According to the World Health Organisation (WHO), iron deficiency anaemia (IDA) is one of the most common health problems worldwide, but in contrast, the recommendations of this mineral are higher in some populations (Ekström et al. Reference Ekström, Hyder, Chowdhury, Chowdhury, Lönnerdal, Habicht and Persson2002), leading in many cases to Fe-overload. Hepatotoxicity is common in patients with Fe supplements, followed by many other pathologies involving cardiovascular, endocrine and muscular systems. Fe excess in the body leads to generation of reactive oxygen species and severe damage to cell constituents (Piloni et al. Reference Piloni, Fermandez, Videla and Puntarulo2013)
Fe is a necessary cofactor in many metabolic processes in the central nervous system, including oxidative phosphorylation, myelin synthesis, neurotransmitter production, nitric oxide metabolism and oxygen transport (Díaz-Castro et al. Reference Díaz-Castro, Pérez-Sánchez, Ramírez, ópez-Frías, López-Aliaga, Nestares, Alférez, Ojeda and Campos2012). The liver has a key role in metabolism, eliminating xenobiotics and endogenous metabolites, and the antioxidant enzymes protect the cells (including hepatocytes) from the oxidative stress (Díaz-Castro et al. Reference Díaz-Castro, Alférez, López-Aliaga, Nestares, Sánchez-Alcover and Campos2013).
Our research group has previously reported that goat milk consumption has positive influence on enzymatic antioxidant defence, even in situation of Fe-overload in comparison with cow milk (Díaz-Castro et al. Reference Díaz-Castro, Pérez-Sánchez, Ramírez, ópez-Frías, López-Aliaga, Nestares, Alférez, Ojeda and Campos2012). In addition, goat milk fat has a higher nutritional quality than cow milk fat (Alférez et al. Reference Alférez, Barrionuevo, López-Aliaga, Sanz Sampelayo, Lisbona, Robles and Campos2001) and improves Fe metabolism (Nestares et al. Reference Nestares, Díaz-Castro, Alférez, Ochoa, Rivas, Hijano and López-Aliaga2008). However, in spite of its nutritional quality and healthy benefits, goat milk folic acid content is much lower than in cow milk (Haenlein, Reference Haenlein2001). The folic acid is an essential water-soluble vitamin that can regulate many cellular pathways such as growth and differentiation, DNA repair, apoptosis and carcinogenesis prevention, and in addition, has antioxidant effects and the capacity of scavenging the excessive free radicals output (Gursu et al. Reference Gursu, Onderci, Gulcu and Sahin2004).
Taking into account the beneficial effects of goat milk previously mentioned and the low folic acid content in this type of milk, the present study was carried out to assess the influence of cow and goat milk-based diets, either with normal-Fe content or Fe-overload and the influence of nutritional folic acid supplement on haematological parameters, some aspects of hepatic physiology and enzymatic antioxidant defence in liver, brain and erythrocyte of control and anaemic rats.
Materials and methods
Animals
All animal care procedures and experimental protocols were approved by the Ethics Committee of the University of Granada in accordance with the European Community guidelines. One hundred and sixty male Wistar albino breed rats (21 d old and weighing 41±5 g), purchased from the University of Granada Laboratory Animal Service (Granada, Spain) were used in the current study.
Experimental design
During a pre-experimental period (PEP) of 40 d rats were divided into two groups: the control group (n=80) receiving a normal Fe diet (45 mg/kg diet) (Reeves et al. Reference Reeves, Nielsen and Fahey1993), and the anaemic group (n=80) receiving a low-Fe diet (5 mg/kg diet), to induce a severe dietary Fe-deficiency according to the method developed previously by us (Pallarés et al. Reference Pallarés, Lisbona, López-Aliaga, Barrionuevo, Alférez and Campos1993). During the course of the study, the animals were placed in individual metabolic cages in an environmentally controlled room with a constant temperature (22±2 °C), humidity (55±5%) and a 12-h light-dark cycle (9:00 to 21:00). Diet intake was controlled, pair feeding all the animals (80% of the average intake) and bidistilled water was available ad libitum. On d 40 of the PEP, all rats per group (controls and anaemics) were anaesthetized by intraperitoneal injection with sodium pentobarbital (Sigma Diagnostics, St Louis, MO, USA), peripheral blood samples from caudal vein with ethylenediaminetetraacetic acid (EDTA) were analysed for haematological parameters.
An experimental period (EP) of 30 d followed in which the control and anaemic groups were further fed with either goat milk (n=80) or cow milk-based diets (n=80) with normal folic acid content (2 mg/kg diet) or folic acid supplemented (40 mg/kg diet) (Achón et al. Reference Achón, Alonso-Aperte, Reyes, Úbeda and Varela-Moreiras2000) and either with normal Fe content (45 mg/kg diet) or Fe overloaded (450 mg/kg) to induce chronic Fe overload (Raja et al. Reference Raja, Simpson and Peters1994) (10 control and 10 anaemic rats for each of the eight milk-based diets; Table 1). On d 30 of the EP, the animals were kept fasting overnight anaesthetised intraperitoneally, totally bled out by cannulation of the abdominal aorta and blood aliquots with EDTA were analysed for haematological parameters. Subsequently, plasma was obtained by centrifugation (1500 g, 4 °C, 10 min). The remaining blood was centrifuged without anticoagulant to separate the red blood cells from the serum and subsequent analysis of serum Fe, ferritin and total Fe-binding capacity (TIBC). The liver and brain were removed and washed with ice-cold saline solution (0·9%, w/v, NaCl). Liver, brain and erythrocyte cytosolic fractions were prepared fresh the same day by successive differential centrifugations, preserving these fractions at −80 °C for further analyses of oxidative damage.
Iron determination in the diets
After mineralisation by a wet method in a sand bath (J.R. Selecta, Barcelona, Spain), Fe concentrations in the experimental diets were determined by atomic absorption spectrophotometry (PerkinElmer Analyst 1100B spectrometer with WinLab32 for AA software, Ueberlingen, Germany). To calibrate the measurements, samples of lyophilised bovine liver (certified reference material BCR 185; Community Bureau of References, Brussels, Belgium) were used to check the Fe recovery (Fe value=209 (sem 5·7) mg/kg, mean (sem) of five determinations, certified value=212 (sem 5·1) mg/kg).
Haematological test
All the haematological parameters studied were measured and calculated by fully-automatic haematology analyser Sysmex K-1000D (Sysmex, Tokyo, Japan).
Serum ferritin
Serum ferritin concentration was determined using the Rat Ferritin ELISA Kit (Biovendor Gmbh, Heidelberg, Germany). The absorbance of the reaction was read at 450 nm using a microplate reader (Bio-rad Laboratories Inc., California, USA).
Serum iron, total iron binding capacity (TIBC) and transferrin saturation
To calculate the rate of transferrin saturation, serum Fe concentration and TIBC were determined using Sigma Diagnostics Iron and TIBC reagents (Sigma Diagnostics). The percentage of transferrin saturation was calculated from the following equation:
Plasma transaminases
Plasma alanine transaminase (ALT) and aspartate aminotransferase (AST) activity were determinedusing a Transaminases kit (Sigma Diagnostics) as previously described (Bergmeyer et al. Reference Bergmeyer, Scheibe and Wahlefeld1978).
Plasma creatinine and creatine kinase (CK)
Creatinine and CK were measured in plasma using a commercial colorimetric kit (Spinreact, Barcelona, Spain).
Liver, brain and erythrocyte cytosolic preparations
The liver, brain and erythrocyte samples were thawed and homogenates were freshly prepared the same day in a Potter homogeniser after the addition of 1·0 ml phosphate buffer per 0·1 g tissue. Successive differential centrifugations (Beckman Coulter Inc., CA, USA) were performed to separate cytosolic fractions, according to DeSandro et al. (Reference DeSandro, Chevrier, Boddaert, Melcion, Cordier, Richert, De Sandro, Chevrier, Boddaert, Melcion, Cordier and Richert1991). The erythrocyte cytosolic fractions were prepared according to Hanahan & Ekholm (Reference Hanahan and Ekholm1974). Cytosolic protein contents were measured as described by Lowry et al. (Reference Lowry, Rosenburgh, Farr and Randall1951).
Antioxidant enzymes activity in cytosolic fractions of liver, brain and erythrocyte
Glutathione peroxidase (GPx) activity was measured by the method of Flohé & Günzler (Reference Flohé and Günzler1984), which is based on the instantaneous formation of oxidised glutathione during the reaction catalysed by GPx. The oxidised glutathione is continually reduced by an excess of glutathione reductase and NADPH. The subsequent oxidation of NADPH to NADP+ was monitored spectrophotometrically (Thermo Spectronic, Rochester, USA) at 340 nm.
Superoxide dismutase (SOD) activity was determined according to Crapo et al. (Reference Crapo, McCord and Fridovich1978) based on its inhibition in the reduction of cytochrome c, measured in a spectrophotometer (Thermo Spectronic, Rochester, USA) at 550 nm. Catalase (CAT) activity was determined following the method described by Aebi (Reference Aebi1984), monitoring at 240 nm in a spectrophotometer (Thermo Spectronic, Rochester, USA) the H2O2 decomposition, as a consequence of the catalytic activity of CAT.
Thiobarbituric acid–reactive substances (TBARS) measurement
The extent of lipid peroxidation was evaluated on liver, brain, erythrocyte and plasma by measuring the concentration of TBARS according to the methods of Yagi (Reference Yagi1976) & Ohkawa et al. (Reference Ohkawa, Ohishi and Yagi1979). The reaction product was extracted and measured by spectrophotometric analysis (Thermo Spectronic) at 532 nm.
Statistical analysis
Data are reported as means±standard error of the mean (sem) during the PEP and means±residual standard deviation (RSD) during the EP. Statistical analyses were performed using SPSS (SPSS, version 20.0, 2012; SPSS Inc., Chicago, IL, USA). The data were analysed by the use of a complete randomised block experiment design, with factorial arrangement (2×2×2×2), 2 (type of diet: goat vs. cow milk diet)×2 (group of animal: control vs. anaemic)×2 (Fe content in the diet: normal-Fe or Fe-overload)×2 (folic acid content in the diet: normal vs. folic acid-supplemented), and by the least squares method (Steel et al. Reference Steel, Torrie, Dickey, Steel, Torrie and Dickey1997). Differences between groups (control vs. anaemic, normal-Fe vs. Fe-overload and normal-folic acid vs. folic acid-supplemented) were tested for statistical significance with Student's t test. Variance analysis by one-way methods was used to compare the different diets supplied. Individual means were tested by pairwise comparison with Tukey's multiple comparison test when main effects and interactions were significant. Differences were considered significant at P<0·05.
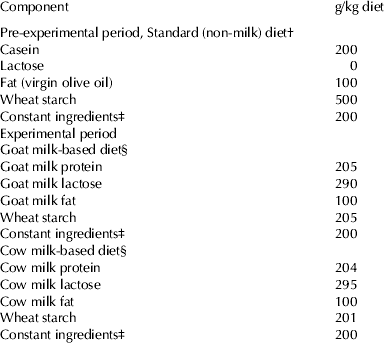
Table 1. Composition of the experimental diets
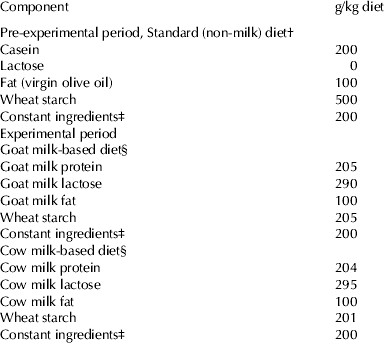
† The diets were prepared according to the recommendations of the AIN-93 for control rats (45 mg Fe/kg diet) (Reeves et al. Reference Reeves, Nielsen and Fahey1993), or with low Fe content (5 mg Fe/kg diet) (Pallarés et al. Reference Pallarés, Lisbona, López-Aliaga, Barrionuevo, Alférez and Campos1993), for Fe-deficient groups
‡ The constant ingredients consisted of (g/kg diet): fibre (micronized cellulose) 50, sucrose 100, choline chloride 2·5, L-cystine 3, mineral premix 35, vitamin premix 10
§ Specific vitamin and mineral premixes supplements for goat milk and cow milk-based diets were formulated taking into account the mineral and vitamin contents of the skimmed milk powder supplied in order to meet these recommendations. The diets were prepared with normal-Fe content (45 mg Fe/kg diet) (Reeves et al. Reference Reeves, Nielsen and Fahey1993), or with high Fe content (450 mg Fe/kg diet) (Raja et al. Reference Raja, Simpson and Peters1994) for Fe-overload groups, and both either with normal folic (2 mg/kg diet) or high folic content (40 mg/kg diet) for folic-acid-supplemented diets (Achón et al. Reference Achón, Alonso-Aperte, Reyes, Úbeda and Varela-Moreiras2000)
Results
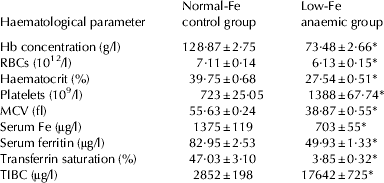
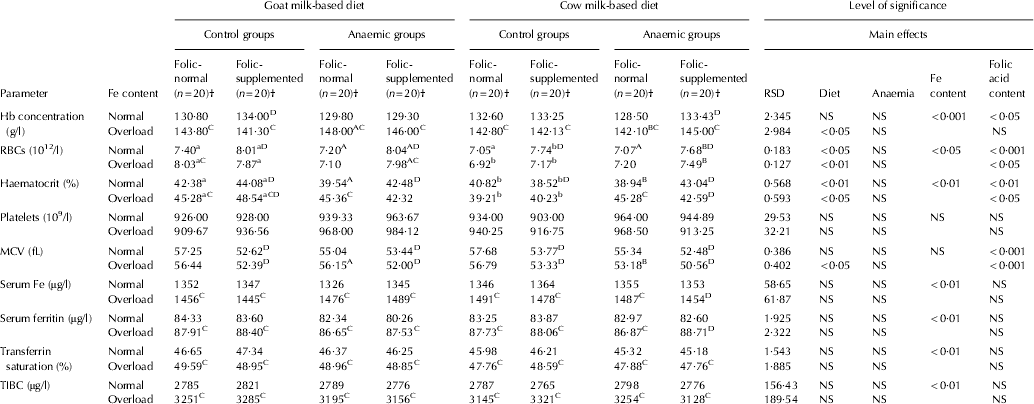
After rats had consumed the low-Fe diet for 40 d, haematological parameters were dramatically different from the controls (P<0·001) and control rats fed normal-Fe diet had all the haematological parameters within normal limits for this species at the end of the PEP (Table 2). After 30 d feeding the milk-based diets (d 70), the haematological parameters were fully recovered with both milk-based diets, either with normal-Fe or Fe-overload. As expected, serum Fe was higher in the Fe-overload groups (P<0·01). Fe-overload also increased haemoglobin (P<0·001), serum ferritin (P<0·01), transferrin saturation (P<0·01) and TIBC (P<0·01). Folic supplementation increased RBC count in the normal-Fe groups (P<0·01) and reduced MCV (P<0·001) (Table 3).
Table 2. Haematological parameters in the pre-experimental period. Values are means±sem for n=80 per group
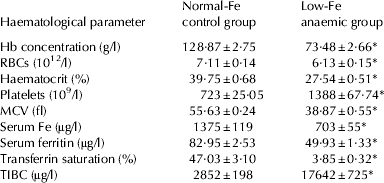
Hb, haemoglobin; RBCs, red blood cells; MCV, mean corpuscular volume; TIBC, total Fe-binding capacity
* Significantly different (P<0·001) from the control group by Student's t test
Table 3. Haematological parameters in the experimental period

RSD, residual standard deviation; NS, not significant
† (n=20), 10 rats with Fe normal and 10 with Fe overload
a,bMean values among groups of controls rats fed with different diet and different lower case letters in the same row indicated significant difference by one-way ANOVA (Tukey's test)
A,BMean values among groups of anaemic rats fed with different diet and different upper case letters in the same row indicated significant difference by one-way ANOVA (Tukey's test)
CMean values were significantly different from the corresponding group of rats fed with normal Fe content at P<0·05 by Student's t test
DMean values were significantly different from the corresponding group of rats fed with normal folic acid content at P<0·05 by Student's t test
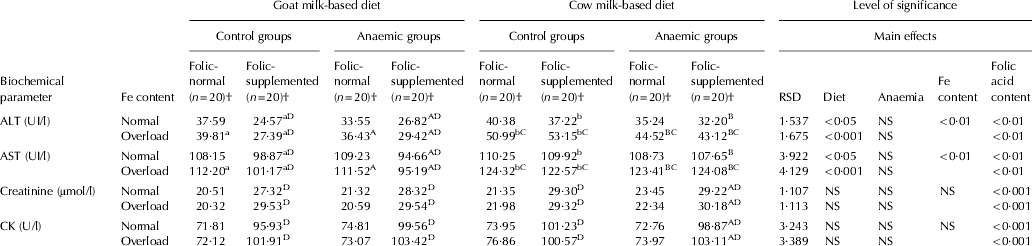
Fe-overloaded cow milk consumption increased ALT and AST levels in comparison with goat milk, in both animal groups (P<0·001). In addition, Fe-overload lead to an increase of ALT and AST with cow milk-based diets compared with normal-Fe content in all experimental conditions (P<0·01), meanwhile no effect of Fe-overload was observed with goat milk. Folate supplement markedly reduced transaminases plasma levels with goat milk-based diet (P<0·001). In addition, supplemental folic acid significantly increased creatinine and CK with both milk-based diets and under all the experimental conditions (P<0·001) (Table 4).
Table 4. Transaminases, creatinine and creatine kinase (CK) in the experimental period

RSD, residual standard deviation; NS, not significant
† (n=20), 10 rats with Fe normal and 10 with Fe overload
a,bMean values among groups of controls rats fed with different diet and different lower case letters in the same row indicated significant difference by one-way ANOVA (Tukey's test)
A,BMean values among groups of anaemic rats fed with different diet and different upper case letters in the same row indicated significant difference by one-way ANOVA (Tukey's test)
CMean values were significantly different from the corresponding group of rats fed with normal Fe content at P<0·05 by Student's t test
DMean values were significantly different from the corresponding group of rats fed with normal folic acid content at P<0·05 by Student's t test
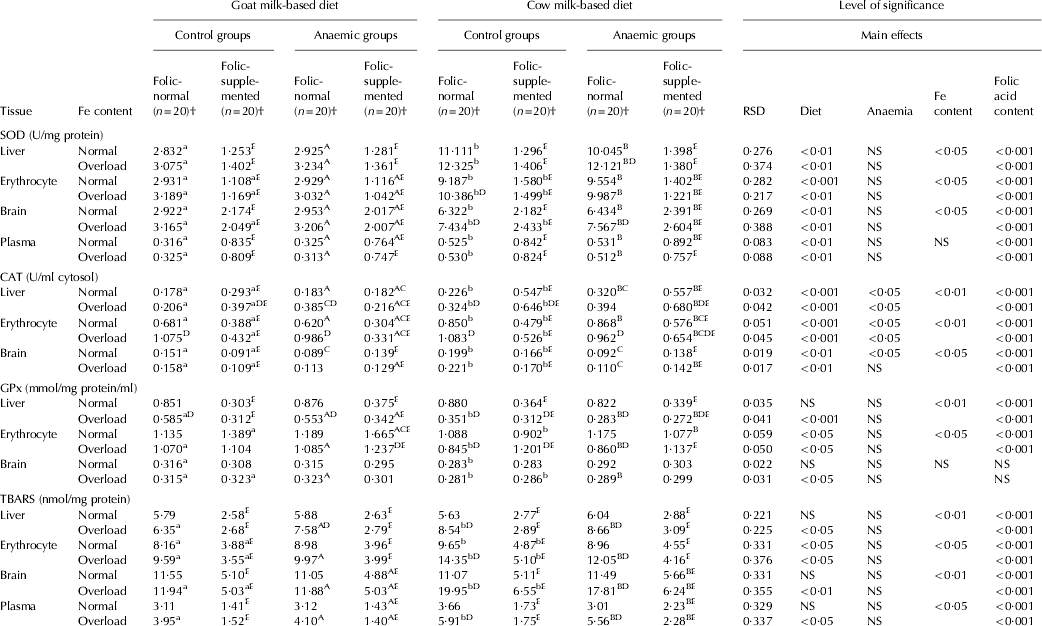
An increase in the SOD activity was observed in the animals fed cow milk diet in all the organs either with normal-Fe or Fe-overload with normal- folic content in comparison with goat milk (P<0·001). Fe-overload also increased SOD activity in erythrocyte and brain for control animals and in liver for anaemic rats fed cow milk, in comparison with normal-Fe content (P<0·05). Folic acid supplementation lead to a decrease on the activity of this enzyme in all the tissues studied except for plasma in the different experimental conditions (P<0·001) (Table 5). A concomitant increment in CAT was also observed in all the tissues of control animals fed cow milk in comparison with goat milk with normal-Fe content (P<0·001 for liver and erythrocyte and P<0·01 for brain) and the same trend is observed for anaemic animals (Table 5).
Table 5. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and thiobarbituric acid-reactive substances (TBARS) in the experimental period

RSD, residual standard deviation; NS, not significant
† (n=20), 10 rats with Fe normal and 10 with Fe overload
a,bMean values among groups of controls rats fed with different diet and different lower case letters in the same row indicated significant difference by one-way ANOVA (Tukey's test)
A,BMean values among groups of anaemic rats fed with different diet and different upper case letters in the same row indicated significant difference by one-way ANOVA (Tukey's test)
CMean values that were significantly different from the corresponding group of control rats at P<0·05 by Student's t test
DMean values were significantly different from the corresponding group of rats fed with normal Fe content at P<0·05 by Student's t test
EMean values were significantly different from the corresponding group of rats fed with normal folic acid content at P<0·05 by Student's t test
In general in all the conditions assayed, Fe-overload increased CAT activity in liver and erythrocyte (P<0·01), and no changes were observed in brain. Folate supplementation leads to an increase in CAT activity in liver and a decrease in erythrocyte and brain (P<0·001). Anaemia diminished CAT activity in brain of animals fed cow milk-based diet either with normal or Fe-overload and normal folic acid content (P<0·001), while its activity is not affected by the folic acid supplementation. In general, GPx activity decreased in the animals fed cow milk with Fe-overload in comparison with goat milk with high Fe content in liver (P<0·001), and erythrocyte (P<0·05) (Table 5). Fe-overload in the milk-based diets decreased GPx activity, especially in liver and erythrocyte of the animals fed cow milk with normal-folate content (P<0·001). Folate supplementation diminished GPx levels in liver (P<0·001), and increased its activity in erythrocyte (P<0·001). In general, anaemia had no effect on enzymatic activities in brain under the different experimental conditions (Table 5).
With regard to the oxidative stress-mediated damage to lipids (Table 5), in general, TBARS levels are higher in erythrocyte (P<0·05), brain (P<0·01) and plasma (P<0·05) for control and anaemic animals fed with Fe-overloaded cow milk-based diet in comparison with goat milk. Anaemia had no effect on TBARS levels in all tissues studied under the different experimental conditions. Fe-overload had a negative influence with cow milk and normal-folic content, increasing lipid peroxidation in erythrocyte (P<0·05), brain (P<0·01) and plasma (P<0·05). Furthermore, supplemental folic acid in the milk-based diets had a beneficial effect, reducing the extent of lipid peroxidation in all the tissues studied (P<0·001) (Table 5).
Discussion
Fe-deficiency occurs because the rats with normal body Fe stores lose a large portion of body Fe before the haemoglobin falls, and that the stores were replenished after feeding the animals during 30 d EP with different milk-based diets either with normal-Fe or Fe-overload. Folic supplementation increased RBC count in the normal-Fe groups, a fact that can be explained by the key role of folate in cell growth and differentiation (Gursu et al. Reference Gursu, Onderci, Gulcu and Sahin2004). The reduction of mean corpuscular volume (MCV) in the folate-supplemented groups can be attributed to the higher cell turnover in this group, giving place to smaller cells (Cook, Reference Cook2005).
After suppling cow milk with Fe-overload, ALT and AST levels increased, indicating hepatocellular damage and release of both transaminases into the bloodstream. Dietary folate supplementation reduced both plasma transaminases levels in animals fed goat milk, suggesting a hepatoprotective effect under basal conditions and more noticeably in situation of Fe-overload. This fact can be explained by the better Zn bioavailability and nutritive utilisation of goat milk fat, which provides lower substrate for hepatic lipid peroxidation and consequently reducing free radicals production in the animals consuming this type of milk (Díaz-Castro et al. Reference Díaz-Castro, Pérez-Sánchez, Ramírez, ópez-Frías, López-Aliaga, Nestares, Alférez, Ojeda and Campos2012).
We have observed that plasma creatinine and CK increased in the groups with folic acid supplementation, probably because of increased production of creatine which is a major consumer of methyl groups and necessary for a correct folate metabolism (Gamble et al. Reference Gamble, Liu, Slavkovich, Pilsner, Ilievski, Factor-Litvak, Levy, Alam, Islam, Parvez, Ahsan and Graziano2007). Creatine increases the muscle mass and energy production. After creatine synthesis, the level of thiodiglycolic acid (TDGA) increases really fast (Navrátil et al. Reference Navrátil, Kohlíková, Petr, Pelclová, Heyrovský and Pristoupilová2010). From an oxidative point of view, the increase in creatinine would be negative, because the oxidative degradation of xenobiotics via TDGA formation decreases the glutathione (GSH), one of the main intracellular antioxidant, therefore, this fact lead to a decrease of all metabolic pathways dependent on GSH (Navrátil et al. Reference Navrátil, Kohlíková, Petr, Pelclová, Heyrovský and Pristoupilová2010). However, we postulate that folate supplementation reduces the GSH consumption and also improves hepatic antioxidant capacity, scavenging the excessive free radicals produced in the course of creatine metabolism, finding supported by the activities of SOD, CAT and GPx recorded in the current study.
In general, Fe-deficiency anaemia did not affect SOD, CAT and GPx activities and, as a consequence, lipid oxidative damage in the tissues studied remain unaltered, results which are in agreement with those reported previously by us, (Díaz-Castro et al. Reference Díaz-Castro, Alférez, López-Aliaga, Nestares, Granados, Barrionuevo and Campos2008).
A remarkable increase in the SOD and CAT activity was observed in the animals fed cow milk diet in all the organs either with normal-Fe or Fe-overload especially with folate supplementation for CAT. This finding indicates that the increase observed in the SOD activity induces a concomitant augmentation in the formation of hydroperoxides and the CAT is increased to scavenge these ROS, needing an increase in the GPx activity to remove the excess of these intermediary products. The lower levels of GPx in all the groups fed Fe overload diets, especially in liver and erythrocyte of the animals fed cow milk, can be due to the reduction of the enzyme in the process of neutralisation of free radicals generated. The beneficial nutritional characteristics of the goat milk previously mentioned (Díaz-Castro et al. Reference Díaz-Castro, Pérez-Sánchez, Ramírez, ópez-Frías, López-Aliaga, Nestares, Alférez, Ojeda and Campos2012) can explain the lower affect on the liver and erythrocytes induced by the Fe overload in animals fed this type of diet. In the current study, folate supplementation decreased SOD, which is the first line of enzymatic defence against ROS. Therefore, oxidative status markers were modified by folic acid supplementation in the sense of lower oxidative stress, which has clear positive effects on enzymatic antioxidant defence.
On the other hand, ROS play a key role in several pathways of the nervous system, while antioxidants neutralise the oxidative stress (Uttara et al. Reference Uttara, Singh, Zamboni and Mahajan2009). The role of antioxidant defence in the brain is not clear and, in this sense, some authors report that the brain is not enriched in antioxidant defences (Uttara et al. Reference Uttara, Singh, Zamboni and Mahajan2009), while other studies report that antioxidants (enzymatic and/or non-enzymatic) protect the brain against induced oxidative damage (Ozturk et al. Reference Ozturk, Demirbilek, Kadir But, Saricicek, Gulec, Akyol and Ozcan Ersoy2005), although the presence of antioxidant enzymes CAT, GPx and SOD in brain region has been previously documented (Alférez et al. Reference Alférez, Díaz-Castro, López-Aliaga, Rodríguez-Ferrer, Pérez-Sánchez and Campos2011). In the current study, in brain and erythrocyte, folate-supplementation led to a reduction in the antioxidant enzymes studied, supporting earlier reports which suggest that the function of the key intracellular antioxidant enzymes may be interdependent (Valko et al. Reference Valko, Leibfritz, Moncol, Cronin, Mazur and Telser2007). This finding can be due to the antioxidant effects of folate and the capacity of scavenging the excessive free radicals output, a fact that can be directly correlated with the lower increase in SOD activity in these tissues, reducing the formation of hydroperoxides. With regard to Fe content in the diet, the slightly modified activities of SOD, CAT and GPx in the nervous tissue, reveals that it is relatively independent of the Fe variations in the organism (Moos et al. Reference Moos, Rosengren Nielsen, Skjørringe and Morgan2007).
The liver is a tissue especially vulnerable to the oxidative damage, which is reflected in the increase of the SOD activity in hepatic cytosol of animals Fed with Fe-overload diets. This hepatic Fe-overload promotes the generation of free radicals, so the upregulation of SOD would be directed to eliminate this high rate of generation of ROS (Díaz-Castro et al. Reference Díaz-Castro, Pérez-Sánchez, Ramírez, ópez-Frías, López-Aliaga, Nestares, Alférez, Ojeda and Campos2012). This situation demands an excessive activation of the protective mechanisms and leads to the progressive inactivation of antioxidant enzymes, fact that might probably justify the reduction in the activity of GPx. The higher levels of CAT in the liver of folate-supplemented animals indicates that folate supplementation causes different effects on antioxidant enzyme activity in various tissues/organs owing to inherent differences in organ pathways regulating folate metabolism as well as antioxidant enzyme synthesis, function, or turnover. The remarkable increase observed in the SOD of normal-folate diets (especially with cow milk) leads to an increment of the formation of hydroperoxides and CAT is also increased to neutralise and to scavenge the excessive production of O2•− in this tissue. The higher levels of CAT found in liver in situation of folate-supplementation can be due to the antioxidant and scavenging effect of folic acid, which protects hepatocytes from oxidative stress by decreasing homocysteine levels that are associated with oxidation and auto-oxidation of homocysteine, and increases the antioxidant activity of CAT (Yamakura et al. Reference Yamakura, Taka, Fujimura and Murayama1998; Handy et al. Reference Handy, Zhang and Loscalzo2005). In addition, folate may exert antioxidant effects independent of homocysteine lowering by inhibiting NADPH oxidase-mediated superoxide anion production (Woo et al. Reference Woo, Prathapasinghe, Siow and O2006) and improves the total plasma antioxidant capacity (Alvares Delfino et al. Reference Alvares Delfino, De Andrade Vianna, Mocelin, Sabbatini Barbosa, Aiko Mise and Matsuo2007), scavenging the excessive free radicals produced due to the increased SOD activity and avoiding the progressive inactivation of CAT. This situation would need a concomitant increase in the GPx activity to remove the excess of these intermediary products highly harmful to the cell, a fact that leads to the progressive inactivation of the antioxidant activities, justifying the reduction in the activity of GPx, especially in the Fe-overload groups.
The increased SOD activity observed in our study with cow milk and Fe-overload may be a compensatory mechanism of the body to cope with the increase in oxidative stress, however, the increase in lipid peroxidation products levels (TBARS) in control and anaemic groups fed cow milk with Fe-overload, suggest an imbalance in the functioning of the enzymatic antioxidant defence system. Nevertheless, this increase is more significant in the animals receiving cow milk diet that in those fed goat milk diet. In addition, folate supplement has beneficial effects, avoiding lipid peroxidation with both milk-based diets. Our results are in agreement with previously reported results highlighting that folic acid scavenges free radicals and their metabolic products, maintaining normal cellular physiology very efficiently and avoiding lipid oxidative damage (Gursu et al. Reference Gursu, Onderci, Gulcu and Sahin2004).
In the light of these results, it would be really positive to include folic acid supplemented goat milk in the diet of population consuming oral Fe supplements, because this would avoid the undesirable effects of Fe excess in the tissues.
Conclusion
In conclusion, Fe-overloaded cow milk induces hepatocellular damage, releasing both transaminases into the bloodstream and also increases oxidative stress in all the tissues, an issue that is not observed with goat milk. Dietary folate-supplemented goat milk reduces both plasma transaminases levels, suggesting a hepatoprotective effect of this type of milk and has beneficial effects in situation of Fe-overload, protecting cell from free radicals oxidative damage, improving the antioxidant enzymes activities and limiting lipid peroxidation
Funding was provided by the Ministry of Science and Innovation, Research Project No. PT_2011_10, Granada Research of Excellence Initiative on BioHealth (GREIB). The authors are grateful to Lactalis Iberia, S.A. (Lugo, Spain) for providing the dairy products used in this study, to Ms Elisa Alcover and Ms Encarnación Rebollo for her competent technical assistance.