of practice for the care and use of animals for scientific purposes, as well as the Queensland Animal Care and Protection Act 2001; Queensland Animal Care and Protection Regulation 2002. This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.













