INTRODUCTION
The myxozoan parasite Tetracapsuloides bryosalmonae causes the economically important proliferative kidney disease (PKD). This disease primarily affects salmonid species, and can cause high levels of mortality on affected trout and salmon farms (Hedrick et al. Reference Hedrick, MacConnell and de Kinkelin1993). While PKD is endemic across much of Western Europe and North America it has recently been associated with losses of wild salmonid populations in these areas suggesting that the disease has a substantial ecological impact (Wahli et al. Reference Wahli, Bernet, Segner, Pugovkin, Burkhardt-Holm, Escher and Schmidt-Posthaus2002; Foott et al. Reference Foott, Stone and Nichols2007; Sterud et al. Reference Sterud, Forseth, Ugedal, Poppe, Jorgensen, Bruheim, Fjeldstad and Mo2007).
PKD is characterized by anaemia and a pronounced inflammation of the posterior and anterior portions of the fish's kidney, together with the spleen. The disease is associated with the PKX stage of T. bryosalmonae development (Seagrave et al. Reference Seagrave, Bucke and Alderman1980; Lom and Dyková, Reference Lom and Dyková2006). This stage undergoes a rapid proliferation within the haematopoietic tissues and consists of a single primary cell containing 1 or more secondary cells at least one of which may contain a tertiary cell within it (Ferguson and Needham, Reference Ferguson and Needham1978). During the course of the infection, the PKX stages become the focus of the inflammatory response. With the discovery of spores within the kidney tubule lumena of infected rainbow trout Oncoryhnchus mykiss in North America, T. bryosalmonae was classified as a member of the Myxozoa. The nature of the PKX stage together with the morphology of the spores initially suggested a relationship with the genus Sphaerospora (Kent and Hedrick, Reference Kent and Hedrick1986, Reference Kent and Hedrick1987). However, as the spores did not possess hardened valves it was initially considered that they were immature and that salmonids were possibly an aberrant host (Kent and Hedrick, Reference Kent and Hedrick1985, Reference Kent and Hedrick1986, Reference Kent and Hedrick1987).
The discovery that T. bryosalmonae and members of the related genus Buddenbrockia, could infect freshwater bryozoans forming spores with cytoplasmic valve cells (Canning et al. Reference Canning, Okamura and Curry1996; Anderson et al. Reference Anderson, Canning and Okamura1999) led to the suggestion that the spores found in fish may be viable (Kent et al. Reference Kent, Khattra, Hervio and Devlin1998, Reference Kent, Khattra, Hedrick and Devlin2000). While exposure of fish to infected bryozoans resulted in PKD (Feist et al. Reference Feist, Longshaw, Canning and Okamura2001), initial attempts to transmit the parasite from fish to bryozoans failed to produce demonstratable infections, suggesting a paucity of viable spores (Morris et al. Reference Morris, Morris and Adams2002; Tops et al. Reference Tops, Baxa, McDowell, Hedrick and Okamura2004). Therefore it was considered that if salmonids were involved in the life cycle they acted as facultative hosts and were not important for the spread and maintenance of the parasite (Henderson and Okamura, Reference Henderson and Okamura2004; Tops et al. Reference Tops, Baxa, McDowell, Hedrick and Okamura2004).
Recently we have demonstrated that T. bryosalmonae can be transmitted to naive bryozoan colonies from infected brown trout Salmo trutta (Morris and Adams, Reference Morris and Adams2006). Little is documented on the development of T. bryosalmonae within the kidney of the fish host with previous studies primarily focussing on the disease pathology/host response and describing the morphology of the PKX stages that cause PKD (Ferguson and Needham, Reference Ferguson and Needham1978; Seagrave et al. Reference Seagrave, Bucke and Alderman1980; Clifton-Hadley et al. Reference Clifton-Hadley, Bucke and Richards1987; MacConnell et al. Reference MacConnell, Smith, Hedrick and Speer1989). While a few papers note the presence and overall morphology of spore and sporogonic stages found within the kidney tubules there are no details on how the spores are formed (Kent and Hedrick, Reference Kent and Hedrick1985, Reference Kent and Hedrick1986; Clifton-Hadley and Feist, Reference Clifton-Hadley and Feist1989; Kent et al. Reference Kent, Khattra, Hedrick and Devlin2000). Antigenic and ultrastructural examinations have suggested that they are derived from the secondary cells found within the PKX stages (Kent and Hedrick, Reference Kent and Hedrick1986; Castagnaro et al. Reference Castagnaro, Marin, Ghittino and Hedrick1991; Morris et al. Reference Morris, Adams and Richards1997). To date, the study of Kent and Hedrick (Reference Kent and Hedrick1986) remains the only attempt to chart the intra-renal development of the parasite through to sporogenesis. These authors acknowledged that much remained unknown and they could not identify any clear developmental sequence.
Here we examine the development T. bryosalmonae within the brown trout using a combination of methods. Through this study, we describe the intra-piscine sporogenesis of T. bryosalmonae, derive insights into the pathogenicity of this parasite and discuss how its development correlates to other myxozoans and the implications of this.
MATERIALS AND METHODS
Forty naïve, fingerling brown trout were obtained from eggs grown in the experimental aquarium facility at the University of Stirling. This facility is specific pathogen-free, being supplied by charcoal filtered mains water. The fish were transferred to 2 experimental flow-through aquaria (20 fish per tank), maintained at a constant temperature of 18°C. The effluent from a tank that contained laboratory grown colonies of the bryozoan Fredericella sultana was then continuously dripped into 1 of the aquaria for 3 days using a peristaltic pump. These F. sultana had been experimentally infected with T. bryosalmonae following a slight modification of the method of Morris and Adams (Reference Morris and Adams2006), which resulted in 100% of colonies becoming infected – the length of tubing connecting the tanks together was kept to an absolute minimum, <0·5 m compared to 4 m in the original experiment. At the time of the brown trout exposure, these infected bryozoans possessed numerous mature and developing spore sacs within them.
The brown trout were then maintained for 7 weeks before 6 were killed from each tank and the kidneys assessed macroscopically for clinical symptoms of PKD. In those kidneys where PKD was evident, the presence of T. bryosalmonae was confirmed by taking impression smears stained with Rapi-Diff (Raymond Arnold Labs). Three infected fish were selected and samples of the posterior kidney were taken for ultrastructural examination and confocal microscopy. In addition, portions of posterior kidney from all of the fish necropsied were collected for immunohistochemistry. After 20 weeks all of the remaining fish were killed and samples taken for immunohistochemistry and PCR. For PCR, DNA was prepared by a standard phenol/chloroform extraction method followed with temporary storage in 80% ethanol at −20°C. The PCR was conducted using the T. bryosalmonae-specific primers 5F, 6R with their recommended cycling conditions (Kent et al. Reference Kent, Khattra, Hervio and Devlin1998) and products examined under fluorescence on a 1·5% agarose gel post-stained with Gel-Red™ (Biotium Labs).
For transmission electron microscopy, a piece of kidney was cut into ~0·5–1 mm3 cubes, fixed in 2·5% glutaraldehyde, post-fixed in osmium tetroxide, and embedded in Spurr's resin. Semi-thin sections were obtained from the blocks and stained using toluidene blue to assess the prevalence of sporogonic stages and PKX. Serial gold sections were cut and floated onto formvar-coated copper grids. They were stained using lead citrate/uranyl actetate and examined on a Technai G2 Spirit electron microscope (FEI instruments) at 120 kV. Additional sections were taken at 5 μm intervals from blocks that possessed parasite stages of particular interest.
For confocal microscopy, tissue was placed into embedding medium (Cryo‘M’ Bed, Bright Laboratories, UK) and rapidly frozen using a cryospray (Bright Laboratories, UK). Cryosections were cut at 10 μm and 20 μm thickness onto silanated slides. They were fixed in cold acetone, desiccated and stored at −80°C before being immunostained. Prior to immunohistochemistry the sections were post-fixed in cold 4% paraformaldehyde for 20 min. The sections were blocked using 10% goat serum, incubated using T. bryosalmonae-reactive antibodies mAb B4 (Morris et al. Reference Morris, Adams and Richards1997) or mAb P01 (Aquatic Diagnostics Ltd, UK) overnight at 4°C, washed in PBS×3, incubated with FITC-labelled anti-mouse IgG (Sigma, UK) for 3 h, washed again and finally mounted using Vectorshield with DAPI as a nuclear stain (Vector Labs, Peterborough, UK). They were examined under a Leica TCSSP2 confocal microscope.
Samples taken for standard immunohistochemistry were fixed in 10% neutral buffered formalin, embedded in paraffin wax and cut at 5 μm onto glass slides. Immunohistochemistry was conducted on the fixed sections using anti-T. bryosalmonae antibodies; mAb B4 (reactive to secondary cells, Morris et al. Reference Morris, Adams and Richards1997) or mAb P01 (reactive to PKX primary cells, Aquatic Diagnostics Ltd). The manufacturer's protocol for mAb P01 was followed for both mAbs but with mAb B4 the antibody was applied as neat hybridoma supernatant rather than reconstituted. Appropriate controls of non-infected brown trout kidney were included in the above experiments to allow comparison of infected and naive kidney tissue.
RESULTS
Of the 6 fish sampled at 7 weeks for ultrastructural and confocal studies, all were infected with both PKX stages and intratubular pseudoplasmodial stages. Macroscopically the kidneys were slightly swollen along their length, corresponding to a grade 1 kidney using the scale of Clifton-Hadley et al. (Reference Clifton-Hadley, Bucke and Richards1987) devised for rainbow trout. All sections examined possessed a mixture of stages reiterating the observations of previous studies (e.g. Kent and Hedrick, Reference Kent and Hedrick1986) that T. bryosalmonae development is asynchronous. Where possible, the terminology used to describe the parasite will adhere to that suggested by Lom and Dyková (Reference Lom and Dyková2006) for myxozoan life cycles.
Ultrastructural observations
Interstitial pre-sporogonic development
Typical PKX stages of T. bryosalmonae as detailed previously were noted throughout the kidney interstitium (Ferguson and Needham, Reference Ferguson and Needham1978; Seagrave et al. Reference Seagrave, Bucke and Alderman1980; Kent and Hedrick, Reference Kent and Hedrick1985, Reference Kent and Hedrick1986). These cells were usually engulfed by at least 1 host phagocyte that was in close contact with the parasite's plasma membrane; the surface of which displayed evidence of endocytosis of the surrounding host material together with short projections that appeared to directly interact with the engulfing cells (Fig. 1A). While for rainbow trout similar projections have been reported as interdigitating with the surrounding phagocyte (Morris et al. Reference Morris, Adams and Richards2000a), only isolated projections were noted in the brown trout. Occasionally within the PKX stage's primary cell, regions of cytoplasm containing sporoplasmosomes and numerous vacuoles, some of which filled with membranous material appeared to be contained within the nucleus (Fig. 1B). Similar pseudoinclusions have only previously been noted under light microscopy within rainbow trout in North America (Kent and Hedrick, Reference Kent and Hedrick1986).

Fig. 1. (A) Surface of Tetracapsuloides bryosalmonae cell doublet. Large arrow indicates endocytosis of surrounding host cell. Small arrows indicate cytoplasmic extensions from parasite pushing into the surrounding cell. (B) Primary cell nucleus of cell doublet demonstrating large pseudoinclusion (I). (C) Cell doublet. Secondary cell (S) appears directly attached to the surrounding primary cell at one point (arrow). (D) Mitosis of secondary cell. Nuclear membrane is not present metaphasic alignment of chromosomes is clearly visible. (E) Cytokinesis of primary cell. Two secondary cells (S), one per each forming cell doublet. Boxed area represents image (F), which is a higher magnification highlighting the central spindle of microtubular elements (*) and ingression of the plasmalemma associated with dense body (arrow).
The most common interstitial PKX stage observed in section was a cell doublet. This consisted of a typical PKX primary cell, with its characteristic sporoplasmosomes, containing a single secondary cell within it. The nucleus of the primary cell was often positioned adjacent to the secondary cell that was separated from the host cell by a fluid-filled vacuole containing membranous elements. In some sections one end of the secondary cell was attached to the primary cell indicating that the vacuole is not continuous around the whole cell (Fig. 1C). Short cytoplasmic extensions, delimited by the enclosing vacuolar membrane were noted emanating from the secondary cell into the primary cell's cytoplasm.
In some PKX cell doublets division of the secondary cell was noted. This occurred through an open mitosis with dissolution of the nuclear membrane and, in keeping with other myxozoan spp., centrioles were absent. Metaphase, with the chromosomes aligned on a central plate, was the only recognizable phase of this division noted (Fig. 1D). While nuclear division of the primary cell was not observed, cytokinesis was, with each resultant primary cell containing a single secondary cell (Fig. 1E). Both of the dividing cells retained contact with the engulfing phagocytes, suggesting that they remain attached to these cells subsequent to division. Although a central spindle composed of microtubules and dense cup-like structures could clearly be ascertained during cytokinesis a contractile ring was not noted. However, off centre to the central spindle, ingression of the plasma membrane was noted associated with an extremely electron-dense structure (Fig. 1F).
The maximum number of secondary cells ever observed within an interstitial PKX stage was 3 (Fig. 2A). Engulfment of one secondary cell by another transformed the PKX stage from a cell doublet into a distinct interstitial developmental stage, referred to here as a PKX pseudoplasmodium. The secondary cell now with a tertiary cell within it (referred here as an S-T doublet) and the remaining single secondary cell represented the cells of the sporoblast. The tertiary cell of the S-T doublet was contained within a tightly fitting vacuole and, in accordance with the primary and secondary cells, short protrusions were noted as emanating from the surface of this cell into the surrounding cytoplasm. However, in some parasites these protrusions appeared to form extensive masses within the secondary cell, close to the tertiary cell (Fig. 2B). These structures contained longitudinally oriented microtubules and were delimited from the enclosing secondary cell by a double membrane. In other S-T doublets large fluid-filled vacuoles existed (Fig. 2C). Both of these structures also occur in PKX pseudoplasmodia from rainbow trout and Arctic charr Salvelinus alpinus (D. J. Morris, unpublished observations), and therefore are a relatively common phenomenon. While they could represent evidence of necrosis we cannot preclude a developmental role.

Fig. 2. (A) PKX pseudoplasmodium. This stage signifies the transition from replicating cell doublets to sporogony. It is composed of a primary cell containing 3 secondary cells (S) within it. (B) PKX pseudoplasmodium demonstrating a primary cell, and S-T doublet. Within the secondary cell of the doublet is a tubular mass (arrow) considered to be derived from the enclosed tertiary cell (T). (C) PKX pseudoplasmodium with a S-T doublet, the secondary cell of which contains a large fluid filled vacuole. (D) PKX pseudoplasmodium against the basal lamina of a kidney tubule (Tu). The parasite is pressed against the lamina, apparently displacing the surrounding phagocyte (arrow). (E) PKX pseudoplasmodium migrating through tubule epithelium. Associated phagocyte (Ph) appears to be in the process of being removed from the parasite. (F) Amoeboid motion of PKX pseudoplasmodium with a clear ectoplasmic extension within the tubule epithelium.
As previously noted by Feist and Bucke (Reference Feist and Bucke1987), many tertiary cells contained prominent bundles of microtubules, a feature not present in the secondary or primary cell.
Migration to tubule lumena
PKX pseudoplasmodia observed in direct contact with the basal lamina of tubules were still associated with host phagocytes (Fig. 2D). There appeared to be an active interaction between the parasite and the basal lamina, with numerous sporoplasmosomes occurring within this area. PKX pseudoplasmodia noted between the tubule epithelial cells were either disassociating from host phagocytes (Fig. 2E), or without these cells. Evidence of amoeboid movement between the tubule cells was noted with distinct ectoplasmic regions evident in some primary cells (Fig. 2F), the parasites finally passing into the tubule lumen (Fig. 3A).

Fig. 3. (A) PKX pseudoplasmodium containing one secondary cell (S) and a S-T doublet. Secondary and tertiary cell of doublet indicated with S and T respectively. Lumen of tubule indicated with Lu. (B) PKX pseudoplasmodium within lumen of tubule. Cytoplasm has become notably electron dense and vacuolated with sporoplasmosomes no longer aligned to cell surface. (C) Pseudoplasmodium within tubule with developing pseudopodia. Contents of vacuoles (Vac) appear to be released into the lumen of tubule (arrow). (D) Pseudoplasmodium connected to epithelium via pseudopodia. This section of parasite has 2 cells within it (S). Note various membranous material within tubule lumen (Lu). (E) Interaction of parasite (P) with micovilli lining tubule demonstrating dilation of these structures. Arrows indicate insertion points of the pseudopodia towards host cell junctions.
Occasional PKX pseudoplasmodia were noted within the proximal tubule lumens. While some of these parasites appeared free of the host, resembling stages observed in the tubule epithelium, others were surrounded by material, presumably composed of dilated host microvilli (see below). The primary cell cytoplasm of these latter parasites was relatively electron dense and possessed many small and large vacoular structures together with sporoplasmosomes within it (Fig. 3B). While the smaller vacuoles were electron lucent the large ones contained granular material and tubular structures. The sporoplasmosomes were notably scattered throughout the cytoplasm and were not obviously associated with the plasma membrane, in contrast to these organelles within the interstitial PKX stages. In addition to the proximal tubule, a single PKX pseudoplasmodium was noted free within a collecting duct. The primary cell cytoplasm of this parasite appeared to be in a state of advanced degeneration, with a notable reduction in matrix density.
The subsequent intra-tubular developmental stages noted during the study were morphologically distinct from the interstitial PKX pseudoplasmodia with a primary cell that did not possess sporoplasmosomes. To distinguish from the preceding PKX stages, they will be referred to simply as pseudoplasmodia.
Interaction of pseudoplasmodia and tubule
The simplest pseudoplasmodium noted comprised of a primary cell containing 1 secondary cell and an S-T doublet both of which were contained within delimiting vacoules. The primary cell cytoplasm possessed numerous lysosomal vacuoles, many containing distinctive dense spicules that appeared to be released into the surrounding environment (Fig. 3C). It was also notable that the tubule lumen contained large amounts of membranes and other material within it (Fig. 3D). The pseudoplasmodia were anchored to the junctions between the tubule cells by fine pseudopodia emanating from the primary cell. These pseudopodia contained prominent bundles of microfilaments within them (Fig. 3E).
Associated with the development of more advanced pseudoplasmodia was the dilatation of the tubular epithelial apices, with a resulting loss of microvilli (Fig. 3E). In most of the parasitized tubules examined, this reaction resulted in the pseudoplasmodia and its anchoring pseudopodia becoming effectively sandwiched within the lumen. Rodlet cells were present within tubular epithelia although an associated disintegration of parasites was not observed. Host tubule cilia appeared to remain intact, passing directly through the dilated epithelium, and surprisingly, some sections suggested that they could also pass through the primary cells as well. Interaction of the pseudoplasmodia with the surrounding host cells was noted with the primary cell endocytosing material directly from the swollen epithelium and the contents of the tubule lumen. Passage of material from the primary cell to the enclosed cells was also by endocytosis (Fig. 4A).

Fig. 4. (A) Endocytosis of material into primary cell of pseudoplasmodium and into contained secondary cell (arrows). (B) Two developing pseudoplasmodia within tubule lumen (indicated by P1 and P2 respectively). Within P1 the secondary cell has divided once resulting in 2 valvogenic cells (V) while the S-T doublet remains (S/T). (C) Synchronized mitosis of valvogenic cells resulting in 4 cells (V). (D) Magnified area of (C) represented by box, indicating convergence of mitotic spindle microtubules but with no associated centriole (arrows). (E) Formation of spore. Two capsulogenic cells (C), one with a developing polar capsule in section, are surrounded by 4 valvogenic cells (V). Primary cell (P) surrounds cells of sporoblast. (F) Mature polar capsule of T. bryosalmonae spore. Spore is still contained within a sporophorous vacuole within the primary cell (P). Arrow indicates junction between capsulogenic cell and valve cell. (G) Oblique section through polar capsule demonstrating platted appearance of polar filament.
Sporogony
The single secondary cell divides twice by an open mitosis to form 4 valvogenic cells (Fig. 4B and C). During this process, stages of both metaphase and anaphase were noted with an observable microtubular spindle converging at multiple sites within the cytoplasm; although the precise anchorage point could not reliably be determined (Fig. 4D). During the formation of the valvogenic cells, the S-T doublet transforms into 2 capsulogenic cells and a sporoplasmogenic cell resulting in the sporoblast comprising of 4 valvogenic cells, 2 capsulogenic cells and 1 sporoplasmogenic cell, all contained within a common vacuole inside the primary cell. Unfortunately, despite the large number of sections examined, the developmental sequence transforming the S-T doublet into the sporoplasmogenic and capsulogenic cells could not be reliably determined.
Polar capsule formation within the two capsulogenic cells appeared to be typical of that associated with other Myxosporea in that an initial primordia composed of dense body-containing amorphous material, developed an external tube surrounded by microtubules which was finally drawn into the body to form an inverted tube. During this process the valvogenic cells reorganized themselves around the sporoplasmogenic and capsulogenic cells (Fig. 4E). When in position, the cell junctions between the valvogenic and capsulogenic cells developed.
Spore maturation
While mature and maturing fish malacospores were noted inside pseudoplasmodial primary cells, they were never observed free in either distal tubules or collecting ducts. Within the pseudoplasmodia, the vacuole surrounding the mature fish malacospores was enlarged and the primary cell cytoplasm appeared reduced giving them an extracted appearance. The fish malacospores had 2 spherical polar capsules within 2 capsule cells, 4 valve cells and a single sporoplasm. The polar capsules were 1·4 nm in diameter, each with 6 turns of the polar filament (Fig. 4F). In many of the capsules the filament was twisted to appear ‘S’ shaped in section; however, in other sections it appeared twisted upon itself presenting a platted appearance (Fig. 4G) in accordance to that observed for the T. bryosalmonae malacospore (Morris and Adams, Reference Morris and Adams2007). The outer surface of the filament connected to the surface of the polar capsule while the contents appeared to be continuous with an amorphous stopper structure. This stopper had a burred structure on the surface of the capsule that appears to be typical of Malacosporea reported to date (Feist and Longshaw, Reference Feist, Longshaw and Woo2006). The internal structure of the polar capsule was not homogenous, with granular regions, electron-dense regions and curious crystalline lattices presumably composed of protein. The polar capsule was delimited from the capsulogenic cell by a granular material and a single membrane. This membrane appeared to extend to cover the burred stopper and protruded from the plasma membrane of the capsulogenic cell. The valve cells were stretched around the sporoplasm, eventually joining each other and the capsulogenic cells together through the formation of sinuous junctions composed of an outer dense adherens junction and an inner gap junction. However, unlike T. bryosalmonae malacospores the valve cells did not overlap the capsulogenic cells but were abutted against them. The cytoplasm of these cells contained a small rounded nucleus, mitochondria and numerous clear vacuoles. The sporoplasm, appeared amoeboid, and extended a distance between the capsulogenic cells. It did not contain a secondary cell but possessed sporoplasmosomes of ~200 nm in diameter within it. These did not align to the membrane and some appeared to contain an electron-lucent bar at one end, reminiscent of similar organelles seen in the presaccular, T. bryosalmonae stages described in F. sultana (Morris and Adams, Reference Morris and Adams2006). However, the density of the majority of these bodies was high, making definitive comparisons difficult. In contrast to the valve and capsulogenic cells the nucleus of the sporoplasm had prominent chromatin and a nucleolus.
Immunohistochemistry
The staining of mAbs P01 (alt. mAb C5) and B4 for the interstitial parasites resembled that already noted for these stages in rainbow trout (Morris et al. Reference Morris, Adams and Richards1997). For the intratubular stages immunohistochemical studies using mAb P01 clearly demonstrated that the early pseudoplasmodial stages attached to the tubular epithelium stained with this mAb (Fig. 5). The staining was primarily localized around the edges of the parasite, including the cytoplasmic extensions that anchored the parasite to the tubule wall. Cryosections, stained with mAb B4 demonstrated that ~50% of the tubules within the posterior kidney were infected with T. bryosalmonae pseudoplasmodia (Fig. 6). The staining of this mAb on these sections also demonstrated a release of the B4 antigen from the pseudoplasmodia which permeated the tubule epithelial cells but did not extend beyond the basal lamina. While some tubules were full of the parasites others had a more localized infection with pseudoplasmodia being present within a restricted area (Figs. 7 and 8). Staining using DAPI demonstrated large numbers of parasite nuclei within the tubules, suggesting that a substantial number of pseudoplasmodia were within the infected tubules. The parasite nuclei were relatively small (3 μm), compared to the host nuclei and could be easily differentiated.

Fig. 5. Immunohistochemistry of Tetracapsula bryosalmonae within kidney tubules stained with mAB P01, considered specific for the primary cell of PKX stage.

Fig. 6. Confocal microscopy of cryosection, immunostained with mAb B4 and DAPI. Around half of the tubules contain masses of parasites.

Fig. 7. Confocal microscopy of cryosection immunostained with mAb B4 demonstrating release of this antigen into the surrounding tubular epithelial cells.

Fig. 8. Same section as imaged in Fig. 7 but with DAPI filter to highlight clusters of stages within tubule. Parasite nuclei indicated with arrow.
Samples taken at 20 weeks for immunohistochemistry demonstrated that while the fish had recovered from the clinical disease pseudoplasmodia remained within the tubule lumens. The PCR generated products of the expected size from all of the samples taken from the exposed tank, while samples from the naïve tank remained negative, further confirming successful T. bryosalmonae transmission.
DISCUSSION
The results of this study have elucidated many of the facets of the intra-renal development of T. bryosalmonae and, in conjunction with previous reports (Canning et al. Reference Canning, Curry, Feist, Longshaw and Okamura2000; Feist et al. Reference Feist, Longshaw, Canning and Okamura2001; Morris and Adams, Reference Morris and Adams2006, Reference Morris and Adams2007), represents the first species of the myxozoan class Malacosporea for which the majority of the developmental life cycle has been charted.
The pathogenicity of T. bryosalmonae within the kidney interstitium of salmonids is well documented (Kent and Hedrick, Reference Kent and Hedrick1986; Clifton-Hadley et al. Reference Clifton-Hadley, Bucke and Richards1987). Recently, it was suggested that there might be 2 strains of T. bryosalmonae, adapted for Atlantic and Pacific salmonids respectively (Morris and Adams, Reference Morris and Adams2006; Grabner and El-Matbouli, Reference Grabner and El-Matbouli2008). Evidence for this includes the lack of spores reported in European rainbow trout, together with aberrant primary cell degeneration and release of secondary cells directly into the host's kidney interstitium (Bucke et al. Reference Bucke, Feist and Clifton-Hadley1991; Morris et al. Reference Morris, Adams and Richards1997). T. bryosalmonae has been reported to form spores within Arctic Charr Salvelinus alpinus in North America, while in the UK sporulation has again not been observed within this species (authors' unpublished observation). In addition, North American descriptions of T. bryosalmonae have included interstitial PKX stages containing 4 or more secondary cells which have not been reported in Europe (Kent and Hedrick, Reference Kent and Hedrick1986; Clifton-Hadley et al. Reference Clifton-Hadley, Bucke and Richards1987; MacConnell et al. Reference MacConnell, Smith, Hedrick and Speer1989; Morris et al. Reference Morris, Adams and Richards1997). Although these stages may represent premature sporulation within the interstitium, the description of malacospores possessing polar capsules with 4 turns of the polar filament is in marked contrast to the 6 turns noted in the UK brown trout (Kent et al. Reference Kent, Khattra, Hedrick and Devlin2000; Hedrick et al. Reference Hedrick, Baxa, de Kinkelin and Okamura2004). Again, this difference in turn number may be artefactual as the polar capsules examined in the North American salmonids appeared relatively poorly fixed (Kent and Hedrick, Reference Kent and Hedrick1985, Reference Kent and Hedrick1986; Kent et al. Reference Kent, Khattra, Hedrick and Devlin2000). While there are striking host differences in which T. bryosalmonae can sporulate, significant geographical separation and the genetic differences reported between the parasites from the two continents (Kent et al. Reference Kent, Khattra, Hervio and Devlin1998; Henderson and Okamura, Reference Henderson and Okamura2004) suggest that further studies to characterize the North American strain of T. bryosalmonae are warranted before the precise relationship of this parasite to the European T. bryosalmonae can be determined.
Before the discovery of parasites of the class Malacosporea, studies on T. bryosalmonae assumed that spore production was an incomplete event with hardened spore valves not being formed (Kent and Hedrick, Reference Kent and Hedrick1986; Feist and Bucke, Reference Feist and Bucke1993). This correlated with observations that the spores and developing pseudoplasmodia were rarely observed in tissue sections of brown trout (Clifton-Hadley and Feist, Reference Clifton-Hadley and Feist1989). From the developmental studies presented here it is clear that each PKX stage observed has the potential to form a spore. The use of specific stains in thick sections as opposed to 5 μm sections demonstrate that far from being unusual, a proportion of brown trout renal tubules can harbour large numbers of pseudoplasmodia, the parasites being highly clustered within the tubules. This indicates that the thinner sections employed in previous studies introduced a reporting bias, underestimating true spore numbers. It would be of interest to examine thick sections of other susceptible fish species to validate the reported low levels of T. bryosalmonae sporogony. The localization of mAb B4 in the cryosections correlates with previous studies in that the B4 antigen is released from myxozoan spp. that are entering a sporogonial phase (Morris et al. Reference Morris, Adams and Richards1997, Reference Morris, Molnar, Longshaw and Adams2006). For T. bryosalmonae the antigen release was focused within the kidney tubule to diffuse through the tubular epithelium. While the function of the B4 antigen is currently unknown this localization does suggest the possibility that the clustering of pseudoplasmodia is through release of substances from established parasites to aid subsequent PKX pseudoplasmodial migration through chemotaxis.
The anchoring of the T. bryosalmonae pseudoplasmodia to the gap junctions between tubular epithelium through pseudopodia is often reported for coelozoic myxozoan species (Canning and Okamura, Reference Canning and Okamura2004). In addition, although swelling of the tubule microvilli represents an unusual pathological response to a myxozoan infection it is not unprecedented e.g. Sphaerospora sp. within bluegill Lepornis macrohirus elicits similar reactions, including the association of rodlet cells (Leino, Reference Leino1996). Examination of published photomicrographs of T. bryosalmonae suggests that similar epithelial responses occur in rainbow trout and Arctic Charr (Kent and Hedrick, Reference Kent and Hedrick1985, Reference Kent and Hedrick1986; Kent et al. Reference Kent, Khattra, Hedrick and Devlin2000).
Previous studies have indicated that the malacospore sporoplasms enter the fish through mucus cells in the skin and possibly the gill (Morris et al. Reference Morris, Adams and Richards2000b; Longshaw et al. Reference Longshaw, le Deuff, Harris and Feist2002). Within the salmonid renal tissue and blood sinuses certain PKX stages have been reported that closely resemble these infective sporoplasms, in that they are cell doublets and possess a fibrous layer immediately beneath the plasma membrane of the primary cell instead of the aligned sporoplasmosomes usually reported (Morris and Adams, Reference Morris and Adams2004, Reference Morris and Adams2007). As migration to the renal tissues from the site of invasion is relatively rapid (Morris et al. Reference Morris, Adams and Richards2000b; Longshaw et al. Reference Longshaw, le Deuff, Harris and Feist2002) the presence of these stages leads to the conclusion that there are no further significant morphologically distinct developmental stages prior to renal infiltration. This is in contrast to the Myxosporea, where the multi-cellular sporoplasm of Myxobolus cerebralis released from a triactinospore undergoes an intracellular developmental stage to result in the formation of cell doublets (El-Matbouli et al. Reference El-Matbouli, Hoffmann and Madnok1995).
The main replicating phase of intra-piscine T. bryosalmonae is the PKX stage cell doublet contained within the haematopoietic tissues. Division of the doublet is through an open mitosis of the secondary cell followed by division of the primary cell, presumably also an open mitosis. The retention of the phagocyte during this process suggests that division of these host cells also occurs, resulting in the parasite always being contained within a host cell. A similar mechanism of host-cell division correlating to the intra-cellular proliferation of cell doublets has also been proposed for Hoferellus carassii (Yokoyama et al. Reference Yokoyama, Ogawa and Wakabayashi1990) suggesting that this process is not unique within the Myxozoa.
While cell doublets have been noted as occurring in the intra-vertebrate development of a number of myxozoans, T. bryosalmonae represents the first species for which their replication has been unequivocally observed. This has important implications for our understanding of other myxozoan life cycles as an alternative theory for myxozoan replication has been proposed. A number of pioneering studies identified unusual blood parasites as being pre-sporogonic stages of Sphaerospora renicola (Lom et al. Reference Lom, Dyková and Pavlásková1983a; Csaba et al. Reference Csaba, Kovács-Gayer, Békési, Bucsek and Molnár1984; Molnár, Reference Molnár1988). These stages existed in a variety of forms from simple cell doublets to larger cells containing many secondary cells, some with tertiary cells. Lom et al. (Reference Lom, Dyková and Pavlásková1983a) speculated that replication of these stages was through the endogenous production of secondary cells within a primary cell: the secondary cells producing tertiary cells within them, finally with the primary cell breaking down to release a mass of cell doublets into the fish to continue the cycle. This mode of replication was subsequently suggested for other members of the genus Sphaerospora and for T. bryosalmonae (Lom et al. Reference Lom, Pavlásková and Dyková1985; Kent and Hedrick, Reference Kent and Hedrick1986). As these stages occurred in tissues (usually blood) that were remote from the final site of sporogenesis this proliferative development was termed ‘extrasporogonic’ (Lom and Dyková, Reference Lom and Dyková1986). El-Matbouli et al. (Reference El-Matbouli, Hoffmann and Madnok1995) examined M. cerebralis and demonstrated that the main proliferative phase of this parasite also appeared to be the cell doublet. However, as cell doublet division was not observed, replication was speculated to be through the formation of tertiary cells in a manner similar to that suggested for the Sphaerospora. Due to lack of evidence for cell doublet division, this hypothesis has generally been accepted as representing a primary mechanism by which myxozoans undergo pre-sporogonic proliferation (Lom and Dyková, Reference Lom and Dyková1992; Feist and Longshaw, Reference Feist, Longshaw and Woo2006). A few reports have also noted possible plasmotomy of large plasmodia in fresh mounts but this needs to be confirmed (Mitchell, Reference Mitchell1978; Diamant et al. Reference Diamant, Whipps and Kent2004). For T. bryosalmonae, release of secondary cells into the surrounding tissues has previously been noted from disintegrating primary cells. However, these secondary cells do not form viable parasites, the release being a reflection of T. bryosalmonae, undergoing an abnormal development (Morris et al. Reference Morris, Adams and Richards1997, Reference Morris, Molnar, Longshaw and Adams2006). This suggests that disintegration of a primary cell to release its cellular contents, as for example observed for Sph. coregoni (El-Matbouli and Hoffmann, Reference El-Matbouli and Hoffmann1996), does not necessarily provide reliable evidence of proliferation.
When considering whether cell doublet replication could be widespread throughout the Myxozoa, it is important to note that division of the T. bryosalmonae primary cell was noted only once in the present study and has never been observed before, despite the numerous published studies relating to the ultrastructure of the PKX stage (Ferguson and Needham, Reference Ferguson and Needham1978; Seagrave et al. Reference Seagrave, Bucke and Alderman1980; Smith et al. Reference Smith, Morrison, Ramsey and Ferguson1984; Kent and Hedrick, Reference Kent and Hedrick1985, Reference Kent and Hedrick1986; Feist and Bucke, Reference Feist and Bucke1987; MacConnell et al. Reference MacConnell, Smith, Hedrick and Speer1989; Castaganaro et al. Reference Castagnaro, Marin, Ghittino and Hedrick1991; Morris et al. Reference Morris, Adams and Richards1997, Reference Morris, Adams and Richards2000a; Kent et al. Reference Kent, Khattra, Hedrick and Devlin2000; Morris and Adams, Reference Morris and Adams2004). As the PKX stage cell doublet undergoes a massive replication, this suggests that recognizable division of a myxozoan cell doublet can be a very transient event, which is extremely difficult to convincingly capture in section and explains why it has never been observed during studies on other species.
Usually in myxozoan species that produce polysporic plasmodia, the envelopment of 1 secondary (generative) cell by another within a primary cell, to form a sporogonic cell doublet is recognized as the initiation of sporogony (Current and Janovy, Reference Current and Janovy1977), and draws correlations to the formation of the tertiary cell during the development of T. bryosalmonae. Baska and Molnar (Reference Baska and Molnár1988) recognized that secondary cells with 2 tertiary cells present within extrasporogonic myxosporean stages were morphologically identical to the earliest stages of Sphaerospora sporogony noted in the kidney tubules and inferred that these ‘triple forms’ eventually form spores. Therefore, if the ‘extrasporogonic’ hypothesis is correct it would suggest that the tertiary cell within myxozoan development has significantly different roles being involved in both pre-sporogonic replication and sporogony. Examination of the development of Sphaeorspora truttae, using a range of methods has provided evidence that, like T. bryosalmonae, the tertiary cell is only involved in sporogony. A combination of in situ hybridization together with light and ultrastructural time-course studies have indicated that early replication of this parasite involves cell doublets (McGeorge et al. Reference McGeorge, Sommerville and Wootten1994; Holzer et al. Reference Holzer, Sommerville and Wootten2003). As the infection develops, these doublets become typical extrasporogonic parasites filling with large numbers of secondary cells. Tertiary cell formation is initiated by secondary cell engulfment and is noted only in the largest parasites, the most mature secondary cells of which contain 2 tertiary cells (triple forms) (McGeorge et al. Reference McGeorge, Sommerville and Wootten1994). These large parasites enter the kidney interstitium, migrate to the tubules, and sporulation is initiated within the tubule (Holzer et al. Reference Holzer, Sommerville and Wootten2003). Crucially, the parasites containing tertiary cells have only been noted towards the end of the replicative phase, correlating with their appearance during T. bryosalmonae development. This means that Sph. truttae cell doublets do not derive from the enlarged extrasporonic stages. In addition, McGeorge et al. (Reference McGeorge, Sommerville and Wootten1994) re-affirmed the morphological similarities between the initial sporogonic stages within the kidney tubule lumen to the triple forms, and noted that the surrounding primary cell of the extrasporogonic parasite was absent, suggesting that they are released from this stage.
The hypothesis that division of cell doublets is the primary mode for the replication of freshwater myxozoans within the fish host, while the formation of tertiary cells is reserved only to herald the onset of sporogony is attractive as it allows for some unification and simplification of myxozoan intra-vertebrate developmental cycles, which to date have appeared rather complex and diverse.
The classification of the Myxozoa through spore morphology has long been recognized as artificial and is not a reliable indicator of phylogeny (Lom et al. Reference Lom, Dyková and Lhotáková1982; Xiao and Desser, Reference Xiao and Desser2000). Lom et al. (Reference Lom, Dyková and Lhotáková1982) suggested that a highly desirable criterion for high-level myxozoan phylogeny would be the origin of the pansporoblast as this appeared to differ significantly between species. Recently, Fiala (Reference Fiala2006) constructed a series of phylogenies based on the available sequences of the 18ssu rDNA gene for the Myxozoa and reiterated previous studies highlighting the importance of tissue specificity, host and geography when examining the phylogenetic relationships of the myxozoan species. The phylogeny he constructed split the Myxozoa into several linages listed in terms of relatedness as; Malacosporea, Sphaerosporid, marine, and freshwater clades. While the latter 2 clades were further subdivided these groupings also contained a few representatives that did not adhere to the clade definition. In examining parasite development between the linages, 5 distinct developmental pathways can be observed and therefore, we will reclassify the clades as I (Malacosporea) to V (freshwater) respectively, thus emphasizing the evolutionary distances between the groups. Using this as a foundation, together with our knowledge of intra-renal T. bryosalmonae development, published reports of other myxozoans, a revised understanding of myxozoan proliferation and the role of the tertiary cell it should be possible to explore the relationships between the Myxozoa through their respective sporogonic sequences. In attempting this endeavour, it is important to bear in mind that all of the descriptions of myxozoan sporogony published to date are incomplete to varying degrees. Most descriptions only note spore form, tissue located, host and geography while ultrastructural data, vital for developmental studies are generally lacking. Even where seemingly comprehensive ultrastructural studies have been conducted important events are often not observed. This includes the present study, where although 48 grids were sequentially examined, each with a minimum of 2 sections per grid conclusive separation of the tertiary and secondary cell during sporogenesis was not determined. It is also notable that the vast majority of these studies, including the genetic analyses, have not been conducted on experimentally infected pathogen-free fish but from tissues collected from fish inhabiting ponds, rivers and the sea where concurrent myxozoan infections are likely to exist. This is especially pertinent for genetic studies as translocation of Myxozoa around the host is regarded as a common phenomenon (Dyková and Lom, Reference Dyková and Lom1988; Eszterbauer and Székely, Reference Eszterbauer and Székely2004; Molnár, Reference Molnár2007) meaning that multiple species may be present within a sample. However, despite these constraints different sporogonic sequences and spore forms can be mapped to the clades of the phylogeny of Fiala (Reference Fiala2006) and these are shown diagrammatically in Fig. 9.
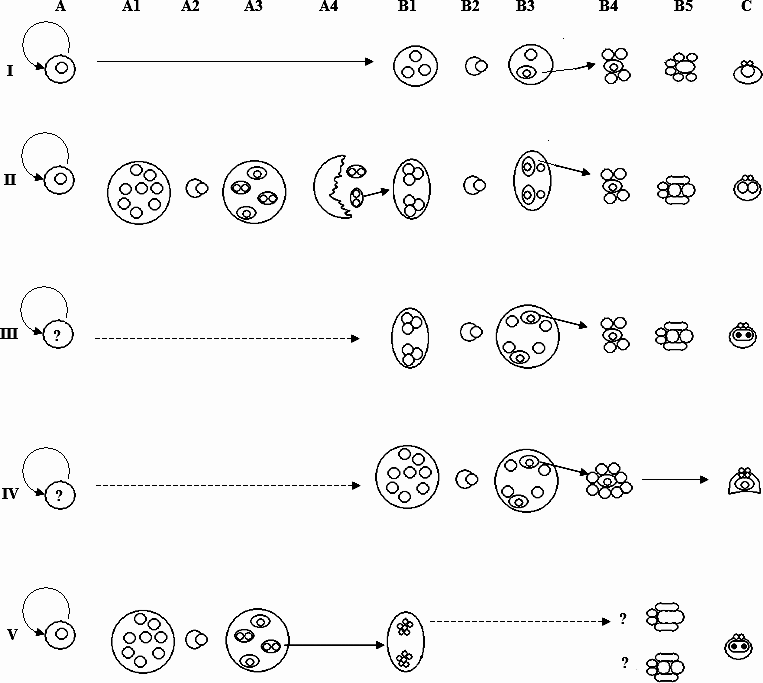
Fig. 9. Diagrammatic representation of suggested myxozoan developmental sequences discussed in text, highlighting differences and similarities between the 5 clades identified in Fiala (Reference Fiala2006). The limitations relating to the necessary generalizations conveyed in this figure are discussed and expanded upon in the text. Solid arrows represent known transitions, broken arrows indicate hypothetical development. I=Malacosporean clade; II=Sphaerosporid clade; III=Marine clade; IV=Kudoid clade; V=Freshwater clade. A. Pre-sporogonic replicative phase; for clades I, II and V this is identified as a cell doublet, while for clades III and IV this stage is currently unknown. Additional pre-sporogonic replicative phases may also occur (see El-Matbouli et al. Reference El-Matbouli, Hoffmann and Madnok1995). A1. Division of secondary cell within doublet to form extrasporogonic stage (clade II) or plasmodium (clade V). A2. Engulfment of one secondary cell by another to form (A3) triple form (clade II) or disporic pansporoblast (clade V). A4 rupture of extrasporogonic stage releasing triple forms at final site of sporogony. B1. Replication of internal cells to form cells of sporoblast(s). B2 engulfment of one internal cell by another. B3. Engulfment produces an S-T doublet (clades I, II, III) or a bi-cellular sporoplasmogenic cell (clade IV) with remaining associated secondary cell(s). A4. Secondary cells continue dividing to form constituents of spore. B5. Differentiation of sporoblasts, S-T doublet disassociates to form capsulogenic and sporoplasmogenic cells of spore (clades I, II, III); clade V either 5 or 6 cells are produced in sporoblast making the constituent cells of the spore. C. Spore maturation: clade I, monosporous, one sporoplasm per spore; clade II usually disporous, 2 sporoplasms per spore; clade III, usually disporous origin of single bi-nucleated sporoplasm through cell fusion; clade IV usually polysporous, single bi-cellular sporoplasm with multiple valve and capsulogenic cells; clade V usually polysporous with 2 spores per pansporoblast, each with single bi-nucleated sporoplasm formed either by cell fusion or endogenous nuclear division.
T. bryosalmonae is the only representative of clade I where the intra-piscine sporogony has been examined, the life cycles of other members of this clade remaining unknown. T. bryosalmonae sporogenesis is initiated in a primary cell by the engulfment of one secondary cell by another resulting in an S-T doublet (eventually forming a single sporoplasm and 2 capsulogenic cells) and a single secondary cell (which forms the surrounding valve cells). As during the latter stages of sporogony all of the cells are obviously delimited from the primary cell by a single membrane it is logical to assume that in the PKX pseudoplasmodium both the secondary cell and S-T doublet are contained within a common, tightly fitting, sporophorous vacuole.
Of clade II, as noted above, Sph. truttae is the only species that has been rigorously examined using a combination of molecular and histological methods. In contrast to clade I, the plasmodia of this clade derived from the replicating cell doublets are comprised of a primary cell containing many secondary cells, and again tertiary cells form by 1 secondary cell engulfing another. However, after engulfment the tertiary cell divides, resulting in triple forms, which escape from the plasmodium at the final site of sporogony (McGeorge et al. Reference McGeorge, Sommerville and Wootten1994). The 2 tertiary cells each contained within a single sporophorous vacuole, become sporoblasts, dividing and differentiating into the valvogenic, capsulogenic and sporoplasmogenic cells that form the mature spores (McGeorge et al. Reference McGeorge, Sommerville and Wootten1994; Holzer et al. Reference Holzer, Sommerville and Wootten2003). Unfortunately, the information regarding actual spore formation for Sph. truttae is incomplete. However, studies on other Sphaerosporids (Lom et al. Reference Lom, Körting and Dyková1983b, Reference Lom, Pavlásková and Dyková1985) suggest that a further cell engulfment occurs within the individual sporoblasts resulting in S-T doublets that are directly associated with sporogony. For some species of clade II, variants of this schema may exist, whereby the tertiary cell within the triple form either does not undergo an initial division resulting in 1 spore or it undergoes an additional division resulting in 4 spores (Hedrick et al. Reference Hedrick, McDowell and Groff1990; Jirků et al. Reference Jirků, Fiala and Modry2007). Sporogony for Sph. truttae is disporic even though the original plasmodial primary cell contains many secondary cells, while the mature spore is composed of hardened valves, 2 polar capsules and 2 sporoplasms.
The large marine clade highlighted by Fiala (Reference Fiala2006) can be split into 2 distinct groupings based on the role of the tertiary cell. Therefore, for the purposes of this discussion, clade III will represent those species of the Parvicapsula, Enteromyxum, Ceratomyxa and marine Myxidium clades, while clade IV will represent the Kudoa clade of Fiala (Reference Fiala2006). In representatives of clade III, during sporogony, the primary cell is retained, but usually contains numerous secondary cells. In a similar process to clade I, sporogony occurs through an association of secondary cells together with an S-T doublet, formed by one secondary cell engulfing another. The tertiary cell of this doublet forms the polar capsules of the spores as observed in Ceratomyxa sparusaurati (Sitjà-Bobadilla et al. Reference Sitjà-Bobadilla, Palenzeula and Alvarez-Pellitero1995). This role of the tertiary cell has previously been suggested for T. bryosalmonae (Feist and Bucke, Reference Feist and Bucke1987) and it is possible, that in addition to clade III, capsulogenesis is the eventual fate of the tertiary cell of the S-T doublets in clades I and II. The spores of clade III species all possess 2 polar capsules and a single binucleated sporoplasm. The origin of this sporoplasm is in some doubt but studies on Ceratomyxa shasta suggest it is through the fusion of 2 sporoplasmic cells (Yamamoto and Sanders, Reference Yamamoto and Saunders1979).
The majority of Clade IV is represented by species of the family Kudoidae (Whipps et al. Reference Whipps, Grossel, Adlard, Yokoyama, Bryant, Munday and Kent2004). Pre-sporogonic proliferative stages have yet to be conclusively determined; however, evidence for their existence does exist (Moran et al. Reference Moran, Margolis, Webster and Kent1999). Like members of clade III, the spores develop through an association of secondary cells enclosed within a common vacuole inside a primary cell. The primary cell being di- or polysporoblastic. However, one of the striking features of this clade is that the infective sporoplasm within the mature spore contains an internal cell (Stehr, Reference Stehr1986; Lom and Dyková, Reference Lom and Dyková1988). It has been suggested that pansporoblast formation, as observed in clade V occurs for Kudoa boopsi, as secondary cell-cell engulfment has been noted during sporogony (Kpatacha et al. Reference Kpatcha, Diebakate, Faye and Toguebaye1999). However, pansporoblast formation is contrary to all other published observations on Kudoid sporogony and the alternative interpretation presented here, is that the engulfment observed in K. boopsi represents the formation of the bi-cellular sporoplasm. In relation to the role of the tertiary cell in other clades, the maintenance of this cell within the mature spore's sporoplasm appears developmentally unique as no other clades produce myxospores that possess this feature. However, during T. bryosalmonae malacospore sporoplasmogenesis, the internal cell forms through engulfment (Morris and Adams, Reference Morris and Adams2007) and internal cells are a feature of actinospore sporoplasms (Lom and Dyková, Reference Lom and Dyková2006). Actinospore/malacospores have evolved to infect hosts through epidermal contact rather than through ingestion. The commonalities of multiple polar capsules/valve cells and multicellular sporoplasms of clade IV species with these spore types suggests that members of this clade may have also evolved to infect hosts through epidermal contact. This leads to two interesting exceptions: Sphaerospora dicentrarchi and a related Sphaerospora sp.
Sphaerospora dicentrarchi is a marine species that produces spores similar to that noted for clade III; however, phylogenetically it is placed within clade IV, as a sister taxon to a further unidentified Sphaerospora sp. (Fiala, Reference Fiala2006). Unfortunately, ultrastructural analysis has not been conducted on this Sphaerospora sp. and therefore its affinities with members of the Kudoidae, or S. dicentrarchi cannot presently be considered. Likewise, the gene sequence for S. dicentrarchi deposited in GenBank (AY278564) is not accompanied with any information as to how it was obtained, or how the parasite was speciated and it is therefore difficult to verify whether this sequence really relates to S. dicentrarchi. Further research is therefore needed before the relationship of these parasites to other members of clade IV can be properly assessed. However, the two sequences do suggest that within clade IV there are species that possess 2 polar capsules and have at least a superficial resemblance to members of the genus Sphaerospora.
Clade V corresponds to the large freshwater clade of Fiala (Reference Fiala2006). However, although this clade includes a large number of diverse genera and species, the overall formation of the spores is similar. The terminology regarding sporogenesis for members of this clade has historically been different from that of the other myxozoan species that develop spores through direct association of secondary cells. For clarity, we will therefore refer to the terminology of clades I–IV when describing clade V. Developing spores are usually formed in large polysporic plasmodia. The plasmodia consist of a primary cell enclosing many secondary (generative) cells. Sporogony initiates when a secondary cell (pericyte) engulfs another to form a tertiary cell (sporogonic). After an initial division, the 2 tertiary cells continue dividing within the secondary cell to form the constituent cells of the spores (sporoblast). The pericyte, together with these sporoblasts, represents a pansporoblast. These pansporoblasts appear analogous to the triple forms associated with species of clade II, but in contrast are not released by the breakdown of the primary cell and the S-T doublet associated with spore formation is not observed. The resultant spores of clade V species possess valvogenic cells that have reduced to form hardened valves, a single binucleate sporoplasm and usually 2 polar capsules. The 2 nuclei of the sporoplasm are stated as deriving from nuclear division (Current and Janovy, Reference Current and Janovy1977) rather than cell fusion as suggested for members of clade III, although this needs to be validated. For some studies on clade V species, 5 sporoblast cells have been reported as forming the spore (Current and Janovy, Reference Current and Janovy1977), while for other species 6 have been noted (El-Matbouli et al. Reference El-Matbouli, Hoffmann and Madnok1995), suggestive of cell fusion.
It is interesting to note that in the 2 freshwater clades (II and V) the valvogenic cells are reduced to form hardened valves, while for examined species in all of the other clades the valvogenic cells form valves which appear more fragile, the valve cells usually retaining nuclei, some cytoplasmic matrix together with only a slight thickening of the plasma membrane surface(s). In relation to this, an intriguing species is Sph. testicularis, which possesses a binucleate sporoplasm and a sporogonial sequence reminiscent of a disporous clade V species, but appears to have purely cytoplasmic valve cells and polar capsules with a burred stopper structure (Sitjà-Bobadilla and Alvarez-Pellitero, Reference Canning, Okamura and Curry1993), both features associated with species of clade I. Unfortunately, at the time of writing, the 18ssu ribosomal gene of Sph. testicularis had yet to be sequenced, but further examination of this unique myxozoan is warranted.
In the phylogeny of Fiala (Reference Fiala2006) 2 notable exceptions exist to the proposed outline of clade V development; Sph. molnari and Sph. renicola. Both of these species produce spores which correspond to those of clade II in that they possess 2 mononucleated sporoplasms, in contrast to the binucleated sporoplasms of clade V (Lom et al. Reference Lom, Dyková and Lhotáková1982; Kaup et al. Reference Kaup, Kuhn and Körting1995). Of these two species, the development of Sph. renicola has been examined in most detail and corresponds to what is known for members of clade II, in contrast to its phylogenetic placement. It is possible that these two species have evolved sporogonic sequences that resemble clade II species through convergent evolution. However, there is reason to question the validity of the 18ssu rDNA gene sequences deposited in GenBank. Like Sph. dicentrarchi there is little information regarding the sequence available for Sph. molnari (AF 378345). However, the type host for this parasite is the common carp Cyprinus carpio from central Europe (Lom et al. Reference Lom, Dyková, Pavlásková and Grupcheva1983c), while the deposited sequence was derived from goldfish Carrassius auratus obtained in Japan (Kent et al. Reference Kent, Andree, Bartholomew, El-Matbouli, Desser, Devlin, Feist, Hedrick, Hoffmann, Khattra, Hallett, Lester, Longshaw, Palenzuela, Siddall and Xiao2001) – a host and locality not usually associated with this parasite. The 18ssu rDNA gene sequence of Sph renicola has been published (Eszterbauer and Székely, Reference Eszterbauer and Székely2004). The authors discussed significant contamination issues encountered during their study, as, in addition to the proposed sequence for Sph. renicola, a further sequence from an undiagnosed myxozoan was also obtained. Therefore, it would be prudent for the sequences of Sph. molnari and Sph. renicola to be re-examined to confirm their phylogenetic placement within the Myxozoa.
In conclusion, examination of T. bryosalmonae suggests that the tertiary cell formed during the intra-vertebrate development of myxozoans is related to sporogony rather than a mode of replication. Sporogonial sequences are proposed corresponding to 5 distinct clades. For clades I, II and III an S-T doublet forms which is directly involved in the development of the spore. Clade IV species (Kudoidae) have a unique sporogony with the tertiary cell forming the internal cell of the bi-cellular sporoplasm: a feature that is in common with the spores produced during the invertebrate phase of myxozoan life cycles. While for clade V, the tertiary cell divides within a pansporoblast to form the spore. We envisage that the different pathways proposed for each of the clades can form a framework from which future studies will build as our understanding of myxozoan biology and phylogeny increases. This will be important if we are to critically evaluate genetic data, develop our understanding of the biological and evolutionary relationships between myxozoans and identify new features that are of taxonomic value.
The authors would like to thank Mr Linton Brown for assistance with cutting and preparing sections for TEM, Dr James Bron for assistance with confocal imaging, Dr Astrid Holzer for translation, and Mr Niall Auchianachie for fish husbandry. This research was funded by a grant from the Biotechnology and Biological Sciences Research Council (BCC 5050421).