Introduction
The importance of post-mastectomy radiation therapy to chest wall and nodal regions at risk is well emphasised in most of the treatment facilities all over the world, even in cases of early breast cancer, though in modern-day practice mastectomy is reserved for locally advanced breast cancer (LABC) only. LABC encompasses a heterogeneous group of patients including those with advanced primary tumour size, extensive or widespread nodal disease and inflammatory breast cancer. Patients who present with LABC are at risk for both distant and locoregional disease recurrences. Reference Huang, Chen, Yang and Shao1 The prognosis of patients with LABC depends on tumour size, extent of lymph node involvement and presence of inflammatory breast cancer. Adjuvant radiotherapy is seen to reduce the incidence of locoregional recurrence from 30 to 10·5% at 20 years and breast cancer deaths by 5·4% at 20 years. Reference Barrett, Dobbs, Morris, Roques, Jamieson, Senior, Silman, Walker, Townson and Ross2 Radiotherapy not only decreases locoregional recurrence but also improves survival which has been unequivocally demonstrated in different landmark randomised studies and meta-analyses [British Columbia Cancer Agency, Danish Breast Cancer Cooperative Group, and the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)]. Reference Ragaz, Olivotto and Spinelli3–Reference McGale, Taylor and Correa6
The art of giving radiation therapy in breast cancer has undergone a paradigm change with anterior photon beams in early days to tangential beams and then to modern conformal radiotherapy treatment techniques in the current era. Traditionally, 50 Gray in 25 fractions is the standard radiotherapy protocol for post-mastectomy patients. Reference MacDonald, Harris and Arthur7 The scope of hypofractionated radiotherapy, incorporating higher dose per fraction and delivering radiation in fewer fractions, arises from the radiobiology of breast tissue. Unlike most of the rapidly proliferating tissues in malignancies, breast cancers behave more akin to resting tissue which was established in the UK Standardisation of Breast Radiotherapy (START) Pilot Trial in 2006· 8 As following the linear quadratic model, the alpha/beta ratio of breast cancer cells is found to be close to 4, which is comparable to that of normal tissue (α/β = 3) than most other malignancies (α/β = 10). Reference Jones, Dale, Deehan, Hopkins and Morgan9 This theoretical applicability of hypofractionation was tested in many trials starting from 80 seconds to modern day. Hypofractionated radiation therapy employing 13–16 fractions after mastectomy has been used in many institutions and has demonstrated equivalent local control, cosmetic outcome and effects on normal tissues in comparison with 50 Gy in 25 fractions. Reference Shaltout and El Razek10 In 2002, a Canadian trial concluded that a lower total dose in a small number of fractions could offer similar rates of tumour control and normal tissue damage as the international standard fractionation schedule of 50 Gy in 25 fractions. Reference Whelan, Mackenzies and Julian11 The Standardisation of Breast Radiotherapy (START) trial was initiated by the UK Co-ordinating Committee for Cancer Research (now National Cancer Institute) to test the effect of radiation schedules using fraction size larger than 2 Gy which was later carried on in many studies. Reference Whelan, Pignol and Levine12–Reference Yarnold, Ashton and Bliss14 In 2008, 10-year follow-up data of START A and START B trials showed that less frequent but higher-dose radiotherapy regimens are as safe and effective as the standard regimen of more frequent lower doses for women with early breast cancer in which most of the patients underwent breast conservative surgery (BCS) receiving whole breast radiation. Reference Bentzen, Agrawal and Aird15,Reference Bentzen, Agrawal and Aird16 Modest increases in fraction size are compared with appropriate downward adjustment of total dose. Reference Clark, Whelan and Levine17–Reference Ollivotto, Weir and Kim-Sing19 The newer 3-week regimen equates to a lower cumulative radiotherapy exposure, thereby less delayed adverse effects compared to a standard 5-week regimen. Reference Whelan, Mackenzies and Julian11 In spite of the existing evidences suggesting that the hypofractionated radiotherapy may also be safe and effective for regional nodal disease and LABC after modified radical mastectomy (MRM), Reference Ragaz, Olivotto and Spinelli3,Reference Wang, Fang and Song20 the use of a hypofractionated schedule in this cohort of patient is not widely documented. Although the American Society of Clinical Oncology recommends radiation therapy even in T1/2 disease with node positivity in patients who had undergone mastectomy, the dose consensus was not unanimous. Reference Recht, Edge and Solin21 Most of the guidelines in USA and UK recommend hypofractionation in conservative surgery, Reference Yarnold22,Reference Smith, Bentzen and Correa23 but most centres in Western countries practise conventional fractionation in post-mastectomy chest-wall, axillary and supraclavicular irradiation wherever applicable. The National Comprehensive Cancer Network recommends hypofractionation in breast conservative treatment only and prefers conventional radiation of dose 45–50·4 Gy in 25–28 fractions for treating chest wall Reference Goetz, Gradishar and Anderson24 and high-risk nodal regions. This is also the standard practice of many Asian countries. Reference Yamauchi, Sekiguchi and Nishioka25
In the modern-day practice of radiotherapy, a software-based treatment planning over a simulated image is being increasingly popularised. Here multiple field matching and beam modification are well evaluated for delivering an optimised dose to the target volume, minimising the dose to organs at risk. Hence, evaluation of an altered fractionation should also be done in light of dosimetric analysis obtained from a software generated data, for speculating its sufficiency and normal tissue complication probability (NTCP).
Therefore, in this study, we aim to compare the conventional radiotherapy regimen delivered over 5 weeks with hypofractionated 3 weeks regimen in patients of breast cancer who had undergone MRM which includes extensive axial lymph node harvesting. The comparison is based on statistical measurement of the dosimetric differences as well as in terms of local control, incidence of early and late radiation toxicities and also disease-free survival (DFS).
Materials and Methods
Acquisition of patients
This prospective and randomised study was undertaken in the department of radiotherapy and oncology, Institute of Postgraduate Medical Education & Research, Kolkata, India. Between December 2014 and December 2017, 240 LABC patients were randomly assigned after primary surgery to receive 50 Gy in 25 # over 5 weeks and 40 Gy in 15 # over 3 weeks, as both of these fractionation schedules are practised at our institute as per the departmental protocol after the approval of the Ethical Committee of the Institute (vide memo no. Inst/IEC/2014/084). Of them, 18 patients were excluded after allotment due to development of comorbidity, withdrawal from study and failure to turn up. Eligible patients were 18–65 years of age, with histologically confirmed infiltrating duct carcinoma of unilateral breast who had undergone MRM with axillary dissection without evidence of distant metastasis or second malignancy, with normal cardiac, renal and pulmonary function tests (PFT) and having Eastern Cooperative Oncology Group (ECOG) Reference Oken, Creech and Tormey26 performance status (PS) 0–2 after they provided proper written informed consent.
Treatment protocol
In the conventional arm (CRA) (Arm 1, n = 102), the patients received 50 Gy in 25 fractions over 5 weeks, and in hypofractionation arm (HFA) (Arm 2, n = 120), 40 Gy in 15 fractions over 3 weeks was delivered. Post-mastectomy radiotherapy was administered daily from Monday to Friday to the chest wall in all patients and supraclavicular and axillary region in high-risk patients. Chemotherapy was administered to all patients, either in form of neo-adjuvant therapy or as adjuvant therapy or both. Trastuzumab was given to her2/neu-positive patients. Chemotherapy and biologic therapy were not given concurrently with radiation therapy. After completion of chemotherapy, all hormonal receptor-positive patients were given proper hormonal therapy.
Objectives of the study
Primary end points are dosimetric analysis between two arms along with locoregional tumour recurrence and normal tissue toxicities. Locoregional relapse is defined by reappearance of cancer at irradiated sites (chest wall, ipsilateral axilla or supraclavicular fossa). Normal tissue effects in chestwall, arm and shoulder were assessed by comparing with baseline by the physician. DFS is defined as time to any breast cancer-related event (local or distal relapse) or death due to any cause from the time of randomisation until the end of follow-up. Cases of ischaemic heart disease and symptomatic lung fibrosis were recorded during follow-up, and their incidence with confirmation of diagnosis using imaging was included.
Treatment planning
Patients were simulated in supine position on the breast board with their arms abducted and externally rotated, and this position was reproduced daily during treatments. The head was turned to opposite side from treated breast to remove chin from beam divergence. The mastectomy scar was delineated with a radioopaque marker to make sure the entire scar was included in the radiation field. The medial, lateral (mid or post axillary line), superior (caudal end of clavicle) and inferior (1–2 cm below opposite inframammary fold) field borders were also delineated with radioopaque markers to guide initial beam placement.
Planning computed tomography (CT) scan was done with Philips Brilliance CT simulator (Phillips Healthcare Inc., Andover, MA, USA), and 3 mm slice were taken and transferred to ONCENTRA™ treatment planning system (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden).
Contouring was done according to Radiation Therapy Oncology Group (RTOG) guideline. 27 The chest wall clinical target volume (CTV) was contoured upto the muscle rib interface to include the pectoralis muscles and the entire scar plus margin. The cranial border extends to the caudal border of the clavicular head and the caudal border was clinically determined based on marker placed at the time of simulation and using contralateral breast as a guide. The planning treatment volume (PTV) was created by expanding the CTV by 0·5 cm.
Axillary lymph node levels 1 to 3 were demarcated relative to pectoralis minor muscle and axillary vessels in which level 1 lies lateral and inferior and level 3 supero-medial. Supraclavicular lymph node lies superior to level 3 and commenced just caudal to the cricoid cartilage and inferiorly to the caudal end clavicular head laterally upto the lateral edge of sternocleido-mastoid muscle, medially excludes thyroid gland and trachea. PTVNODE was created by expanding the CTVNODE by 0·5 cm.
The chest wall was treated with two opposing parallel tangential fields. The tangents were placed at an angle to avoid contralateral breast. Supraclavicular and level 3 lymph node were treated with single oblique anterior portal often angled 10–15° away from midline to avoid overlapping with the spinal cord and oesophagus. An optional posterior axillary boost was given to those patients who had inter-axillary fold distance more than 10 cm.
A chest wall bolus (0·5 cm) was used to increase the skin dose for those who had an increased risk of chest wall recurrence (close margin, large tumour, inflammatory carcinoma).
Beam planning of chest wall followed tangential opposed beam couple, but modified according to dose painting and dose volume histograms. Wedges were used wherever necessary. The supraclavicular and/or axillary field was antero-posteriorly directed or 50 lateralised to prevent thyroid gland irradiation. All the fields were properly matched at junction. Radiation was delivered through BHABATRON-II (BARC, India) telecobalt (Co60) machine (Figure 1).

Figure 1. Sample treatment plan of a left-sided disease.
Follow-up
Patients were assessed clinically, and blood assays, PFT, electrocardiogram (ECG) and echocardiography were done before radiotherapy. The patients were evaluated clinically and with laboratory investigations once per week during radiotherapy and 2 weeks after completion of treatment. Acute radiation toxicity was assessed according to the common terminology criteria for adverse events (CTCAE) version 4.0, 28 and late radiation reaction was measured by using RTOG/European Organization For Research and Treatment of Cancer (EORTC) late radiation morbidity scale. Reference Cox, Stetz and Pajak29 All patients were followed up at radiotherapy and oncology outpatient department every month for a period of 6 months thereafter and 3 monthly till closure of the study. During follow-up, all symptomatic or clinically suspicious patients were evaluated radiologically for lung and with ECG, echocardiography and laboratory markers for cardiac toxicity.
Statistical analysis
The statistical analysis was done in IBM® SPSS version 25. The two arms are compared for demographic characters, planning variables, toxicity and treatment outcomes. Non-parametric data are compared with chi square test or Fisher exact test, wherever applicable. The means of the parametric data were compared using t-test for two independent variables. Actuarial rates of recurrence were calculated by the Kaplan–Meier method. p-Values of less than 0·05 were considered significant.
Results
Demographic characteristics
Patients’ demographics, disease and treatment characteristics were well balanced between the two groups (Table 1). The mean age of patients was 46 years (range 25–70 years). Thirty (29·4%) patients in CRA and 55 (45·8%) patients in HFA had T3 disease. Forty-one (40·2%) patients had T4 disease in CRA and 19 (15·8%) patients had T4 disease in other arm. In CRA, 21 (20·6%) had N2 and 11 (10·8%) patients had N3 disease. In HFA, 32 (26·7%) patients and 6 (5%) patients had N2 and N3 disease, respectively. Totally, 31 (30·4%) patients in CRA and 23 (19·2%) patients in HFA had grade 2 disease. Estrogen receptor (ER) positivity is in higher range than progesterone receptor (PR) positivity in 55 (53·9%) patients in CRA and 52 (43·3%) in HFA. Almost half of the patients in both arms were her2/neu positive. Adequate lymph node dissection was done in most of the patients. A median value of 14 lymph nodes (95% confidence interval (CI) = 13–15) was dissected in CRA and 18 lymph nodes (95% CI = 15–19) in HFA, the later having significantly higher value.
Table 1. Baseline characteristics

Notes: a Chi square test/Fisher exact test.
b Proportional z test.
c t-test of two samples.
Dosimetric comparison
The biological effective doses in the arms vary as well as the equivalent dose of 2 Gy per fraction (EQD2). The calculation and comparisons are made on the basis of EQD2, equated on HFA. The breast tissue tumour cells have α/β ratio close to 4, which is meant for subclinical neoplastic cells present postoperatively and post chemotherapy. Normal tissue toxicity depends on their biologic property to radiation, having α/β = 3. The prescribed dose has low biologically effective dose (BED) in HFA (66·7 Gy), thus also exerts low EQD2 dose on subclinical tumour cell (44·47 Gy versus 50 Gy in CRA) and normal tissue (45·36 versus 50 Gy in CRA). This is reflected in data outcomes of optimised forward planning also. The minimum dose covering 90% of the planning target volume, that is, PTV (D90) is high and equivalent in both the arms of treatment (conventional: 92·04% with 95% CI = 91·07–93·01%, hypofractionation: 92·5% with 95% CI = 91·58–93·42%; p = 0·49). But there is highly significant difference between the arms while prescribing dose to CTV. Maximum CTV dose in 2 Gy equivalent has a mean value of 50·68 Gy in HFA in contrast to a mean value of 55·11 Gy in CRA (p < 0·001), and the values of average CTV dose have mean value of 44·84 Gy and 48·44 Gy, respectively (p < 0·001). There is similar pattern of dose distribution in risk organ, lung being the major one. With equivalent central lung distance (CLD) (p = 0·91), maximum lung dose is significantly smaller in hypofractionation (47·75 Gy versus 51·58 Gy in coneventionl fractionation; p < 0·001), so is average lung dose (9·90 Gy versus 10·84 Gy in conventional fractionation; p = 0·06). However, volume of lung receiving 20 Gy or higher dose is similar in both arms (p = 0·21) and that of heart receiving 30 Gy or higher doses are also differing insignificantly in the two treatment arms (p = 0·34). Table 2 summarises the dosimetric comparison of two treatment arms.
Table 2. Radiation therapy technical details and outcome a
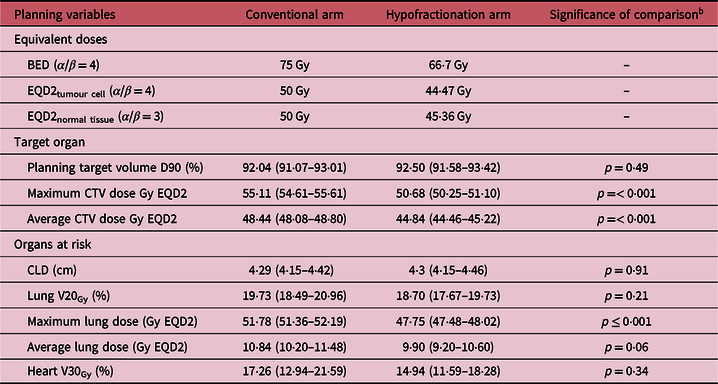
Notes:
a Values in parentheses indicate 95% confidence interval.
b t-test of independent samples.
Abbreviations: BED, biologically effective dose; EQD2, equivalent dose in 2 Gy fractions; CTV, clinical target volume; CLD, central lung distance.
Toxicity
HFA, having lesser dose administration as per planning, showed lesser grade 3 or higher toxicity events (10 versus 12·7%), but this advantage is minor and insignificant (p = 0·52). Also to mention that high-grade toxicity appeared a bit later in HFA with mean time to produce maximal toxicity at any organ being 10 weeks (versus 7 weeks in CRA, Mantel–Cox log rank test p value = 0·245). Acute toxicity appeared in similar times in the two treatment arms, but grade 3 or higher late toxicity appeared as late as 29 weeks in HFA in the form of cardiomyopathy in one patient. In CRA, the appearance of grade 3 or higher toxicity is limited in the range of 2–19 weeks; almost no patient showed delayed grade 3 or higher toxicity in this arm. In CRA, 4·9% patients had grade 3 or higher skin toxicity, which is the most common cause of toxicity in this arm. Though skin toxicity has very low incidence in hypofractionation, they have insignificant difference (p = 0·17). Lymphedema has been found to be the most common high-grade toxicity in HFA seen in 4·2% of the patients. A moderate frequency of lung toxicity occurred in both the arms: 3·9% in CRA and 3·3% in HFA. Only one patient in HFA required hospitalisation for lung opacification after radiation. Cardiotoxicity was seen in relatively few patients: only two in CRA and one in HFA. There was no cardiovascular mortality in either of arms during the follow-up. It is important to note that the organ-related toxicities had more grave form in relatively early phase of follow-up and all within 6 months of therapy, but there were many low-grade pneumonitis appeared late in both the arms, most of which resolved spontaneously. There was no statistical difference in incidence of lung toxicity (p = 0·82) or cardiotoxicity (p = 0·47) between the two arms. The hazard ratio of toxicity is 0·64 (95% CI = 0·28–1·45) with mean high-grade toxicity-free survival is insignificantly (Mantel–Cox log rank test; p = 0·406) higher in HFA (227 weeks, 95% CI = 216–237 weeks) than in CRA (214 weeks, 95% CI = 200–228 weeks) (see Table 3). In Figure 2, comparative hazard functions and Kaplan–Meier survival curves for toxicity-free survival are shown.
Table 3. Treatment outcomes a

Notes:
a Range values in parentheses indicate 95% confidence interval.
b Wilcoxon non-parametric test of comparison of ordinal data.
c Proportional z test.

Figure 2. Hazard function of toxicity and survival curve of toxicity-free survival.
Recurrence
Median follow-up period for conventional radiation was 178 weeks (range 42–241 weeks) and that in HFA was 182 weeks (range 40–147 weeks; p = 0·78) (Table 4). Three-year recurrence rate is low in both the arms: 4·9% in CRA and 5·8% in HFA, which is comparable (p = 0·76). Median time to recur is insignificantly (p = 0·89) higher in CRA (128 days) than in HFA (109 days). There is very low lymphatic failure in HFA; yet, this difference is also insignificant (p = 0·12). There are only two mortality events during the time of follow-up, both occurring in hypofractionation. Mean DFS is 230 weeks in CRA and 235 weeks in HFA (hazard ratio 1·01, 95% CI = 0·32–3·19), Mantel–Cox log rank test for comparison of survival yielded no significant difference (p = 0·987) (Figure 3).
Table 4. Treatment outcomes


Figure 3. Kaplan–Meier survival curve of disease-free survival.
Discussion
The treatment outcome of LABC patient depends upon multidisciplinary approach as per the expertise of the clinical oncologist and surgical oncologist. The impact of post-mastectomy radiation therapy on local control and overall survival was supported by the results of the EBCTCG meta-analysis of 78-prospective randomised clinical trials. The 5-year local recurrence risks in lymph node negative patients with and without post-mastectomy radiation therapy were 2 and 6%, respectively, with a 3·6% decrement in 15-year breast cancer mortality. In lymph node-positive patients, the 5-year local recurrence risks were 6 and 23%, respectively, which translated into a 5·4% benefit in 15-year breast cancer mortality. Reference McGale and Taylor30 START A trial randomised 2,236 patients at 17 centres in UK with early breast cancer after primary surgery to receive radiotherapy with 2 Gy (45 Gy / 25#) versus 3 Gy (39 Gy / 13#) versus 3·2 Gy (41·6 Gy / 13#) in same treatment time of 5 weeks. After a median follow-up of 5 years, the estimated absolute difference in 5 years locoregional relapse rates compared with 50 Gy was 0·2% (95% CI 1·3 to 2·6%) after 41·6 Gy and 0·9% (95% CI − 0·8 to 3·7%) after 3·9 Gy. Similarly, START B trial randomised 2,215 patients of early breast cancer at 23 centres in UK to receive 50 Gy/25# at 2 Gy/# over 5 weeks versus 40 Gy / 16# at 2·67 Gy/# over 3 weeks. The difference in locoregional relapse was not statistically significant. After a median follow-up of 6 years, locoregional tumour relapse rates were comparable with slighty superior cosmetic outcome in HFA. Interestingly, the HFA in the START B trial had lower rate of distant metastasis and overall mortality compared with the conventional fractionation arm. Reference Bentzen, Agrawal and Aird15,Reference Bentzen, Agrawal and Aird16 The results of these trials standardised hypofractionation as the mainstay radiation modality in early breast cancer even in mastectomy. In a phase II trial by Khan et al., a hypofractionation of 3·33 Gy per fraction, delivered in 11 fraction, showed promising outcome in a 32-week follow-up period in stage II and IIIa breast cancer. Reference Khan, Poppe and Goyal31 But in node positive or tumour stage more than 3, use of hypofractionation has not been well established in any randomised trial. Reference Wang, Fang and Song20
Most of the studies dealing with outcomes of hypofractionation on remnant breast tissue in BCT or chest wall and nodal areas in post-mastectomy cases discussed the impact of altered fractionation in terms of toxicity and recurrence events. But the context of dosimetric analysis is much worthy to bring forward in these discussions as both of the said outcomes are largely dose dependant. From computation of equivalent doses, we see that planned EQD2 is lesser in case of hypofractionation than that of conventional fractionation. Hence, the CTV has got significantly less equivalent dose than CRA, in spite of very similar coverage of 90% isodose volume. This has partly been deliberated in START B trial where the current dose schedule used in this study was compared with CRA. There it was found that hypofractionation came out with better breast tissue preservation, lessening breast shrinkage, telangiectasia and edema without compromising locoregional control. Reference Bentzen, Agrawal and Aird16 The Canadian trial rather used an extra fraction (42·5 Gy/16 fractions), elevating the equivalent dose near to that of conventional radiation. But it did not report any additional benefit over 40 Gy in 15 fractions in terms of DFS. In fact, there is no large trial comparing these two hypofractionation schedules. Theoretically in the EQD2 interval between 45 and 50 Gy, a small reduction in dose reduces probability of toxicity (NTCP) more than reducing the probability of subclinical tumour control. Reference Yarnold, Bentzen, Coles and Haviland32 In reality, we see that, along with significant lesser dose planned for CTV, the EQD2 on lung is also significantly less, both in terms of maximum dose and average dose. But in the optimised plan, there is no difference in V20Gy of lung and V30Gy of heart in the two treatment arms, both of which might have acted as the main predictor of indifference of pulmonary and cardiac toxicity in the two treatment arms. In a review by Youssef and Stanford (2018), where they scrutinised on different fractionation in breast conservative treatment, they argued that giving less total dose in hypofractionation certainly improved cosmesis, but this outcome may be variable. Reference Youssef and Stanford33 In post-mastectomy patients, we may not be more interested in cosmesis except in implants. The current study, thus, is important in regard to dosimetric consideration where a lesser dose is planned to reduce overall toxicity without inviting higher risk of locoregional failure.
This study shows that the incidence of high-grade toxicity is very comparable in both arms, which overall is very low indeed. Lymphedema followed by lung toxicity was most prevalent both of which had occurrence rates less than 5% in both the arms. Skin toxicity, though insignificant, was less in HFA (1·7 versus 4·9%). Lack of cardiovascular morbidity after radiation proves better outcome of a software planned radiation, especially in left-sided disease. However, Darby et al. commented that the incidence of ischemic heart disease after radiation can occur as late as 20 years after exposure. Reference Darby, Ewertz and McGale34 This study also proves that the toxicity is more dependent on V20 or V30 volumes of lung and heart respectively than absolute dose (maximum or average).
Locoregional control was also similar in both arms of this study, though lymphatic failure was less in hypofractionation (0·8 versus 3·9%). A small study was done in north eastern region of India by Bhattacharyya et al. where comparison was made between 40 Gy in 15 fractions and 50 Gy in 25 fractions. They demonstrated, in a post-mastectomy setting, hypo-fractionated radiotherapy (HFRT) is comparable to conventional fraction radiotherapy (CFRT) in terms of safety and efficacy and inferred that hypofractionation will be more convenient for patients and care givers and hence can be a routine standard practice. Reference Bhattacharyya, Kalita and Medhi35 A recently published study by Wang et al. compared high-risk breast cancer patients treated with a hypofractionation schedule of 43·5 Gy in 15 fractions given over 3 weeks to a conventional schedule with 820 patients in a single centre in China. Reference Wang, Fang and Song20 They proved non-inferiority of outcome and equivalence of toxicity in the altered fractionation scheme. They did a longer follow-up with median of 58·5 months. A 5-year locoregional recurrence was 8·3% in HFA and 8·1% in the CRA. However, they did not compare dosimetric outcomes of the two fractionation schedules. The 3-year recurrence rate found in this study is 5·8% for hypofractionation and 4·9% in conventional fractionation.
This non-inferior outcome of hypofractionation has placed it as standard of treatment in high-volume centres like the one of ours and also has added benefit of improved patient compliance and overall cost. Rastogi et al. compared the outcomes of 50 Gy in 25 fractions and 42·72 Gy in 16 fractions with a median of 20-month follow-up and concluded that the well-tolerated hypofractionation schedule also exerted statistically non-inferior locoregional control. They inferred that hypofractionation can help in the allotment of more patients in a calendar year while decreasing the waiting list. Reference Rastogi, Jain, Bhatnagar, Bhaskar, Gupta and Sharma36
The major limitation of this study is the short follow-up period. But during an epidemiologic study on breast cancer being conducted in our institute, we found that there was a high degree of patients lost to follow-up after 4–4·5 years of first clinical contact. Hence, we limited our observation to 4 years for better data reliability. However, there is always a scope for larger multi-institutional study with longer follow-up, especially to see the differences on DFS. Another limitation of this study is exclusion of the role of nodal involvement, nodal dissection and radiation dose incident on nodal regions at risk. There are a few data on adequacy of hypofractionated radiation in node-positive breast cancer where axillary dissection was incomplete. In this study, the axillary dissection rate was found to be higher in HFA, though its effect on DFS is undetermined. There is room for multivariate analysis of DFS in altered fractionation in post-mastectomy breast cancer in this regard. This study is also limited as it does not include evaluation of patients with breast prosthesis/implant as there were no patients included in this study who had undergone this procedure.
Conclusion
This study evaluating the effectiveness and acceptability of hypofractionation in LABC has shown that the biologic effective dose in hypofractionation with 40 Gy in 15 fractions is lesser than that of conventional fractionation resulting in lower lung dose as well. But the toxicity events in the two types of radiation were similar, probably owing to comparable V20Gy/ V30Gy volumes of organs at risk. Also there are statistically alike locoregional recurrence event and distant failure rate between the two modalities of radiation both of which were very low. Also the DFS was found to be indistinguishable. But due to the logistic advantage and better patient compliance in hypofractionated radiation, it should be considered as standard of care.
Acknowledgements
None.
Funding
This study was conducted in a Government-funded institution and used institutional protocol for treatment. No external funding was involved.
Conflict of Interests
All authors declare that they have no conflicts of interest.










