Emotional contagion refers to the tendency to automatically assume some degree of the emotions of those with whom we interact (Hatfield, Cacioppo, & Rapson, Reference Hatfield, Cacioppo and Rapson1994). Individuals with autism spectrum disorder (ASD) tend to demonstrate decreased emotional contagion and empathy under some circumstances (Baron-Cohen & Wheelwright, Reference Baron-Cohen and Wheelwright2004). A better understanding of how and when emotional contagion is triggered in individuals with ASD, and its potential relationship to empathic development, has implications for both understanding the neural bases of the disorder and optimizing early intervention.
One mechanism by which emotional contagion may come about is via the underlying process of mimicry (Hatfield et al., Reference Hatfield, Cacioppo and Rapson1994). In contrast to imitation, which involves the conscious, effortful reproduction of another's behavior, mimicry refers to nonvolitional “matching” behavior (Want & Harris, Reference Want and Harris2002). Electromyography (EMG) research reveals that as human beings engage in face to face interaction, we unconsciously mimic (often at a level undetectable to the naked eye) one another's posture, facial expressions, vocal prosody, speech patterns, gestures, and emotional expressions (e.g., Niedenthal, Barsalou, Ric, & Krauth-Gruber, Reference Niedenthal, Barsalou, Ric and Krauth-Gruber2005). The classic James–Lange hypothesis asserts that the observer perceives the target's posture and expressions, her musculature mimics that of the other, and the brain interprets feedback from its own musculature as signaling the emotion in question (e.g., James, Reference James1890). Alternatively, the observer may represent and recognize the emotion that he/she infers from the other's actions; the recognition of that emotion may overflow its neuronal representation and become embodied in the observer's musculature, causing mimicry (Preston & de Waal, Reference Preston and de Waal2002). The embodiment may subsequently “feed back” centrally to generate or enhance the relevant emotion (Moody, McIntosh, Mann, & Weisser, Reference Moody, McIntosh, Mann and Weisser2007).
This emotional contagion or “embodied communication” (Kinsbourne & Jordan, Reference Kinsbourne and Jordan2009) between observer and target is often described as “emotional” or “affective” empathy (Davis, Reference Davis1996; Preston & de Waal, Reference Preston and de Waal2002; although some definitions differ, e.g., see De Vignemont & Singer, Reference De Vignemont and Singer2006) and has been proposed to be a crucial component in developing cognitive empathy (Decety & Jackson, Reference Decety and Jackson2004; Preston & de Waal, Reference Preston and de Waal2002; Singer & Lamm, Reference Singer and Lamm2009), in which deficits among individuals with ASD are well documented (Baron-Cohen, Reference Baron-Cohen, Baron-Cohen, Tager-Flusberg and Cohen2000). The amount of automatic mimicry, observed via behavioral coding (Sonnby-Borgstrom, Reference Sonnby-Borgstrom2002), and measured in movements of the facial muscles via EMG (Andreasson & Dimburg, Reference Andreasson and Dimberg2008), both correlate positively with an individual's level of emotional empathy (taking on the emotions of others; Andreasson & Dimburg, Reference Andreasson and Dimberg2008; Kaplan & Iacoboni, Reference Kaplan and Iacoboni2006; Sonnby-Borgstrom, Reference Sonnby-Borgstrom2002), as well as cognitive empathy (taking on the perspective of others; Chartrand & Bargh, Reference Chartrand and Bargh1999).
Because mimicry automatically occurs when a person observes or engages with another person, neuroimaging studies in which participants passively observe the actions or the emotions of others presumably capture the neural correlates of nonvolitional mimicry, which consistently reveal common coding for first- and third-person experiences. Such common coding has been reported for disgust (Wicker et al., Reference Wicker, Keysers, Plailly, Royet, Gallese and Rizzolatti2003), pain (Singer et al., Reference Singer, Seymour, O'Doherty, Kaube, Dolan and Frith2004), touch (Keysers et al., Reference Keysers, Wicker, Gazzola, Anton, Fogassi and Gallese2004), emotional body language (de Gelder & Hadjikhani, Reference de Gelder and Hadjikhani2006), and emotional expressions (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, Reference Carr, Iacoboni, Dubeau, Mazziotta and Lenzi2003) in the insula and anterior cingulate, while common coding for contagious actions, such as yawning and laughter, are observed in the inferior frontal cortices and sensorimotor cortices (Haker, Kawohl, Herwig, & Rossler, Reference Haker, Kawohl, Herwig and Rössler2013; Meyer, Zysset, von Cramon, & Alter, Reference Meyer, Zysset, von Cramon and Alter2005) with some studies showing insula activation. The insula (particularly, the anterior insular cortex) has been suggested to mediate the transformation of a perceived action into an emotional experience by relaying information about action representation from the superior temporal and inferior frontal cortices (sometimes referred to as “the mirror neuron system”) to the limbic system (Carr et al., Reference Carr, Iacoboni, Dubeau, Mazziotta and Lenzi2003). This relay is proposed to allow information about the actions of others (e.g., a facial expression) to provoke an emotional experience (e.g., emotional empathy), with the resulting shared neural representation providing an “embodied theory of mind” (Corradi-Dell'Acqua, Hofstetter, & Vuilleumier, Reference Corradi-Dell'Acqua, Hofstetter and Vuilleumier2011; Gu et al., Reference Gu, Eilam-Stock, Zhou, Anagnostou, Kolevzon, Soorya and Fan2015; Singer & Lamm, Reference Singer and Lamm2009; Wicker et al., Reference Wicker, Keysers, Plailly, Royet, Gallese and Rizzolatti2003). The passive viewing of contagious actions and emotional expressions while undergoing functional magnetic resonance imaging reveals that individuals with greater activation in shared neural representations (i.e., greater embodiment of the target's state) are also associated with individual levels of emotional empathy (Gazzola, Aziz-Zadeh, & Keysers, Reference Gazzola, Aziz-Zadeh and Keysers2006; Kaplan & Iacoboni, Reference Kaplan and Iacoboni2006), cognitive empathy (Gazzola et al., Reference Gazzola, Aziz-Zadeh and Keysers2006; Platek, Myers, Critton, & Gallup, Reference Platek, Myers, Critton and Gallup2003), or both (Arnott, Singhal, & Goodale, Reference Arnott, Singhal and Goodale2009; Pfeifer, Iacoboni, Mazziotta, & Dapretto, Reference Pfeifer, Iacoboni, Maziotta and Dapretto2008).
Some researchers have proposed that the empathy deficits observed in individuals with ASD can be attributed to a “broken mirror” system (Iacoboni & Dapretto, Reference Iacoboni and Dapretto2006; Oberman & Ramachandran, Reference Oberman and Ramachandran2007; Ramachandran & Oberman, Reference Ramachandran and Oberman2006; Rizzolatti & Fabbri-Destro, Reference Rizzolatti and Fabbri-Destro2010; Williams, Whiten, Suddendor, & Perrett, Reference Williams, Whiten, Suddendorf and Perrett2001), or abnormalities in cortical and subcortical networks involved in emotion contagion (Hadjikhani et al., Reference Hadjikhani, Joseph, Manoach, Naik, Snyder, Dominick and de Gelder2009), which deny individuals with ASD the experience of shared neural representations and emotions during face to face interactions; however, these notions are hotly debated (see Leighton, Bird, Charman, & Heyes, Reference Leighton, Bird, Charman and Heyes2008; Southgate & Hamilton, Reference Southgate and Hamilton2008).
Contagious Yawning
Contagious yawning is a type of mimicry in that it is a matching behavior that is produced automatically. However, unlike the miniscule muscular movements that mimicry typically entails, yawns are large, obvious sequences of movements. The explanation for this may derive from the fact that yawning is a fixed action pattern (Provine, Reference Provine1986), which is a species-typical behavioral sequence that is indivisible and, once initiated, runs to completion. Mimicking the first part of a fixed action pattern may trigger the release of the entire behavior.
In addition to being a form of mimicry, contagious yawning may involve an emotional component. Deputte (Reference Deputte1994) identified two contexts for yawns: the “rest yawn,” when there is a change in arousal level, and the “emotion yawn,” which is used as an unconscious communication of psychological decompression after a state of high alert. The construct of an emotion yawn suggests that contagious yawning may be considered a form of emotional contagion. Yawning is similar to other contagious acts (e.g., crying and laughing) in that it produces a distinct sound, as well as a distinct facial expression (Provine, Reference Provine1996). In contrast, yawning may not signal an emotion but may simply be a facial expression that is unintentionally mimicked, as are other nonemotional facial expressions (Heyes, Reference Heyes2001). Ubiquitous facial mimicry may be adaptive because it facilitates contagion of “true” emotions. In either case, the disruption of mimicry, which may be demonstrated by a disruption in contagious yawning, should have consequences for emotional resonance with others. People with high levels of empathy exhibit greater amounts contagious yawning (Norscia & Palagi, Reference Norscia and Palagi2011; Platek et al., Reference Platek, Myers, Critton and Gallup2003).
Automatic Mimicry and Emotional Contagion in ASD
Whereas TD individuals tend to automatically attend to and mimic emotional cues, individuals with ASD may show decreased (McIntosh, Reichmann-Decker, Winkielman, & Wilbarger, Reference McIntosh, Reichmann-Decker, Winkielman and Wilbarger2006; Yoshimura, Sato, Uono, & Toichi, Reference Yoshimura, Sato, Uono and Toichi2015) and atypical (Beall, Moody, McIntosh, Hepburn, & Reed, Reference Beall, Moody, McIntosh, Hepburn and Reed2008; Oberman, Winkielman, & Ramachandran, Reference Oberman, Winkielman and Ramachandran2009) spontaneous mimicry and decreased emotional contagion. Helt, Eigsti, Snyder, and Fein (Reference Helt, Eigsti, Snyder and Fein2010) assessed contagious yawning in children with ASD under naturalistic circumstances and reported its occurrence to be very low in children with mild ASD symptomology and absent in children with moderate to severe symptomology. Similarly, laboratory studies exposing individuals with ASD to prerecorded video (Senju et al., Reference Senju, Maeda, Kikuchi, Hasegawa, Tojo and Osanai2007) and audio (Giganti & Esposito Ziello, Reference Giganti and Esposito Ziello2009) clips show markedly decreased rates of contagious yawning in individuals with ASD compared with their typicslly developing (TD) peers. Finally, Scambler, Hepburn, Rutherford, Wehner, and Rogers (Reference Scambler, Hepburn, Rutherford, Wehner and Rogers2006) presented strong facial and bodily models of joy, disgust, pain, and fear, and reported that children with ASD responded with significantly fewer instances of emotional contagion than controls.
Sims, Van Reekum, Johnstone, and Chakrabarti (Reference Sims, Van Reekum, Johnstone and Chakrabarti2012) found that TD participants with low (but not high) ASD traits demonstrated greater mimicry for faces that they had been trained to associate with high (vs. low) reward values, indicating that, in general, people will tend to show greater mimicry to faces they find more rewarding. In contrast, participants with high ASD traits showed similar amounts of mimicry to target stimuli regardless of reward value, indicating that individuals with ASD may be less sensitive than their peers to cues associated with social reward. This evidence aligns with theories that posit that children with ASD experience reduced social reward sensitivity, which manifests in reduced ability to affectively tag socially relevant stimuli (Dawson, Bernier, & Ring, Reference Dawson, Bernier and Ring2012; Fein, Pennington, Markowitz, Braverman, & Waterhouse, Reference Fein, Pennington, Markowitz, Braverman and Waterhouse1986; Klin, Jones, Schultz, & Volkmar, Reference Klin, Jones, Schultz and Volkmar2003; Waterhouse, Fein, & Modahl, Reference Waterhouse, Fein and Modhal1996).
Although behavioral evidence consistently reveals that children with ASD show less emotional contagion than their TD peers, other studies suggest the possibility of intact or even heightened emotional contagion among individuals with ASD. Hadjikhani et al. (Reference Hadjikhani, Joseph, Manoach, Naik, Snyder, Dominick and de Gelder2009) showed that brain activation in individuals with ASD did not differ from controls when participants viewed fear or pain (Hadjikhani et al., Reference Hadjikhani, Zürcher, Rogier, Hippolyte, Lemonnier, Ruest and Prkachin2014). Blair (Reference Blair1999) reported that children with ASD showed typical psychophysiological responses to images of distressed people. Meanwhile, Gu et al. (Reference Gu, Eilam-Stock, Zhou, Anagnostou, Kolevzon, Soorya and Fan2015) reported that individuals with ASD showed heightened autonomic arousal (though reduced embodiment, or shared neural representation) when viewing others in pain. Self-report questionnaires, such as the Interpersonal Reactivity Index and the Multifaceted Empathy Test, have revealed impairments in cognitive empathy, but either normal (Dziobek et al., Reference Dziobek, Rogers, Fleck, Bahnemann, Heekeren, Wolf and Convit2008) or heightened emotional empathy among adults with Asperger syndrome (Rogers, Dziobek, Hassenstab, Wolf, & Convit, Reference Rogers, Dziobek, Hassenstab, Wolf and Convit2007). Finally, Magnee, de Gelder, van Engeland, and Kemner (Reference Magnee, de Gelder, van Engeland and Kemner2007) used EMG to measure subtle emotional responses in a small group of high-functioning individuals with ASD. The researchers reported that the participants with ASD showed heightened electromyographic responsiveness both to happy and to fearful faces compared to controls. This evidence of heightened emotional empathy aligns with theories that posit that children with ASD begin life with normal social attention and then defensively turn their attention away from social stimuli because it is overarousing (Kinsbourne, Reference Kinsbourne and Fein2011; Markram, Rinaldi, & Markram, Reference Markram, Rinaldi and Markram2007; Smith, Reference Smith2009).
Eye Gaze, Mimicry, and ASD
Regardless of the underlying cause of impaired social attention in ASD, it undoubtedly plays a role in the reduced emotional contagion previously observed in individuals with ASD. Research in another area of social cognition, face perception, has demonstrated that under default circumstances, individuals with ASD show reduced fusiform face activity (FFA; Schultz et al., Reference Schultz, Gauthier, Klin, Fullbright, Anderson, Volkmar and Gore2000) compared to controls; however, if participants are continuously cued to the eye region, these group differences disappear (Hadjikhani et al., Reference Hadjikhani, Joseph, Snyder, Chabris, Clark, Steele and Tager-Flusberg2004). Individuals with ASD have been documented to show very reduced attention to another's pain (Bacon, Fein, Morris, & Waterhouse, Reference Bacon, Fein, Morris and Waterhouse1998; Sigman, Dissanayake, Corona, & Espinosa, Reference Sigman, Dissanayake, Corona and Espinosa2003). However, Scambler et al. (Reference Scambler, Hepburn, Rutherford, Wehner and Rogers2006) gained the child's attention before assessing emotional contagion, and found it still reduced, supporting the notion that social attention cannot account for all of the reduction in emotional contagion.
Eye contact with the target has been suggested to trigger contagious yawning. For example, Provine (Reference Provine1989) demonstrated that TD individuals will yawn contagiously if shown the disembodied eyes of a yawning face, but not the disembodied mouths. To test the possibility that diminished contagious yawning in ASD is due to diminished eye gaze, Senju et al. (Reference Senju, Kikuchi, Akechi, Hasegawa, Tojo and Osanai2009) instructed children to fixate on the eyes of yawning faces and found no group differences in the rate of contagious yawning between TD and ASD children; however, as the TD group yawned the same amount, to yawn and control stimuli these results are difficult to interpret. Further complicating the picture, Giganti and Esposito Ziello (Reference Giganti and Esposito Ziello2009) found that individuals with ASD also showed reduced yawn contagion when presented with an auditory only yawning stimulus indicating that lack of eye gaze cannot completely explain diminished yawn contagion in ASD.
Stimulus Familiarity
Research on other aspects of social–emotional functioning in ASD has also revealed particularly strong effects of stimulus familiarity in this population. Individuals with ASD have been reported to show enhanced language, social skills, cognitive test scores, and even skin conductance responses when “high-interest” stimuli are used (Koegel, Koegel, & Smith, Reference Koegel, Koegel and Smith1997; Pierce & Schreibman, Reference Pierce and Schreibman1997; van Engeland, Roelofs, Verbaten, & Slangen, Reference van Engeland, Roelofs, Verbaten and Slangen1991). Although Shultz et al., (2000) reported diminished FFA activation in response to faces in ASD, Pierce, Haist, Sedaghat, and Courchesne (Reference Pierce, Haist, Sedaghat and Courchesne2004) found that participants with ASD showed normal FFA activity to familiar faces. Finally, although Bernier, Dawson, Webb, and Murias (Reference Bernier, Dawson, Webb and Murias2007) and Oberman et al. (Reference Oberman, Hubbard, McCleery, Altschuler, Ramachandran and Pineda2005) reported diminished mu wave suppression (taken to indicate “mirror” response) in participants with ASD when viewing the hand motions of strangers, Oberman, Ramachandran, and Pineda (Reference Oberman, Ramachandran and Pineda2008) reported normal mu wave suppression in participants with ASD when viewing the hand movements of their family members.
Finally, mimicry studies on TD adults indicate that preexisting rapport increases the amount of mimicry one will show with an interaction partner (Likowski, Muhlberger, Seibt, Pauli, & Weyers, Reference Likowski, Muhlberger, Seibt, Pauli and Weyers2008; McIntosh, Reference McIntosh2006; Tickle-Degnen, Reference Tickle-Degnen, Manusov and Patterson2006), and that individuals are most likely to contagiously yawn when exposed to the yawn of a relative (Norscia & Palagi, Reference Norscia and Palagi2011), indicating that among the general population the effects of social reward may be more pronounced than the effects of attention when it comes to mimicry. We know of no previous research that has explored the impact of stimulus familiarity on emotional contagion in individuals with ASD.
Current Study
In summary, the majority of the literature shows reduced emotional contagion in ASD. This has been attributed to attaching less reward value to social stimuli, showing reduced social attention to the target stimuli, or innate neurological differences in the common coding of actions and emotions. However, a handful of studies implicate intact emotional contagion in ASD, and research in other aspects of social cognition reveal that individuals with ASD appear to be disproportionately influenced by study design, such as the familiarity or importance of the target, and the task instructions.
Contagious yawning and laughing offer a noninvasive approach (well tolerated by children with ASD) to the study of mimicry (i.e., mirrorlike phenomena; Provine Reference Provine2005b). Laughter and yawning were chosen as the target responses because they share several traits: (a) they have emotional content; (b) they occur under both spontaneous and contagious conditions (Provine, Reference Provine2005b); (c) their spontaneous occurrence has been shown not to be diminished in children with ASD (Giganti & Esposito Ziello, Reference Giganti and Esposito Ziello2009; Hudenko, Stone, & Bachorowski, Reference Hudenko, Stone and Bacharowski2009); and (d) TD individuals show similar amounts of mimicry to both (as opposed to frowning; Estow, Jamieson, & Yates, Reference Estow, Jamieson and Yates2007).
We explored emotional contagion in ASD under different conditions, including manipulating instructions to maximize eye gaze, and stimulus familiarity. We hypothesized that if differences are primarily mediated by eye gaze/social attention, then manipulating task instructions to maximize eye gaze to target should minimize group differences, as should delivering the stimuli in an auditory-only condition. If differences are mediated by decreased reward value of social stimuli, then increasing the familiarity, and thus the social reward value, of the stimuli should minimize group differences. Finally, if reduced emotional contagion is mainly due to innate neurological differences in the mirror neuron areas of the brain, then individuals with ASD should show reduced emotional contagion across all conditions.
Method
Participants
Participants were 60 children, ages 8–17, with a prior diagnosis of ASD, confirmed by the experimenter (M.H.) using the Autism Diagnostic Observation Scale (ADOS) and clinical judgment based on the DSM-5. There was also a TD control group (n = 60), ages 5–13, matched to the ASD group for both sex and mental age within 6 months using the Stanford Binet. Both groups were recruited through information tables set up at various local events (e.g., the Connecticut Special Olympics and the Autism Speaks Hartford walk). See Table 1 for participant characteristics.
Table 1. Demographic characteristics of participants
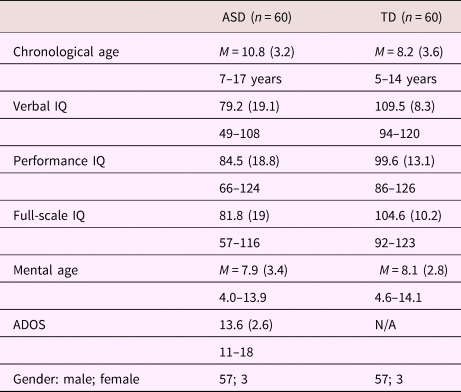
Note: ASD, children with autism spectrum disorders. TD, typically developing children. IQ, intelligence quotient. ADOS, Autism Diagnostic Observation Scale. Scores given as: Mean (SD), range.
Measures
ADOS
The ADOS (Lord, Rutter, DiLavore, & Risi, Reference Lord, Rutter, DiLavore and Risi2000) consists of a structured play session that is scored for ASD and was used to confirm existing diagnoses. Modules 2 (for children with phrase speech) and 3 (for children with fluent speech) were used.
Stanford–Binet Intelligence Scale: Fifth Edition Abbreviated IQ scales (Roid, Reference Roid2003)
Children provide word definitions, and solve a series of picture puzzles, yielding a performance IQ, a verbal IQ, and mental age.
Procedure
Each child viewed the videos in a private room in his/her home, sitting 18 inches from a 23.6-inch monitor and speakers. Audio level was preset, but participants were given the option to adjust the volume during the video introduction. Participants were told that they would be viewing a series of faces making different expressions. Children then viewed a series of video clips of individuals yawning and laughing under six different experimental conditions, randomized for order:
1. Sight only (clips of individuals laughing or yawning in which the sound had been removed)
2. Sound only (audio clips of laughter or yawning in which the video had been removed)
3. Sight + sound (clips of individuals facing the camera and included both audio and visual components)
4. Naturalistic (clips of individuals laughing or yawning with both audio and visual components but rather than close up shots of one person facing the camera, they were taken in a naturalistic context—two individuals talking at a table and one laughing, a mother putting her child to bed and the child yawning, etc.)
5. Cued attention to eyes (the clips were identical to those in Condition 3 but participants were asked to press a button indicating whether the target's eyes were blue or brown, forcing at least brief eye gaze)
6. Familiar/parent targets (the clips were identical in format to those in Condition 3 except that the stimuli were the participants’ parents)
The stimuli for Conditions 1, 2, 3, and 5 were created by having 18 adults and 18 children (ages 8–13) wear wireless earbuds that played sounds of yawning and laughter taken from the internet while looking into the video camera. Thus, the videos of yawns and laughs (both under weak voluntary control; see Provine Reference Provine2005a) were themselves contagious yawns and laughs produced in response to auditory stimuli. Laughs and yawns judged to be genuine by the “actor” and the experimenter were used in a pilot study with undergraduates to ensure their contagious properties, and any clips that did not produce contagious responses in the pilot group were discarded, for a final set of 32 yawn clips and 32 laugh clips (8 male children, 8 female children, 8 male adults, and 8 female adults). This pool of video clips was used for Conditions 1 (the sound was removed from the clips), 2 (the audio portion only of the clips were used), 3 (the clips were used as described), and 5 (the clips were used as described). The blocked stimuli for each of these four conditions included 8 clips, half adults and half children, half male and half female.
The stimuli for Condition 6 were created in the same manner using the individual participant's parents. To ensure that these videos with parent targets were like those used in other conditions, we conducted a pilot study with (n = 12) undergraduates. The results of this pilot study indicated that undergraduates experienced just as much contagious laughter, t (11) = 1.33, p = .36, and yawning, t (11) = 0.87, p = .59, when viewing a compilation of the videos of a parent as they did when viewing the videos of experimenter confederate stimuli (used for Condition 3).
The stimuli for Condition 4 were created by capturing a large corpus of videos of friend–friend and parent–child dyads in naturalistic contexts and then editing the videos in length to leave only spontaneously captured laughter and yawns. These videos were piloted with undergraduates in the same manner described above, and the 16 clips (8 yawn, 8 laughter) that produced the most contagion in the pilot group were selected for use in Condition 4.
Thus, the final studies were each 2 (group) × 6 (condition) designs: one for yawning and one for laughter. Each of the 12 blocked video and/or audio clips contained 8 yawns (M = 6.5 s each) or laughs (M = 3 s each) with fixation stars shown at eye level between contagious stimuli clips within each blocks, lasting approximately 90 s. (Note that a laugh was defined as a laugh bout, i.e., the laughter produced by one continuous vocalization; e.g., ha-ha-ha-ha-ha). The order of the 12 blocked videos (conditions) was randomized for each participant. Interim blank screens were presented between blocked conditions for a total of 20 min of viewing time.
Contagious laughs occur almost immediately after the stimulus laugh, and there appears to be a gradual decrease in the tendency to laugh contagiously as subsequent laugh stimuli are presented (Provine, Reference Provine2012). In contrast, contagious yawns may occur up to 90 s after the target yawn (Helt et al., Reference Helt, Eigsti, Snyder and Fein2010), and there may be a gradual increase in the probability of yawning during the seconds after the observed yawn (Provine, Reference Provine2012). The current videos were blocked in order to account for this lag time in contagious yawning and to provide the maximal opportunity for individuals to show a low base rate behavior without interference from the arousal of laughing. After the data was collected, we ensured the manipulations had worked to induce matching emotions by comparing the mean number of yawns exhibited during yawn stimuli (M = 2.6) to the mean number of yawns during the laughter stimuli (0.78), as well as the mean number of laughs during the laughter stimuli (4.1) to the mean number of laughs during the yawn video (0.69).
The computer recorded a video of each participant's face as he/she watched the videos. Each video was coded for yawning and laughing by two independent raters blind to group status, as well as to which type of stimulus the participant was watching at the time of the coding. The dependent variable was the mean number of matching responses (contagious yawns or laughs) that occurred in each group during each condition. Yawn coding criteria required the presence of all of the physical manifestations of a yawn (Provine, Reference Provine2005a): open mouth or jaw clench (associated with attempted suppression of yawn), narrowed eyes, indrawn breath, and observable vibration of throat and shoulders. Laughter coding criteria involved counting laugh bouts (i.e., laughter episodes typically produced during one exhalation) whenever a participant demonstrated (a) rapid intensification of positive facial expression accompanied by (b) a voiced or unvoiced exhalation of breath and (c) observable shaking or vibration of throat and shoulders. Interrater reliability was 100% for yawning, and 91% for laughter.
Results
Yawning
A mixed between- and within-subjects analysis of covariance was conducted to assess the impact of condition (sight, sound, sight + sound, naturalistic, cued-to-eyes, and parents) on the mean number of contagious yawns exhibited per group, covarying chronological age, and IQ. Preliminary checks were conducted to ensure that there was no violation of the assumptions of normality, linearity, homogeneity of variances, homogeneity of regression slopes, and reliable measurement of the covariate.
After adjusting for age and IQ, there remained a significant main effect for condition, F (5, 114) = 5.184, p < .0001, partial η2 = .197 (see Figure 1), and for group, F (1, 118) = 14.103, p < .0001, partial η2 = .203, with the ASD group showing less contagious yawning. There was also a significant interaction between stimulus condition and group, Pillai's trace = .116, F (5, 114) = 2.996, p = .014, partial η2 = .116.

Figure 1. Mean number of yawns per group by stimulus condition. Error bars represent 95% confidence intervals.
One-way repeated-measures analyses of variance on yawning rates showed no significant effects of condition for the TD group Pillai's trace = .095, F (5, 55) = 1.161, p = .340, but a significant effect for condition in the ASD group, Pillai's trace = .312, F (5, 55) = 4.979, p = .001. Pairwise comparisons (Bonferroni-adjusted) indicated that children with ASD yawned significantly more in the parent condition than in the sight only condition, t (59) = 4.243, p ≤ .0001, the sound only condition, t (59) = 3.94, p ≤ .0001, the naturalistic condition, t (59) = 3.595, p = .001, and the sight + sound condition, t (59) = 3.069, p =.003, and there was a trend-level difference for more yawning to parents than to cued-to-eyes, t (59) = 1.646, p = .10. Participants with ASD yawned significantly more in the cued-to-eyes condition than in the sight only, t (59) = 3.5, p = .001, sound only, t (59) = 2.96, p = .004, sight + sound, t (59) = 1.99, p = .052, and naturalistic conditions, t (59) = 2.67, p = .01.
Independent t tests between groups indicated that the TD group showed significantly more yawning than the ASD group in the sight condition, t (118) = 5.95, p < .0001, sound condition, t (118) = 4.003, p < .0001, naturalistic condition, t (118) = 4.155, p < .0001, and sight + sound condition, t (118) = 4.035, p < .0001, but not in the cued-to-eyes condition, t (118) = 1.289, p = .200, or the parent condition, t (118) = –0.084, p = .933.
Laughter
The same analyses were run on contagious laughter and preliminary assumptions were met. Once again, there was a significant main effect for condition, Pillai's trace = .329, F (5, 114) = 11.363, p < .0001, partial η2 = .333 (see Figure 2) and for group, F (1, 118) = 13.2, p < .0001, partial η2 = .108, with the ASD group showing less contagious laughter overall. There was also a significant interaction between condition and group, Pillai's trace = .156, F (5, 114) = 4.411, p = .002, η2 squared = .156. Again, the means (see Figure 2) indicated that the ASD group, but not the TD group, showed more contagious laughter under some conditions than others.

Figure 2. Mean number of yawns per group by stimulus condition. Error bars represent 95% confidence intervals.
Confirming this, one-way repeated measures analyses of variance showed no significant effect for condition in the TD group, Pillai's trace = .153, F (5, 55) = 1.985, p = .105, but a significant effect for condition in ASD, Pillai's trace = .189, F (5, 55) = 2.562, p = .037. Pairwise comparisons in ASD showed more laughter in the parent condition than in all other conditions: sight, t (59) = 8.75; sound, t (59) = 7.08; naturalistic, t (59) = 5.738; sight + sound, t (59) = 6.15; cued-to-eyes, t (59) = 4.81; all p < .0001. Participants with ASD laughed significantly more in the cued-to-eyes condition than in the sight, t (59) = 3.9, p < .0001, and sound, t (59) = –2.46, p ≤ .017, conditions.
Independent t tests between groups indicated more laughter in TD than ASD groups in the sight, t (118) = 5.280, p < .0001, sound, t (118) = 4.099, p < .0001, sight + sound, t (118) = 3.191, p = .002, and naturalistic conditions, t (118) = 2.955, p = .004, but not in the cued-to-eyes condition, t (118) = –1.740, p = .086, or in the parent condition, t (118) = 0.767, p = .445.
Correlations between contagion and ASD severity
ASD symptom severity (as measured by ADOS scores put into an algorithm developed by Gotham, Pickles, & Lord, Reference Gotham, Pickles and Lord2009) and contagious yawning (total number of contagious yawns across conditions) were negatively correlated, r = –.345, p = .007, so worse symptoms were associated with less contagious yawning, as were ASD symptom severity and contagious laughter, r = –.333, p = .009. This relationship was even stronger in the “naturalistic” condition alone (yawn r = –.632, p < .0001; laugh r = –.590, p < .0001) and weaker in the parent condition (yawn r = –.236, p = .010; laugh r = –.145, p = .035). The relationship between yawn contagion and laugh contagion was also significant (TD: r = .401, p = .002; ASD: r = .377, p = .003).
Percentage of each group showing emotional contagion
Contagious yawning and laughter were also coded as dichotomous variables (the individual either did or did not yawn or laugh more when presented with the matching stimuli than when presented with nonmatching stimuli for each condition. This produced a percentage of participants in each group showing emotional contagion under each condition (see Figures 3 and 4). Chi square goodness-of-fit testing revealed that fewer children with ASD showed emotionally contagious responses than did TD children in most conditions: yawn sight, χ2 (1, 120) = 28.5, p < .0001; laugh sight, χ2 (1, 120) = 26.4, p < .0001; yawn sound, χ2 (1, 120) = 30.0, p < .0001; laugh sound, χ2 (1, 120) = 16.8, p < .0001; yawn naturalistic, χ2 (1, 120) = 19.2, p < .0001; laugh naturalistic, χ2 (1, 120) = 26.4, p < .0001; yawn with sight + sound, χ2 (1, 120) = 23.5, p < .0001; laugh with sight + sound, χ2 (1, 120) = 27.6, p < .0001; yawn cue-to-eyes, χ2 (1, 120) = 12.2, p < .0001; laugh cue-to-eyes, χ2 (1, 120) = 14.4, p < .0001. The only condition with no group difference was parents as stimuli, in which cases the groups only showed nonsignificant trends toward difference: yawn parents, χ2 (1, 120) = 3.357, p = .066; laugh parents, χ2 (1, 120) = 1.6, p = .10.

Figure 3. Percentage of each group showing contagious yawning by stimulus condition.

Figure 4. Percentage of each group showing contagious laughter by stimulus condition.
Discussion
Consistent with a great deal of previous research, children with ASD showed diminished emotional contagion compared to their TD peers under most conditions. However, when they were exposed to the familiar, and presumably rewarding, stimuli of their parents, or eye contact with the stimulus was cued via task instructions, they demonstrated rates of emotional contagion approaching or comparable to their TD peers. These findings offer a new perspective as to whether emotional empathy is reduced or intact among children with ASD, showing that children with ASD may show either typical or atypical rates of emotional contagion depending upon the circumstances. The finding that emotional contagion is intact in children under some circumstances is inconsistent with the hypothesis that they have innate abnormalities in neural systems associated with emotional contagion, which prevent its expression. Rather, it seems that reduced emotional contagion may be secondary to problems with social orienting and attention, which were partially overcome in the cued to eyes condition, and completely overcome in the parents condition.
The TD group displayed roughly the same amount of emotional contagion across conditions. In contrast, the ASD group displayed highly varied amounts of emotional contagion depending on the condition. For participants with ASD, cueing-to-eyes resulted in more yawning and laughter contagion compared to other conditions (although not as powerfully as the parent stimuli) and eliminated significant differences in yawning contagion between groups. The latter finding is consistent with that of Senju et al. (Reference Senju, Kikuchi, Akechi, Hasegawa, Tojo and Osanai2009) as well as the hypothesis that reduced social attention, in particular to the eyes, at least partially mediates the diminished emotional contagion previously reported among individuals with ASD. Continuous cueing to the eyes has previously been found to result in normal neural activation during face processing in an ASD population (Hadjikhani et al., Reference Hadjikhani, Joseph, Snyder, Chabris, Clark, Steele and Tager-Flusberg2004). The striking increase in rates of emotional contagion among children with ASD when eye gaze is cued supports the importance of teaching children with ASD early and often to attend to the eyes of others via explicit instruction or prompting (e.g., holding a desired object near the other's eyes) and delivering early intervention face-to-face so as to improve opportunities for automatic affective exchange.
However, the only conditions in which the ASD and TD means were truly comparable, and which produced the same percentage of contagious ASD responders as TD responders, were the conditions in which the child's parent served as the stimulus. These results align with previous research reporting normal neural responses to faces and actions in individuals with ASD only in studies in which the stimuli were composed of familiar individuals (Oberman et al., Reference Oberman, Ramachandran and Pineda2008; Pierce et al., Reference Pierce, Haist, Sedaghat and Courchesne2004). Typical levels of emotional contagion between children with ASD and their parents is also consistent with previous research showing that children with ASD are more likely to laugh at cartoons if viewing them with their parents (Helt & Fein, Reference Helt and Fein2016). In addition, previous research suggests that children with ASD display increased ability to match facial and vocal expressions of emotion when familiar adults are used as stimuli (Kaana-Kalman & Goldman, Reference Kaana-Kalman and Goldman2008). Furthermore, children with ASD show improved social interaction skills (Knott, Lewis, & Williams, Reference Knott, Lewis and Williams1995) as well as increased rates of physical and eye contact (Kasari, Sigman, & Yirmiya, Reference Kasari, Sigman and Yirmiya1993) when interacting with familiar, as opposed to unfamiliar, individuals.
ASD symptom severity was inversely correlated to both contagious yawning and laughter. This relationship was stronger in the “naturalistic” conditions and weaker in the parent condition, indicating that the amount of emotional contagion that participants with ASD show to strangers is more strongly indicative of their core ASD symptomology than is the amount of emotional contagion they show with their parents. Contagion for both laughter and yawning were also positively correlated with one another, showed similar patterns across conditions, and were both negatively correlated with ASD severity, consistent with the idea that emotional contagion is, to some degree, a unified construct relying on similar component processes, one that is disrupted with strangers to a greater degree in individuals with more severe ASD symptoms.
Candidate mechanisms
There are multiple reasons that the parent stimuli could have elicited normal levels of emotional contagion from children with ASD, including increased attention to parents, increased feelings of social reward and desire to affiliate toward parents, a less efficient and automatic embodied response to unfamiliar others due to impoverished social experience, or a combination of all of the above.
First of all, perhaps the initial cueing to the eyes was not enough to sustain the children's motivation to continue to attend to the eye region, whereas the connection with the parent provided an ongoing source of motivation and reward to continue to attend. Alternatively, it is possible that these effects demonstrate what happens when the uncomfortable arousal or anxiety of viewing strangers is removed for the group with ASD. Dalton et al. (Reference Dalton, Nacewicz, Johnstone, Schaefer, Gernsbacher, Goldsmith and Davidson2005) reported that in individuals with ASD, but not controls, the amount of eye gaze was significantly correlated with amygdala activation while viewing both emotional and nonemotional faces, suggesting that for individuals with ASD, eye gaze fixation is associated with emotional arousal. Perhaps even when participants with ASD are cued to the eyes of others, there remains an aversion to looking for any longer than possible. Future research should engage eye tracking and skin conductance to address these questions.
Second, these effects could be interpreted as evidence that children with ASD place normal social reward value on their interactions with their parents and reduced social reward value on unfamiliar others. Research on healthy adults reveals that one aspect of automatic facial mimicry is the (unconscious) desire to affiliate with the target (Lakin & Chartrand, Reference Lakin and Chartrand2003; Stel & Vonk, Reference Stel and Vonk2010). Meanwhile, previous research on individuals with ASD and high ASD traits indicate atypical sensitivity to the social rewards of mimicking and being mimicked by strangers (Hsu, Neufeld, & Charkrabati, Reference Hsu, Neufeld and Chakrabarti2018; Sims et al., Reference Sims, Van Reekum, Johnstone and Chakrabarti2012). Although a core symptom of the disorder is reduced affiliation with others, children with ASD tend to have typical attachment rates to their parents (Capps, Sigman, & Mundy, Reference Capps, Sigman and Mundv1994; Rutgers, Bakermans-Kranenburg, van Ijzendoorn, & Van Berckelaer-Onnes, Reference Rutgers, Bakermans-Kranenburg, van IJzendoorn and Van Berckelaer-Onnes2004). Parents are presumably sources of reward of many kinds, in addition to being familiar. In order to disentangle the effects of familiarity from those of social reward, future studies should compare emotional contagion to strangers, to rewarding familiar adults, and to familiar but not rewarding adults. Nevertheless, these results imply that increasing the social reward value of the target may result in normal emotional empathy levels among children with ASD.
Finally, it is possible that multiple factors are contributing to the current findings. Emotional contagion is not an all-or-none phenomenon; familiarity, intensity, salience, and attention may modulate responses (Hatfield et al., Reference Hatfield, Cacioppo and Rapson1994) just as they do for other forms of empathy (e.g., Avenanti, Paluello, Bufalari, & Aglioti, Reference Avenanti, Paluello, Bufalari and Aglioti2006) and face perception (e.g., Vuilleumier, Aromony, Driver, & Dolan, Reference Vuilleumier, Armony, Driver and Dolan2001). This definition of emotional empathy involves a representation of the target's inner state, which automatically activates in the observer a representation of the observed state. These representations, unless inhibited, prime associated autonomic and somatic responses in the observer (Baron-Cohen & Wheelwright, Reference Baron-Cohen and Wheelwright2004). Perhaps, rather than being fully automatic, emotional contagion involves some form of rapid, nonconscious, contextual appraisal, and individuals with ASD have stricter parameters for evaluating an emotional cue in a manner consistent with a subsequent empathic response (rather than inhibiting either the response or the attention to the emotional cue). If social signals are uncomfortably arousing, then perhaps only the abovementioned factors are enough to overcome the tendency for subtle forms of attentional avoidance. Perhaps, rather than the scope of mirror neuron functioning being innately specified, mirroring processes become automatic by means of a stream of continuous emotional exchange with others early in development (Heyes, Reference Heyes2010), and so a history of impoverished social attention (due to ASD symptoms) results in a less efficient and automatic activation of shared neural representations with others. In other words, TD individuals may develop an “enactive mind” (Klin et al., Reference Klin, Jones, Schultz and Volkmar2003), facilitating automatic processing of the social signals of most people most of the time, at least once they have reached the age of 5. In contrast, children with ASD, having missed out on an early developmental milieu consisting of a continuous stream of emotional contagion (feeling at least a little bit of what most of their interaction partners are feeling) may seek out and attend to a different set of environmental cues as salient and rewarding, and only automatically affectively familiar people and certain types of emotional signals as behaviorally relevant stimuli, and others only when given explicit cueing or instructions.
Clinical implications
The results of the current study reinforce that it is crucial to enhance attentional and motivational salience for children with ASD, in order to measure their true abilities (i.e., in order to tease apart what they can do from what they tend to do); this applies to both laboratory and educational settings. In addition, gaining the child's attention and creating opportunities for affective exchange may form a particularly important part of intervention or therapy carried out by unfamiliar individuals. Although it is always important for parents to be involved in their children's therapies no matter what disorder is being treated, this may be especially so in the case of children with ASD, who may only show their true capacity for empathic engagement with their parents.
Limitations and future directions
There are multiple factors that limit interpretability of the current work. First, it is possible that the fact that our groups are matched for mental, rather than chronological, age either exaggerated or minimized group differences, as previous studies have reported mixed effects about the influence of each (Helt et al., Reference Helt, Eigsti, Snyder and Fein2010; Senju et al., Reference Senju, Kikuchi, Akechi, Hasegawa, Tojo and Osanai2009). Second, having participants watch video clips of targets does not constitute a true social interaction; additional research might avoid this problem by using a more interactive design, perhaps involving trained confederates displaying differing facial expressions rather than videotaped targets. Third and finally, participants with ASD will likely always experience more arousal and anxiety during experimental testing due to change in routine, interacting with experimenters, and so on, which may affect their results.
In our future work, we hope to explore emotional contagion competency in newly diagnosed toddlers, and track whether quality or frequency of early emotional contagion appears to be a turnkey ability that allows the development of other, later developing, social competencies (e.g., theory of mind). Improvement in emotional contagion, particularly with strangers as stimuli, might serve as a marker of effective treatment. In addition, detailed phenotyping data may elucidate whether emotional contagion is altered with strangers in all individuals with ASD or only in a subgroup. Finally, future research in this area may benefit from the incorporation of eye tracking, EMG, and skin conductance to measure eye gaze, mimicry, and arousal level with more precision than was possible in the current study.
Conclusions
In summary, these results reveal an intact island of empathetic competence among individuals with ASD when interacting with very familiar others. This is the first study to show that emotional contagion in the same group of individuals with ASD may be either reduced or intact, depending upon the context, and that it is the amount of emotional contagion observed under naturalistic circumstances that is most strongly related to ASD severity. It seems that prompting eye gaze to stimuli greatly diminishes, but does not obliterate, group differences in emotional contagion in ASD individuals, and that small remaining differences may be driven by group differences in social reward valuation (which are magnified in children with greater ASD severity). It may be that enhancing eye contact in the moment cannot erase the effects of a lifetime of avoiding eye contact, and that even when social attention is engaged, small differences may remain in the tendency of those with ASD to have efficient automatic emotional contagion (or “embodied communication”) with unfamiliar others. The continued study of empathic processes is of central importance to understanding ASD.
Funding Statement
This research was partially supported by a predoctoral mentor-based grant awarded to Deborah A. Fein and Molly S. Helt by Autism Speaks.
Conflict of Interest
The authors declare that they have no competing or potential conflicts of interests.







