Introduction
Hesperantha coccinea (Backh. & Harv.) Goldblatt & J.C. Manning (syn. Schizostylis coccinea Backh. & Harv.) is widely cultivated as an herbaceous ornamental plant and also used in floristry. H. coccinea is a diploid, monocotyledonous flowering plant in the family Iridaceae, subfamily Crocoideae, tribe Croceae (Reeves et al., Reference Reeves, Chase, Goldblatt, Rudall, Fay, Cox, Lejeune and Souza-Chies2001; Goldblatt and Manning, Reference Goldblatt and Manning2008). Originally placed in a separate monospecific genus, Schizostylis, the species is now included in the genus Hesperantha (Goldblatt and Manning, Reference Goldblatt and Manning1996; Goldblatt, Reference Goldblatt2003), containing about 77 species. The principal morphological characters that differentiate H. coccinea from other species in Hesperantha are its short rhizomatous rootstock, thought to be an adaptation to its wet habitat, and red (occasionally pink) flowers, pollinated by Aeropetes tulbaghia. However, these characters were not deemed sufficient to maintain it in a separate genus. Goldblatt and Manning (Reference Goldblatt and Manning1996) suggested that the rhizome was acquired secondarily by loss of the corm and in many other respects, including arrangement and position of style branches, articulated, twisted anthers and chromosome number (x = 13), S. coccinea is nested in Hesperantha.
Popular names for H. coccinea include river lily, scarlet rover lily, crimson flag or Kaffir lily, and in horticulture the generic name Schizostylis is still commonly used. The species is indigenous to southern Africa and is distributed from the Drakensberg Escarpment of Eastern Cape Province to Mozambique. In the northern hemisphere it produces flowers from late summer to early winter (Huxley, Reference Huxley1992). After introduction to warm areas in southern USA and Australia, it has become naturalized and even invasive in some areas.
H. coccinea cultivars can be described by their flower colour, number of flowers per raceme, length of the perianth and other minor morphological characters. However, there are few characters and many similarities between cultivars. Moreover, characters such as flower colour and plant size are not constant across environments and plants do not flower at the same time. Consequently, it is practically impossible to know whether a new cultivar is indeed new and could be awarded Plant Breeders' Rights (PBR).
In addition to multiple names for cultivars, mutation and sporting add another layer of complexity. In general, new cultivars arise through selections within offspring of a segregating cross. However, in some species, sports can arise that differ from the originating cultivar in one or a few characteristics, such as flower colour, e.g. in Chrysanthemum L. (Becher et al., Reference Becher, Steinmetz, Weising, Boury, Peltier, Renou, Kahl and Wolff2000). Sporting generates cultivars that are said to be ‘essentially derived’. Sports are based on changes occurring in buds, usually through chimaeral rearrangements of cell layers that are genetically different, or infrequently because of a mutation in somatic tissue. Even if a mutation is involved in the generation of a new cultivar through sporting, genetic markers generally do not allow discriminating between two cultivars that are essentially derived (e.g. Esselink et al., Reference Esselink, Smulders and Vosman2003), although there are a few studies showing that molecular differences can sometimes be detected between sports (e.g. Wolff, Reference Wolff1996).
Plant breeders can protect their newly developed or selected cultivars through plant patents in the USA, whereas in many other countries, PBR can be obtained. In the EU, cultivars can only be awarded PBR grants if they are distinct, uniform and stable (DUS). Cultivated plants not requiring DUS trials may be registered with an International Cultivar Registration Authority (ICRA), but this does not confer any legal protection over the name or plant. The chief aim is to prevent the duplicated use of cultivar and group epithets within a defined denomination class, and to ensure that names are in accord with the latest edition of the International Code of Nomenclature for Cultivated Plants (see Brickell et al., Reference Brickell, Baum, Hetterscheid, Leslie, McNeill, Trehane, Vrugtman and Wiersema2004). However, it is still possible that multiple names may be given to what appears to be the same cultivar, and often there is an additional marketing name. To solve this confusion, DNA fingerprinting and a database can be a useful tool to determine which cultivars are genetically distinguishable, which are likely to be sports, and at the same time help in protecting plants with PBRs against illegal propagation and sale.
Microsatellites are highly variable codominant markers that are ideal for identification purposes, such as cultivar identification (Weising et al., Reference Weising, Nybom, Wolff and Kahl2005). A disadvantage of microsatellites is that they need to be developed for every species, because cross-species amplification is often limited (Weising et al., Reference Weising, Nybom, Wolff and Kahl2005, but see Hale et al., Reference Hale, Borland and Wolff2005). For this reason, other generally applicable markers, such as randomly amplified polymorphic DNAs and amplified fragment length polymorphism (AFLPs), have been used for horticultural species that are not highly commercial (e.g. Pharmawati et al., Reference Pharmawati, Yan and Finnegan2005; Lubell et al., Reference Lubell, Brand and Lehrer2008). However, there are now also a number of garden species that have been studied using microsatellites, such as New Guinea Impatiens (Parks et al., Reference Parks, Moyer and Lyerly2006), pampas grass (Cortaderia, Ahmad et al., Reference Ahmad, Okada, Firestone, Mallek and Jasieniuk2006) and garden roses (Rosa, Scariot et al., Reference Scariot, Akkak and Botta2006).
The objectives of this research were to develop microsatellite markers for H. coccinea for DNA fingerprinting and to investigate whether a database can be created to assist cultivar comparison and identification. Molecular markers may also shed light on whether sporting has given rise to new cultivars. Although H. coccinea can reproduce asexually from rhizomes, new cultivars will generally arise through sexual reproduction, which may even happen in suitable garden environments (Kennedy and Wolff pers. obs.). In addition, the microsatellite markers may provide insight into the breeding system of the species and cultivars in cultivation as well as in their natural habitat.
Materials and methods
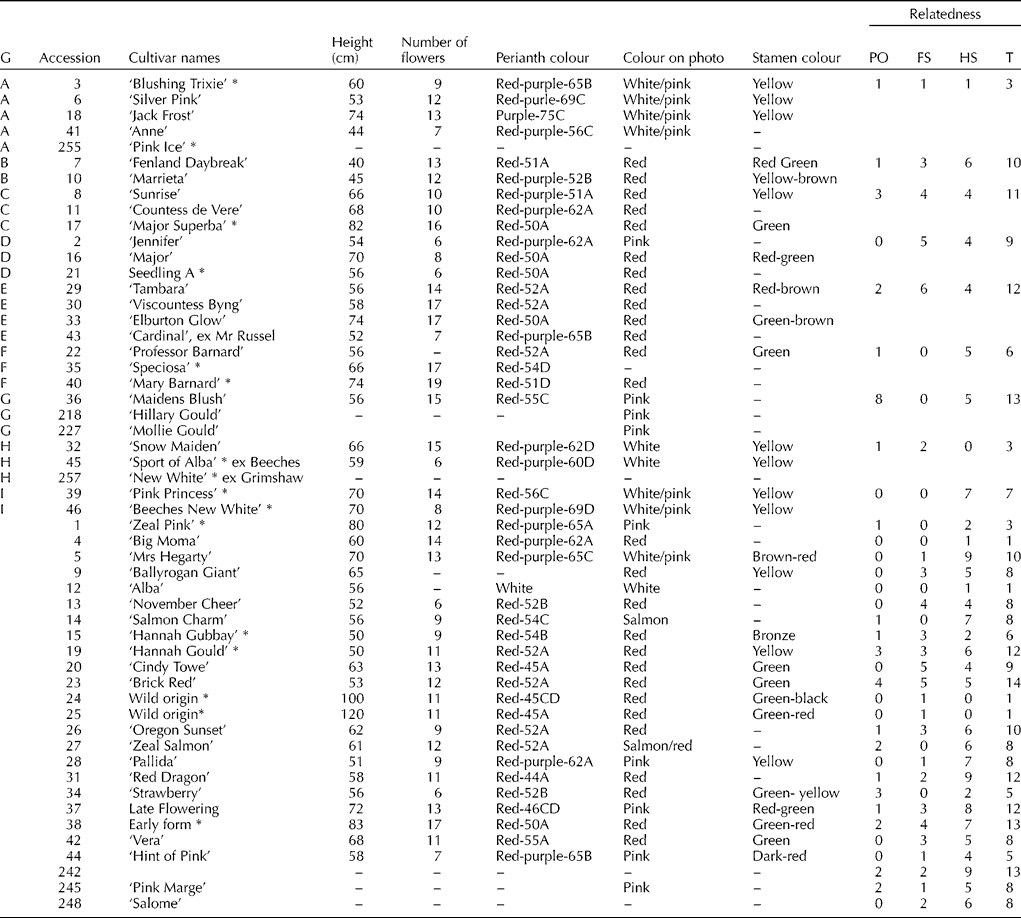
The ICRA for Hesperantha is the Royal General Bulb growers' Association in the Netherlands and the ICRA group is ‘Bulbous, cormous and tuberous-rooted ornamental plants’. However, in the UK, the National Council for the Conservation of Plants and Gardens (NCCPG) has two National Collections of the species, one in Devon and one in SW Scotland, to ensure that the diversity within the species is maintained. Material of 53 accessions was obtained from the NCCPG National Collection, formerly held by Mr A. Kennedy, now by Mr T. Ewing in Newton Stewart, Dumfriesshire (Scotland). There are 43 cultivars listed in the 2008 RHS Plantfinder (Lord et al., Reference Lord, Armitage, Cubey, Edwards, Lancaster and Merrick2008), of which 40 were included in the current study (Table 1). Accession numbers 24 and 25 are plants obtained from a breeder in Devon, and originally collected by Grimshaw and Linden (collection number 144C) from South Africa, Eastern Cape region. Several phenotypic characters were recorded from plants growing at the National Collection site in Scotland, including flower colour, height and number of flowers (Table 1). The RHS Colour Chart was used to describe the perianth colour when making herbarium vouchers. Voucher specimens made for the accessions used in this study are deposited in the herbarium at the Royal Botanic Garden Edinburgh (E).
Table 1 Accessions of Hesperantha coccinea grouped by genotype (G), with some of their phenotypic characteristics and their level of relatedness with other accessions

PO, parent–offspring; FS, full sib; HS, half sib; T, total over all relationships. The level of relatedness is the total number of other accessions with which a PO, FS, HS relationship was likely for that particular genotype. The cultivars indicated with * are not listed in the 2007/08 RHS Plantfinder (Lord et al., Reference Lord, Armitage, Cubey, Edwards, Lancaster and Merrick2008).
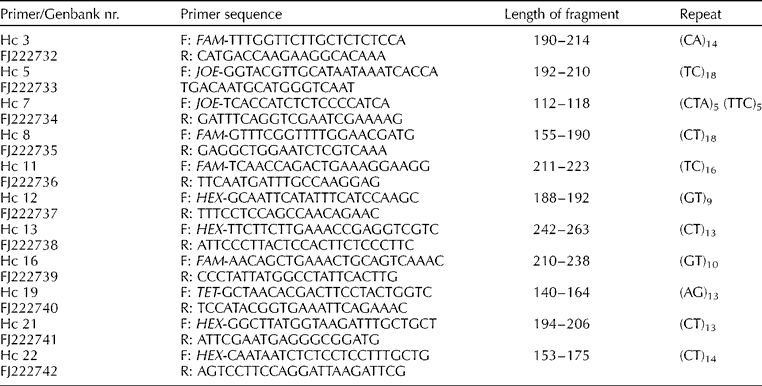
For each cultivar, leaf material was collected from a single plant and dried in silica gel. DNA was extracted using a CTAB method (Weising et al., Reference Weising, Nybom, Wolff and Kahl2005). A dinucleotide and a trinucleotide-enriched library was constructed using a filter hybridization and enrichment method modified from Edwards et al. (Reference Edwards, Barker, Daly, Jones and Karp1996) and Hale et al. (Reference Hale, Bevan and Wolff2001). Genomic DNA was extracted from Hesperantha coccinea accession ‘Major’ and digested with Mbo I. The resulting DNA fragments were then ligated to SauL linkers and amplified via PCR cycling using SauL A. The SauL linker is composed of the SauLA and SauLB oligos, 5′ GCG GTA CCC GGG AAG CTT GG 3′ and 5′ GAT CCC AAG CTT CCC GGG TAC CGC 3′, respectively. Fragments were enriched by hybridizing DNA against 1 cm2 pieces of Hybond® N+ membranes on which 10 μg of [CA]15 and [GA]15 or [ATG]8 and [AAG]8 had been fixed. Bound DNA was eluted with H2O by heating to 98°C, and amplified via PCR. This enrichment process was repeated once. Fragments were cloned into the vector Ready-To-Go™ pUC18 BamH I/BAP (Amersham Pharmacia Biotech, GE Healthcare, Little Chalfont, Buckinghamshire, UK) and transformed into competent Escherichia coli cells (Qbiogene Inc., MP Biomedicals, Irvine, CA, USA). Resultant colonies, with inserts greater than 250 bp, were identified using PCR with M13 primers and gel electrophoresis. M13-amplified inserts were sequenced using the BigDye Terminator Ready Reaction kit v3.0 or v3.1 (Applied Biosystems, Forster City, CA, USA) and subsequently analyzed on an ABIPrism® 3100 genetic analyser.
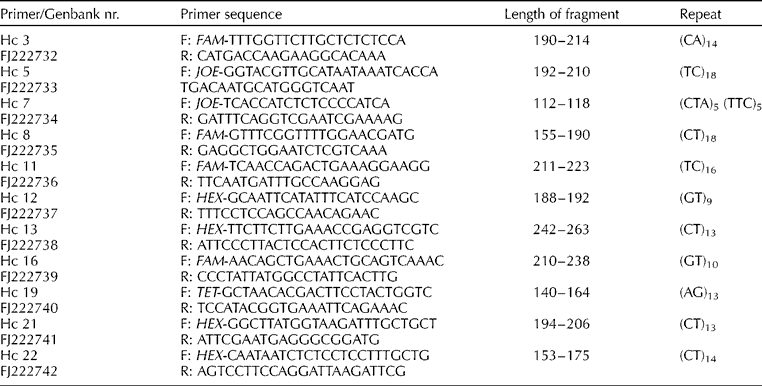
Primers were designed for sequences containing a microsatellite region with six or more repeats using Primer3 for amplification of the microsatellite region (Rozen and Skaletsky, Reference Rozen, Skaletsky, Krawetz and Misener2000). Fluorescently labelled forward primers were used to detect the size of the amplified fragments (see Table 2). The loci were amplified in 10 μl reactions containing PCR buffer (16 mM (NH4)2SO4, 67 mM Tris–HCl and 0.01% Tween-20), 200 μM each dNTP, 2 mM MgCl2, 1 pmol of each primer, 0.5 U Taq DNA polymerase (Bioline, London, UK) and approximately 2 ng DNA. Fluorescently labelled primers were used to amplify the loci on a MJ Research PTC100 thermocycler: an initial denaturing step of 95°C (5 min) was followed by 20 cycles at 95°C (15 s), 52°C (15 s) and 72°C (15 s), then 15 cycles of 89°C (15 s), 50°C (15 s) and 72°C (15 s) followed by a final extension at 72°C for 30 min. PCR fragments were analyzed on an ABI3100 Gene Analyser, using GENESCAN software (Applied Biosystems) with ROX500 (Applied Biosystems) as size standard.
Table 2 Microsatellite primers developed for Hesperantha coccinea and their characteristics
Diversity estimates, number of alleles as well as observed and expected heterozygosity were calculated using Genalex (Peakall and Smouse, Reference Peakall and Smouse2006). This program was also used to detect identical genotypes (multi-locus matching) and to perform a principal coordinate analysis (PCA) for visualization of genetic distances between cultivars. Genetic distances were calculated using Genalex, following Smouse and Peakall (Reference Smouse and Peakall1999). Arlequin was used to detect linkage disequilibrium (LD) and deviation from Hardy Weinberg equilibrium, using a Markov Chain exact test, with 1,000,000 chain and 10,000 dememorization steps (Excoffier et al., Reference Excoffier, Laval and Schneider2005). Relatedness of accessions was estimated using the computer program ML Relate (Kalinowski et al., Reference Kalinowski, Wagner and Taper2006). This program uses microsatellite genotypic data to estimate the genealogical relationship or relatedness between individuals of unknown ancestry, following a maximum likelihood method.
Results
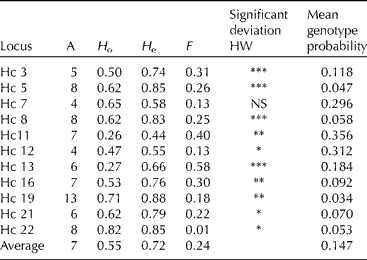
The enrichment process was successful with 70% of the clones from the dinucleotide-enriched library and 50% of the clones from the trinucleotide library containing a microsatellite region with at least six repeats. Primers were designed for 25 sequences. Out of those 25 primer pairs, three amplified a monomorphic band, four did not amplify a product, seven gave a complex pattern that was deemed too difficult to score reliably, while 11 were polymorphic, amplifying reliably and with a clear, scoreable pattern. The level of polymorphism was considerable, with on average seven alleles per locus (range 4–13; Table 3). Genotypes of the accessions are available on request.
Table 3 Diversity measures for 11 microsatellite primers in 34 different Hesperantha coccinea genotypes
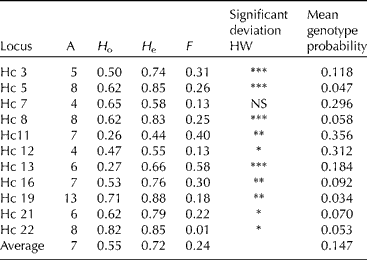
A, number of alleles detected; H o, observed heterozygosity; H e, expected heterozygosity; F, the inbreeding coefficient. The significance of the deviation from Hardy Weinberg is indicated as ***P < 0.001, **P < 0.01, *P < 0.05. Also, the probability obtaining a certain genotype, as average over the genotypes, per locus and in total is presented.
Multi-locus matching showed that among the 53 accessions tested, there were nine groups of two to five accessions with an identical multi-locus genotype, named A–I (Table 1). Flower colour was very similar within each genotype (clone), with for example white/light pink accessions largely grouping together in genotypes A, H and I. However, in group D, two accessions were red (Major and Seedling A) and one was pink (‘Jennifer’). Other characters, such as height, were in some cases similar within genotypes (genotype B and I), but rather different for others (e.g. genotype A). The number of flowers was low in genotype D, high in F, but rather mixed in genotypes A and E. The remaining 25 accessions all had unique genotypes that differed for two or more loci from other accessions. The accessions with an identical genotype are likely to be clones of each other, or may be essentially derived (sports). In subsequent genetic analyses, only one accession per genotype was used to prevent unequal weighting. This reduced the number of samples to 34 unique genotypes, each differing from every other genotype for at least two loci.
Results from population genetic analyses (below) must be treated with caution as the collection studied here is not sourced from a randomly breeding population, but has originated from different breeders who may have had starting material from various wild populations or plants that have been cultivated for many decades. The average expected heterozygosity across all accessions was 0.72 (range 0.55–0.88; Table 3). All loci, except Hc 7, were not in HW equilibrium (P < 0.05), with all of those loci showing a heterozygote deficit. This can be explained by non-random mating (population structure), null alleles and/or a (partly) selfing mating system. This collection of accessions is not a randomly mating population, and therefore this can explain at least part of the deficit of heterozygotes. For one locus, Hc 13, a null allele has been detected, because one accession had a homozygote null genotype for that locus. The current data do not allow us to exclude null alleles at low frequencies for the other loci. However, it is also likely that some degree of selfing occurs as plants bear viable seeds even when there are no other plants around for fertilization. It must be noted that the presence of null alleles is not a significant problem when the markers are used for cultivar identification purposes, but could potentially be a problem for population genetic studies.
Markers were tested for LD, and it appeared that many loci are in LD. Out of 110, 106 possible pairs were in LD at the 5% level, and 102 out of 110 at the 1% level. This result can be expected when dealing with accessions that are not a random-mating population as they originate from various, genetically different, garden locations and wild populations.
The probability of obtaining a particular genotype due to chance for each of the markers and as an average over all markers, based on allele frequencies in the accessions tested, is shown in Table 3. Using all 11 markers together, the probability of obtaining a certain multi-locus genotype by chance alone was 0.9 × 10− 11, as an average over all genotypes (range 1 × 10− 9 to 1 × 10− 22). As an example, the probability of obtaining the genotype of group D by chance is very small, namely 4 × 10− 11, assuming random mating. The probability of obtaining a particular genotype, as an average over all genotypes, using the most informative markers would be 0.002 with only Hc19, a probability of 1 × 10− 4 with Hc19 and Hc5, and a probability of 1 × 10− 5 with Hc19, Hc5 and Hc22. To distinguish all accessions, except accessions D and 20, only a subset of the four most informative markers (Hc19, Hc5, Hc22 and HC8) is needed.
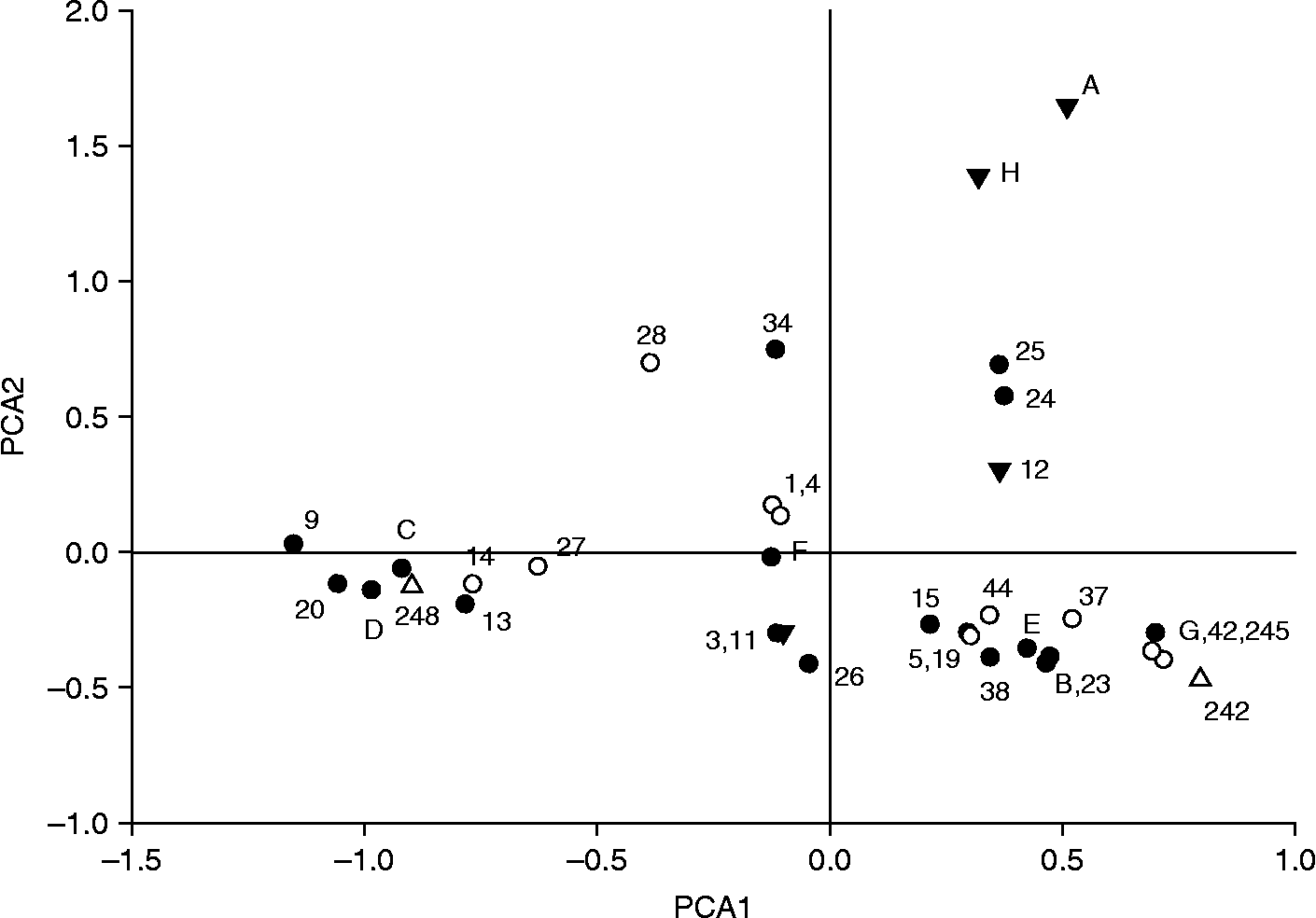
The genetic similarity of cultivars can be visualized by PCA analysis and by construction of a dendrogram. Both methods were used for the current dataset and the implications from both methods were similar. We present only the result of a PCA analysis because it best depicts the genetic similarities, and because it is more appropriate as the accessions do not all share recent genealogical ties. PCA analysis, based on covariance, showed that the first three axes explained a large proportion of the variation, namely a total of 69% (29, 23 and 17% for the first three axes). Figure 1 shows a scatterplot of the accessions in relation to axes 1 and 2. Some clear groupings were observed, for example, accessions 24 and 25, both from a breeder in Devon and collected from a wild population from the Eastern Cape Province in South Africa, are very near each other in the graph, and separated from the other accessions in the UK. These two wild accessions have rather different genotypes from the other accessions, with unique alleles for four out of the 11 loci, and they also stand out phenotypically as they are much taller than all other accessions. Also, genotypes A and H take up a separate position, close together, with the only genotypic difference being five loci for which H is heterozygous, while A is homozygous for those loci. The other accessions largely form two larger clusters along axis 1. The symbols for the accessions indicate their flower colour, red, white, pink/salmon, and it is clear that accessions with a particular colour do not all group together. Similarly, accessions did not group together in the PCA for other characteristics, such as flower size, flower number or stamen colour.
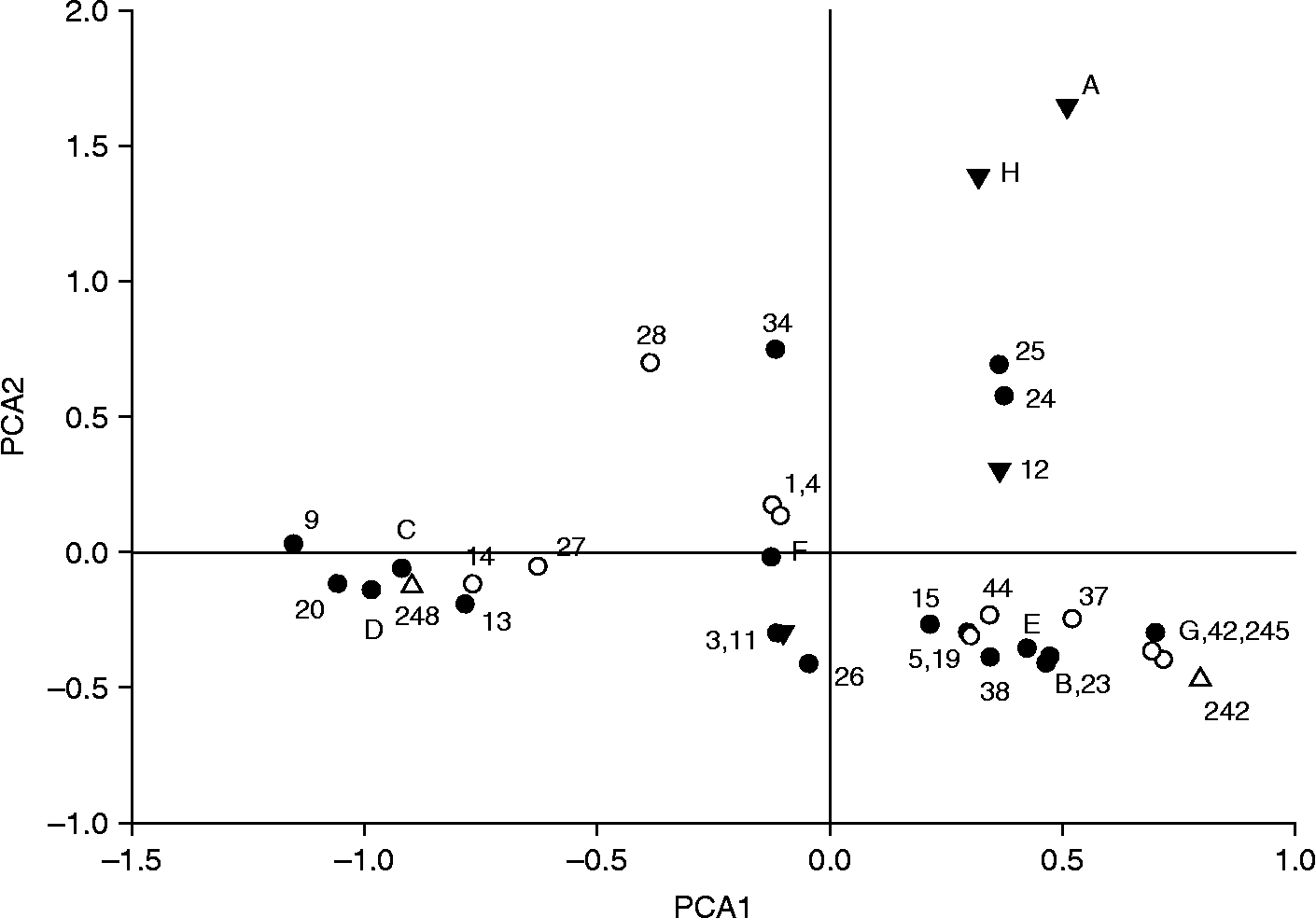
Fig. 1 Distribution of Hesperantha coccinea genotypes in a scatterplot of axes 1 and 2 of a principal coordinate analysis (PCA), accounting for 52% of the total variation in the dataset. The symbols denote the flower colour of the accession, with closed circles being red, open circles being salmon and pink, closed triangles being white or very light pink and open triangles accessions with unknown flower colour.
ML Relate determines whether any pair of accessions is likely to be related as half sibs, full sibs or as parent–offspring, and which of those three relationships is the most likely. On average half sibs share a quarter of their alleles, whereas with full sibs and parent–offspring relationships half of the alleles are shared. In addition, parents and offspring must have at least one allele in common for every locus. Accessions that are part of a genealogically closely related group of accessions will have a large number of likely relationships (Table 1). For example, accession 23, ‘Brick Red’, has 14 possible genealogical ties (half sib, full sib or parent–offspring) with other accessions tested, and G, 38 (‘Early form’) and 242 each have 13 possible ties with other accessions. At the other end of the spectrum, the two accessions obtained from the wild (24 and 25) are only related to each other, as full sib or as parent offspring, but they have no ties with other accessions.
Discussion
DNA fingerprinting using microsatellites showed high polymorphism in H. coccinea, making the markers ideal tools for cultivar identification in the species. The level of polymorphism observed, with average H e of 0.72, indicates that a relatively large number of plants are likely to have been collected from the original South African populations, in one or more collection trips. The high level of polymorphism is also partly due to its breeding system, which we can presume to be mixed mating. From observations of seed set in isolated plants, it seems likely that selfing is possible. However, considering the level of variation (H e) and the level of observed heterozygosity (0.55), it is most likely that the species reproduces predominantly through outcrossing. However, the interpretation of statistics such as H o and H e must be interpreted with care since the accessions analyzed are not a randomly mating population. Using the equation: outcrossing rate t = (1 − F)/(1+F), the average inbreeding coefficient (F) of 0.24 indicates an outcrossing rate of 61%, (Wright, Reference Wright1969; Rousset and Raymond, Reference Rousset and Raymond1995). However, as explained above, this must be interpreted with great care.
The level of molecular marker variation seen in H. coccinea is higher than seen in some other garden species. For example, in New Guinea Impatiens, there are 7 loci out of 14 that are polymorphic, with 2–6 alleles per locus (Parks et al., Reference Parks, Moyer and Lyerly2006). On the other hand, variation appears to be higher in roses, where for Rosa multiflora Thunb., an average of 8.4 alleles per locus (Kimura et al., Reference Kimura, Nishitani, Iketani, Ban and Yamamoto2006) and for old garden roses 13.7 alleles per locus (Scariot et al., Reference Scariot, Akkak and Botta2006) were found. For the old garden roses, this probably reflects the broad genetic basis (7 botanical sections and 13 horticultural groups) from which these roses are derived (Scariot et al., Reference Scariot, Akkak and Botta2006).
The 11 microsatellite loci showed that there were nine genotypic sets, each with two to five accessions sharing an identical multi-locus genotype. Flower colour within each genotype is extremely similar, with one exception, namely group D. Other characters, such as height and number of flowers, were variable within many genotype groups. It is currently impossible to know for certain whether cultivars with identical genotypes simply have more than one name or whether they are essentially derived from each other. Extensive analysis of the stability and distinctness of morphological characters is required to assess this. In group D, two cultivars are red and one is pink. This is either caused by sporting or identity by chance. Considering that the probability of obtaining the genotype of group D by chance is 4 × 10− 11, it seems likely that sporting has occurred in this case. This is the first evidence that sporting may occur in this species. The high level of variation of the markers makes it unlikely that those with an identical 11-locus genotype are actually genetically different and could have been distinguished with a larger number of markers. This is because the current set of markers gives an average of identity by chance of 0.9 × 10− 11, and each genotype pair has a different genotype for at least two loci.
Both PCA analyses and clustering (dendrograms) can show how similar or different cultivars within a species are, although it is more common to use dendrograms. For example, in New Guinea Impatiens, AFLPs and microsatellites were equally valuable for cultivar identification, but it was clear that AFLPs showed clustering of cultivars by breeder, whereas microsatellites did not group cultivars that were genealogically related (Parks et al., Reference Parks, Moyer and Lyerly2006). In ornamental pampas grass, Cortaderia selloana, a UPGMA cluster analysis of microsatellite data grouped cultivars corresponding to origin and morphological characteristics (Ahmad et al., Reference Ahmad, Okada, Firestone, Mallek and Jasieniuk2006). Also, in old garden roses, a dendrogram constructed by cluster analysis grouped the genotypes into seven major clusters that were consistent with the generic sections and horticultural groups to which they had been assigned (Scariot et al., Reference Scariot, Akkak and Botta2006).
Here, we have used a PCA to visualize genetic similarity. The groupings may reflect a common origin of accessions, which in many cases is difficult to confirm from historical records. It is likely that cultivars that originated from one breeder or garden have a common genetic background. This is the most likely explanation for the position of the two accessions from the wild, separate from the other cultivars. The ML determination of significant relatedness between cultivars, at half sib, full sib or parent–offspring level gives a more detailed picture of relatedness than the PCA.
The easiest to analyze were those accessions that have few significant relationships among those analyzed. Accession 4, ‘Big Moma’, has possibly only one half sib relationship with any of the other accessions. Also, accession 12, ‘Alba’, is only possibly related to accession 1, ‘Zeal Pink’, as a half sib. Other white (or very pale pink) accessions, e.g. A and H group, seem not to have many sib or parent–offspring relationships with most of the accessions tested here, i.e. they form a separate group.
Related to the A and H group is accession 34 (‘Strawberry’), which has a potential parent–offspring relationship with A, C and H. On closer inspection, it seems that C is very likely to be one of its parents, because Strawberry has at least one allele at each locus identical to C, and those alleles are absent from A and H. Accession groups A and H are equally likely to be the other parent of accession 34 (Strawberry). Accession 34 has a red flower, as does C (its putative parent), whereas the other possible parent (A or H) is almost colourless. This would be expected, as the allele for red flowers is likely to be dominant over the alleles for white or lightly coloured flowers, because being white or colourless generally is caused by the absence of an enzyme producing a pigment.
Accession 45, ‘Sport of Alba’, is not a sport of accession 12, Alba, as the genotypes are quite different, but it could be identical to, or a sport of, accession 32, ‘Snow Maiden’. Straley (Reference Straley1989) states that the oldest cultivar is accession 5, ‘Mrs Hegarty’, and was among the red forms in Ireland in the early 1900s. However, this cultivar is only possibly a full sib of accessions in group B, so does not seem to be basic to a large number of current cultivars.
Major (syn ‘Gigantea’) is said to be similar to wild-type red forms, but with larger and brighter flowers. Accession 14, ‘Salmon Charm’, is claimed to be a sport of Major, but their genotypes do not reflect this: they are different. However, accession 14, Salmon Charm, is possibly related to accession group C as parent–offspring. Therefore, it is likely that a seed of Major, resulting from outcrossing, fell and germinated near the original plant and gave rise to Salmon Charm, instead of it seemingly being a sport.
‘November Cheer’ (accession 13) is claimed to be a sport of Major or a hybrid with a pink cultivar (Straley, Reference Straley1989). However, November Cheer (13) is not a sport of C or D as it differs at many loci from C and D, but it may be a full sib of either C (includes ‘Major Superba’), D (includes Major) and/or with accession 9 (‘Ballyrogan Giant’) or accession 20 (‘Cindy Towe’). ‘Tambar’ was named in 1970 from plants collected in Zimbabwe [Rhodesia], and is in group E. It could be one of the cultivars basic to many others. However, accession group G seems to be more crucial to other cultivars in the set as it has a possible parent–offspring relationship with nine other cultivars or cultivar groups, namely, B, E, F, 1, 15, 19, 23, 26 and 28. The two accessions collected from the wild, 24 and 25, are clearly genetically different from the remainder of the group accessions tested, and, therefore, the population where accessions 24 and 25 were collected is not a likely source of any of the current cultivars.
The markers can now be used to investigate which geographic regions gave rise to the current cultivars. The morphological measurements taken from the herbarium samples showed that the cultivated accessions were morphologically rather diverse. However, stronger evidence from wild-collected plants would be needed to suggest whether the species could be split up into subspecies, or that hybridization with related taxa has given rise to the great diversity in cultivated accessions in flower colour and shape. For example, hybridization with Hesperantha baurii Baker, which has pink flowers, and Hesperantha erecta Benth & Hook.f. and Hesperantha cucullata Klatt, which have a white or cream-coloured perianth, seems unlikely, because in the wild they do not flower at the same time and also the stature of white or pink cultivars is typical of H. coccinea and not of the other three related species. In addition, the genetic markers do not indicate that the current group of cultivars is heterogeneous: the accessions with white, red, pink and salmon-coloured flower forms do not form separate groups in the PCA. Therefore, there is currently no indication of subspecies distinction or hybridization with other species. To obtain a fuller picture of cultivated H. coccinea, all existing cultivars and samples from the wild, from various populations and closely related species, need to be analyzed.
In conclusion, the level of polymorphism in H. coccinea is high, even though the total number of known cultivars is less than one hundred. The markers developed allowed us to reliably identify different genotypes and several cultivars with different names appeared to have the same multilocus genotype. The markers will be useful to further describe variation and relatedness in H. coccinea cultivars and plants collected from the wild.
Acknowledgements
We would like to thank Mr Alan Kennedy and Mr Tim Ewing for materials and their valuable discussions. We are grateful to Dr James Cullen and the Stanley Smith (UK) Horticultural Trust as well as the NCCPG Dumfries and Galloway Group for funding. We would also like to acknowledge the Royal Botanic Garden Edinburgh for allowing the Edinburgh authors time to collect and process the voucher material and Ms Susyn Andrews for her valuable comments on the manuscript. In addition, we are very grateful for two reviewers and an editor, whose comments have greatly improved the quality of the manuscript.