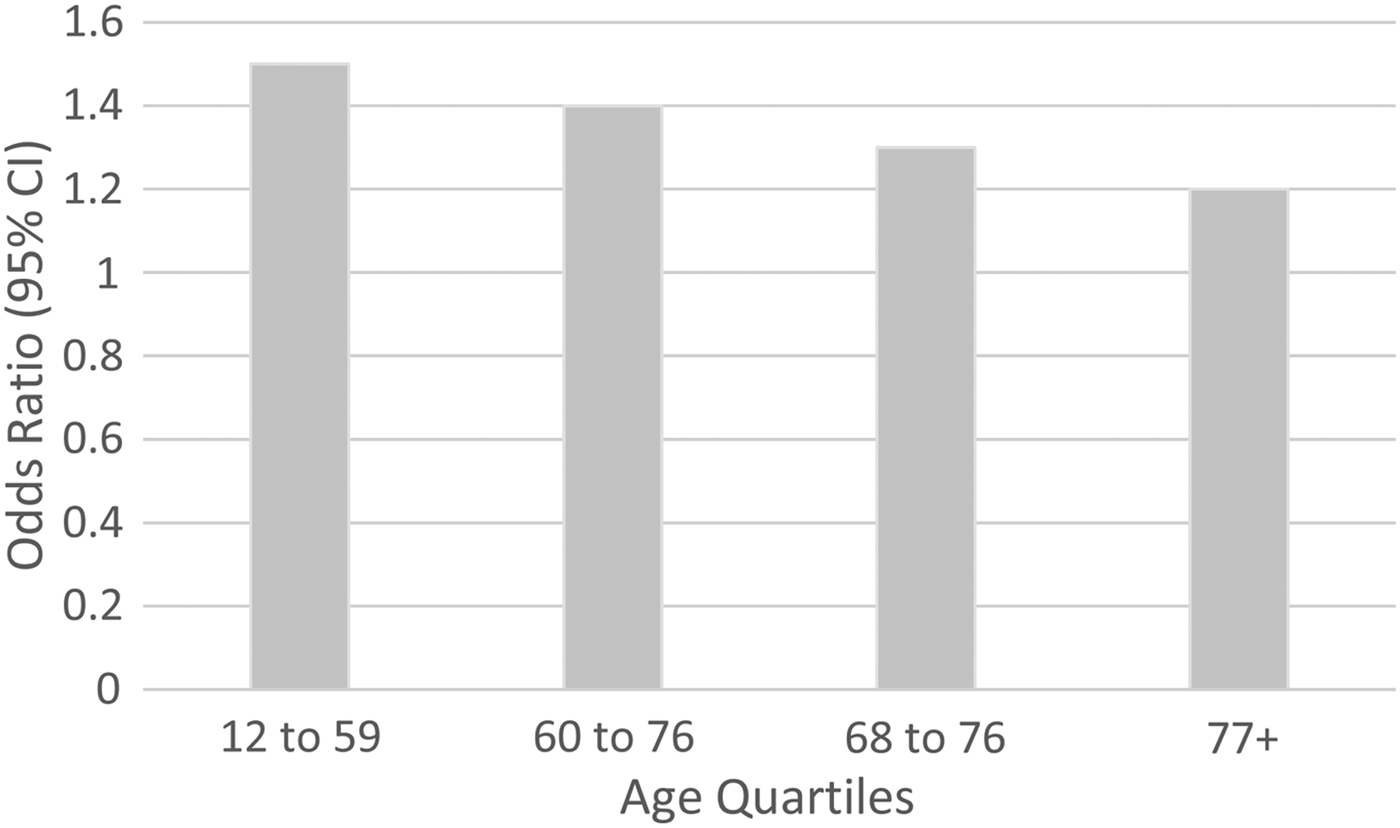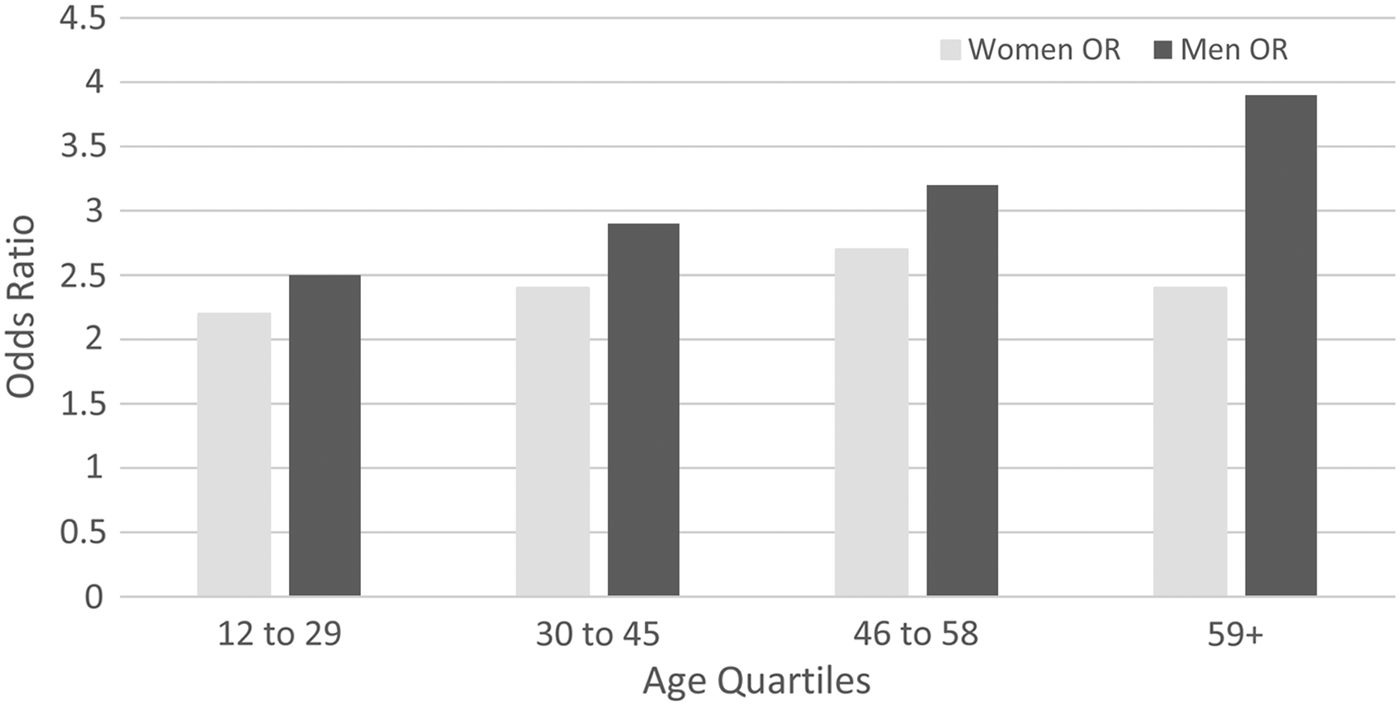Background
The association of major depressive episodes (MDE) with chronic medical conditions has been replicated in several population studies (Wells et al. Reference Wells, Goldin and Burnam1988; Moldin et al. Reference Moldin, Scheftner, Rice, Nelson, Knesevich and Akiskal1993; Patten, Reference Patten1999; Gagnon & Patten, Reference Gagnon and Patten2002). Access to very large datasets has allowed two studies to assess the strength of association between various medical conditions and MDE using probability samples (Patten et al. Reference Patten, Beck, Kassam, Williams, Barbui and Metz2005; Scott et al. Reference Scott, Bruffaerts, Tsang, Ormel, Alonso, Angermeyer, Benjet, Bromet, De Girolamo, De Graaf, Gasquet, Gureje, Haro, Kessler, Levinson, Mneimheh, Oakley Brown, Posada-Villa, Stein, Takeshima and Von Korff2007). These two studies produced generally consistent results; the strongest associations were for painful conditions (e.g., multiple pains, fibromyalgia, headaches, back pain), intermediate associations were found for conditions prominently characterised by inflammation (e.g., arthritis, asthma, heart disease) and weaker associations were observed for other conditions such as cancer, diabetes and hypertension. These associations likely arise from a complex interplay of personal vulnerabilities, the psychosocial challenges of coping with the illnesses and biological effects.
A more detailed understanding of these associations has been elusive. For example, whereas some studies have reported age and sex adjusted analyses, (Patten et al. Reference Patten, Beck, Kassam, Williams, Barbui and Metz2005; Scott et al. Reference Scott, Bruffaerts, Tsang, Ormel, Alonso, Angermeyer, Benjet, Bromet, De Girolamo, De Graaf, Gasquet, Gureje, Haro, Kessler, Levinson, Mneimheh, Oakley Brown, Posada-Villa, Stein, Takeshima and Von Korff2007) it is conceivable that the associations are modified by age and sex, a possibility that has not generally been evaluated. Also, since younger age is a risk factor for MDE, confounding effects of age and sex may differ depending on the age of onset of various medical conditions. Detailed investigation of these patterns has been impeded by the limited sample sizes of individual surveys. The goal of the current study was to overcome these challenges by meta-analytic pooling of data from multiple nationally-representative surveys.
Systematic reviews of chronic condition-MDE associations have generally not used meta-analytic techniques because they have needed to rely on a heterogeneous literature of published studies (e.g., Pincus et al. Reference Pincus, Burton, Vogel and Field2002; Hackett & Anderson, Reference Hackett and Anderson2005; Radat & Swendsen, Reference Radat and Swendsen2005; Renn et al. Reference Renn, Feliciano and Segal2011; Roy & Lloyd, Reference Roy and Lloyd2012; Walker et al. Reference Walker, Holm Hansen, Martin, Sawhney, Thekkumpurath, Beale, Symeonides, Wall, Murray and Sharpe2013; Lacey et al. Reference Lacey, Salzberg and D'souza2015). For a few individual conditions, meta-analyses have been conducted. For example, one systematic review used meta-analysis to pool the results of 12 studies of migraine and depression, reporting a pooled odds ratio of 2.2 (Antonaci et al. Reference Antonaci, Nappi, Galli, Manzoni, Calabresi and Costa2011). However, it was not possible to use age- and sex-specific estimates as these were not consistently reported in the constituent studies. A systematic review of the epilepsy literature encountered similar limitations (Fiest et al. Reference Fiest, Dykeman, Patten, Wiebe, Kaplan, Maxwell, Bulloch and Jette2013). In a systematic review of the rheumatoid arthritis literature, Matcham et al. (Reference Matcham, Rayner, Steer and Hotopf2013) attempted to examine the roles of age and sex but without access to individual-level data could only do so by examining the mean age of participants in the various studies. A pooled analysis of World Mental Health survey data (Scott et al. Reference Scott, Bruffaerts, Tsang, Ormel, Alonso, Angermeyer, Benjet, Bromet, De Girolamo, De Graaf, Gasquet, Gureje, Haro, Kessler, Levinson, Mneimheh, Oakley Brown, Posada-Villa, Stein, Takeshima and Von Korff2007) incorporated age and sex adjustments but did not evaluate effect modification nor the issue of age or sex confounding, e.g., through comparison of adjusted and unadjusted estimates. Interactions of race and ethnicity have been examined more often than those for age and sex, for examples see review of cardiovascular risk studies by Assari (Reference Assari2016). Investigating a related topic, Assari & Lankarani (Reference Assari and Lankarani2015) found an association between obesity and weight-loss intention that was stronger in women than men.
The literature associating various chronic medical conditions with depression has used a variety of measures and concepts of depression (e.g., symptom scales, diagnostic measures), contributing to heterogeneity in this literature. For example, Antonaci et al.’s (Reference Antonaci, Nappi, Galli, Manzoni, Calabresi and Costa2011) review of migraine studies found prevalence estimates for depression ranging from 3.4 to 24.4% (Antonaci et al. Reference Antonaci, Nappi, Galli, Manzoni, Calabresi and Costa2011). The study by Fiest et al. (Reference Fiest, Dykeman, Patten, Wiebe, Kaplan, Maxwell, Bulloch and Jette2013) found estimates in epilepsy ranging from 4.1 to 36.5%. However, in addition to measurement, there are other sources of heterogeneity in this literature. The rheumatoid arthritis review by Matcham et al. (Reference Matcham, Rayner, Steer and Hotopf2013) reported significant heterogeneity even across studies using the same instruments. Overall, prevalence estimates were found to range from 0.04 to 66.3%. These wide ranges illustrate the importance of using individual-level data from methodologically homogeneous studies in representative samples.
The objective of this study was to use available datasets from large population surveys to describe the strength of association between MDE and a set of chronic medical conditions, delineating for the first time potential modifying or confounding roles of age and sex.
Methods
This study used data from 11 population-based surveys arising from three linked sources, all with the Canadian household population as their target population: (1) a general health survey called the National Population Health Survey (NPHS) (Statistics Canada, 2012), (2) general health cycles of the Canadian Community Health Survey (CCHS) (Beland, Reference Beland2002) and (3) two mental health–focused iterations of the CCHS (CCHS 1.2 and CCHS-MH) (Pearson et al. Reference Pearson, Janz and Ali2014). The NPHS produced suitable cross-sectional datasets in 1996 and 1998, both using the Composite International Diagnostic Interview Short Form for Major Depression (CIDI-SFMD) (Kessler et al. Reference Kessler, Andrews, Mroczek, Ustun and Wittchen1998), a brief validated (Patten et al. Reference Patten, Brandon-Christie, Devji and Sedmak2000) predictive interview for past-year MDE. The CCHS is a survey program that started in 2001 with a general health survey called the CCHS 1.1. The program has since produced six additional survey datasets. These include the CCHS 2.1 (conducted in 2003), CCHS 3.1 (conducted in 2005), three additional surveys named after 2-year periods in which their data were collected: CCHS 2007/2008, CCHS 2009/2010 and CCHS 2011/2012, and finally a CCHS survey conducted in 2013. These surveys also included the CIDI-SFMD, but only as optional content. This means that the instrument was included in some, but not all, of the ten Canadian provinces in each survey. Finally, there have been two national mental health–focused CCHS surveys, each of which included a Canadian adaptation of the WHO Mental Health CIDI (WMH-CIDI), a structured, validated (Haro et al. Reference Haro, Arbabzadeh-Bouchez, Brugha, De Girolamo, Guyer, Jin, Lepine, Mazzi, Reneses, Vilagut, Sampson and Kessler2006) full-length diagnostic interview designed for use in epidemiological studies (Kessler & Ustun, Reference Kessler and Ustun2004). These surveys were conducted in 2002 (Gravel & Béland, Reference Gravel and Béland2005) and 2012 (Patten et al. Reference Patten, Williams, Lavorato, Wang, Mcdonald and Bulloch2014; Pearson et al. Reference Pearson, Janz and Ali2014). The eligible age range for the two mental health surveys was 15+, but the other surveys included respondents aged 12+. All of the CCHS surveys used independent samples, so the chance of individual people being in multiple samples is low.
All of these surveys collected data about professionally diagnosed long-term medical conditions. The relevant module started with ‘Now I'd like to ask about certain chronic health conditions which you may have. We are interested in ‘long-term’ conditions which are expected to last or have already lasted 6 months or more and that have been diagnosed by a health professional.’ This was followed by questions about specific conditions such as ‘Do you have epilepsy?’ Conditions that were consistently covered and therefore included in the current analysis were: high blood pressure, cancer, (effects of a) stroke, arthritis, cataracts, chronic obstructive pulmonary disease, diabetes, epilepsy, heart disease, migraine and thyroid disease.
The analysis used methods of individual-level meta-analysis, adopting a ‘2 stage’ approach following the terminology of Thomas et al. (Reference Thomas, Radji and Benedetti2014). Rather than creating a large, pooled dataset, the role of age and sex was explored within individual surveys by deriving unadjusted as well as age and/or sex stratified or adjusted estimates and/or estimates of regression coefficients for interaction terms. Relevant parameters were then pooled, hence the description as a ‘two stage’ strategy (Rao et al. Reference Rao, Graubard, Schmid, Morton, Louis, Zaslavsky and Finkelstein2008). The motivation for this ‘two-stage’ approach (rather than a pooled analysis, which would be a ‘one stage’ approach) was driven by the complex sampling procedures used in the surveys. To deal with design effects (clustering, stratified sampling) replicate bootstrap sampling weights calculated by Statistics Canada must be used for variance estimation. By conducting the analysis using individual-level variables at the survey level, and then pooling the resulting estimates, these statistical procedures could be implemented exactly according to recommended protocols.
We first examined whether either age or sex interacted with the long-term condition in question by examining 3-way interaction terms (medical condition by age by sex) in the various surveys, with age treated as a continuous variable. In the absence of 3-way interactions, 2-way interactions between age, sex and the medical conditions were examined. When interactions were found, stratified estimates were calculated within age ranges (using quartiles, tertiles or median ages for the condition in question, as sample size permitted) to create a graphical depiction of the interaction. When interactions were negligible or weak, estimates with and without adjustment for age and sex were made in order to detect confounding. Where there was evidence of confounding (pooled adjusted and unadjusted estimates differed) additional modelling was carried out to determine whether the confounding was due to age, sex or both. Note that the term confounding is used here without reference to the direction of change with adjustment. Some authors prefer the term ‘suppression’ for situations in which adjustment strengthens the relationship between an independent and dependent variable (MacKinnon et al. Reference MacKinnon, Krull and Lockwood2000), but this convention is not adopted here.
Pooling was always preceded by a series of preliminary steps. Random effects meta-regression with survey year as a predictor variable was used to ensure that there were no time trends. Also, a meta-regression model was used to explore for the differences depending on the measurement strategy employed in the individual surveys (since some of the studies used the CIDI-SFMD and some the WMH-CIDI). However, there were no secular trends identified, nor any difference depending on the interview used. Hence, these preliminary analyses are not further discussed in this paper. All of the models were accompanied by an examination of heterogeneity using the I 2 statistic (which quantifies heterogeneity as a percentage of variability in the estimates) and the Q-statistic, which provided a statistical test for heterogeneity. I 2 <50% is generally regarded as low. We also report τ 2 values (the variance of the distribution of random effects) whenever it was not negligible. The meta-analyses used Stata Corporation (2015) ‘metan’ command and were conducted in the Prairie Regional Data Centre in Calgary, Canada. Under the current regulatory framework governing use of these datasets, approval by an Ethics Review Board is not required (Interagency Advisory Panel on Research Ethics, 2014).
Results
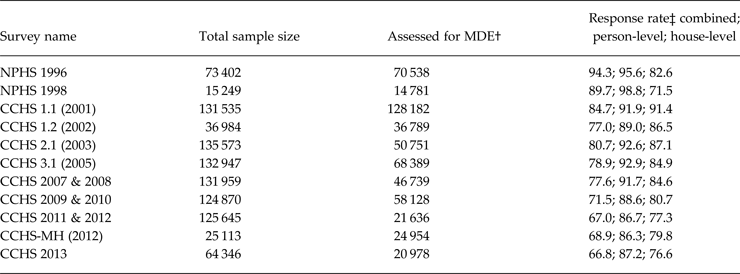
Table 1 shows the number of available observations from the surveys used in the analysis.
Table 1. Sample size available in the surveys included in the pooled analysis*

* Available surveys for: (a) COPD: CCHS 1.1, 1.2, 3.1, 2007 & 2008, 2009 & 2010, 2011 & 2012 and CCHS 2013; (b) cataracts: NPHS 1996 & 1998, CCHS 1.1, 1.2, 2.1 & 3.1; (c) epilepsy: NPHS 1996 & 1998, CCHS 1.1, 1.2, 2.1 & 3.1 and CCHS-MH; (d) stroke: all surveys except CCHS 2013; (d) thyroid disease: same as epilepsy, except not the CCHS-MH; for all other conditions all surveys were included.
† The chronic disease questions were core content, asked of all respondents. Note that while counts are reported here, all estimates were weighted to the national household population.
‡ As reported by Statistics Canada in reference documentation (Canada level). This is the overall response rate and may not include non-response to specific items.
In the high blood pressure analysis, initial meta-analytic models confirmed that there was no 3-way (age by sex by high blood pressure) interaction (z = −1.398, p = 0.162), but a significant age by condition interaction was identified (z = −14.109, p < 0.001). In order to depict the interaction, pooled estimates in age quartiles were estimated. All of these age-specific estimates were homogeneous except that for the 77+ age group, where there was a significant Q-test (Q = 26.8, d.f. = 10, p = 0.003). The random effects model was associated with an I 2 of 62.7% and a τ 2 value of 0.25 in this stratum. As seen in Fig. 1, the ORs were modest in magnitude, ranging from 1.5 in the 12–59 year age category (95% CI 1.4–1.6, p < 0.001) to 1.2 in the 77+ age category (95% CI 0.8–2.0, p = 0.336).
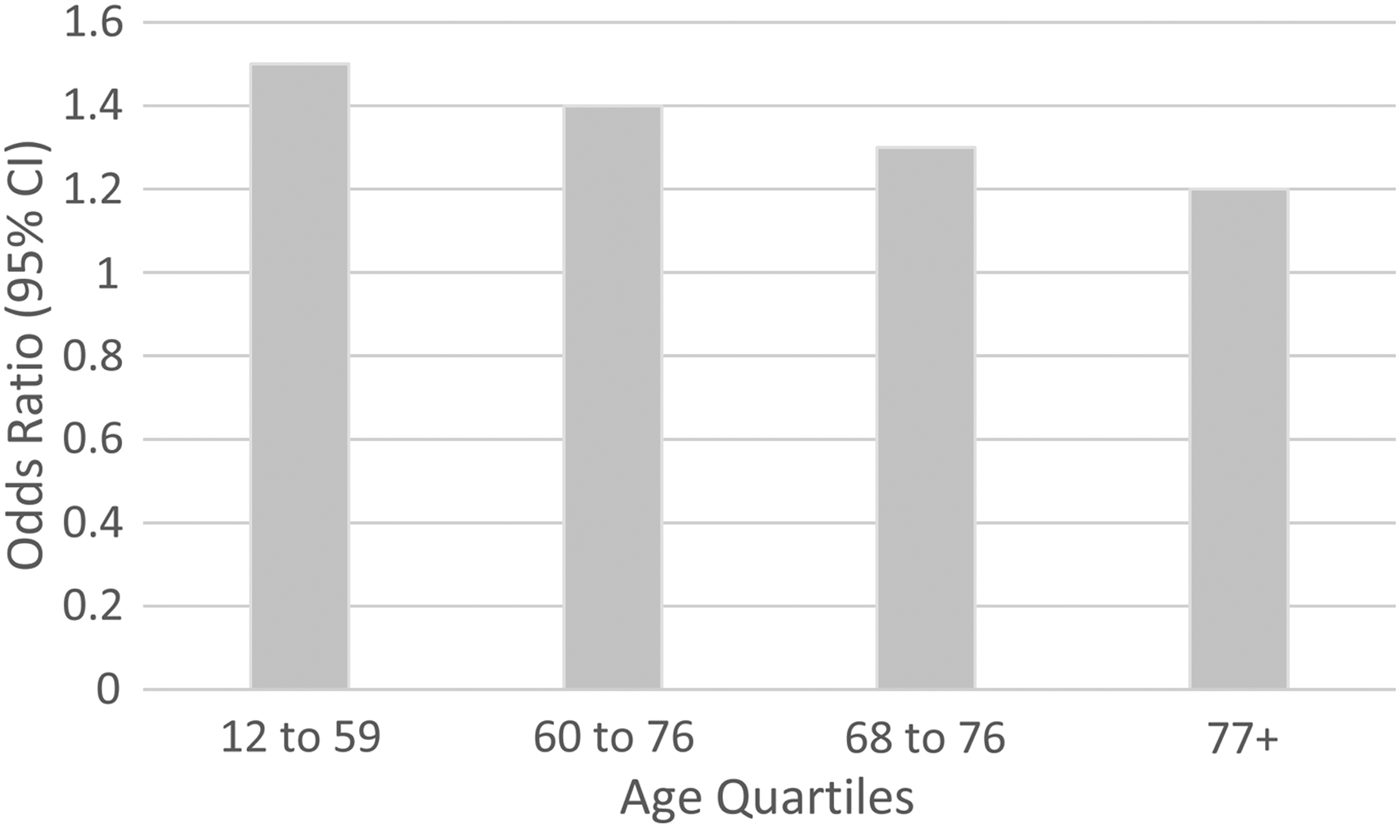
Fig. 1. Association between high blood pressure and MDE, by age.
For cancer, an age by condition interaction was again seen (z = −8.634, p < 0.001). In order to depict the interaction, the samples were stratified at values above and below 70, which was the median age for those with cancer in the sample. The association was found to be stronger (OR of 1.7, 95% CI 1.4–2.1) in the below-median (12–69 year) age stratum whereas in the 70+ group it was 1.3 (95% CI 1.0–1.8). Although the older stratum's CI reaches the null value of OR = 1, the stratum-specific association was significant (p = 0.036). In the meta-analytical modelling both estimates were homogeneous (I 2 < 42% in both models, neither Q-test was significant).
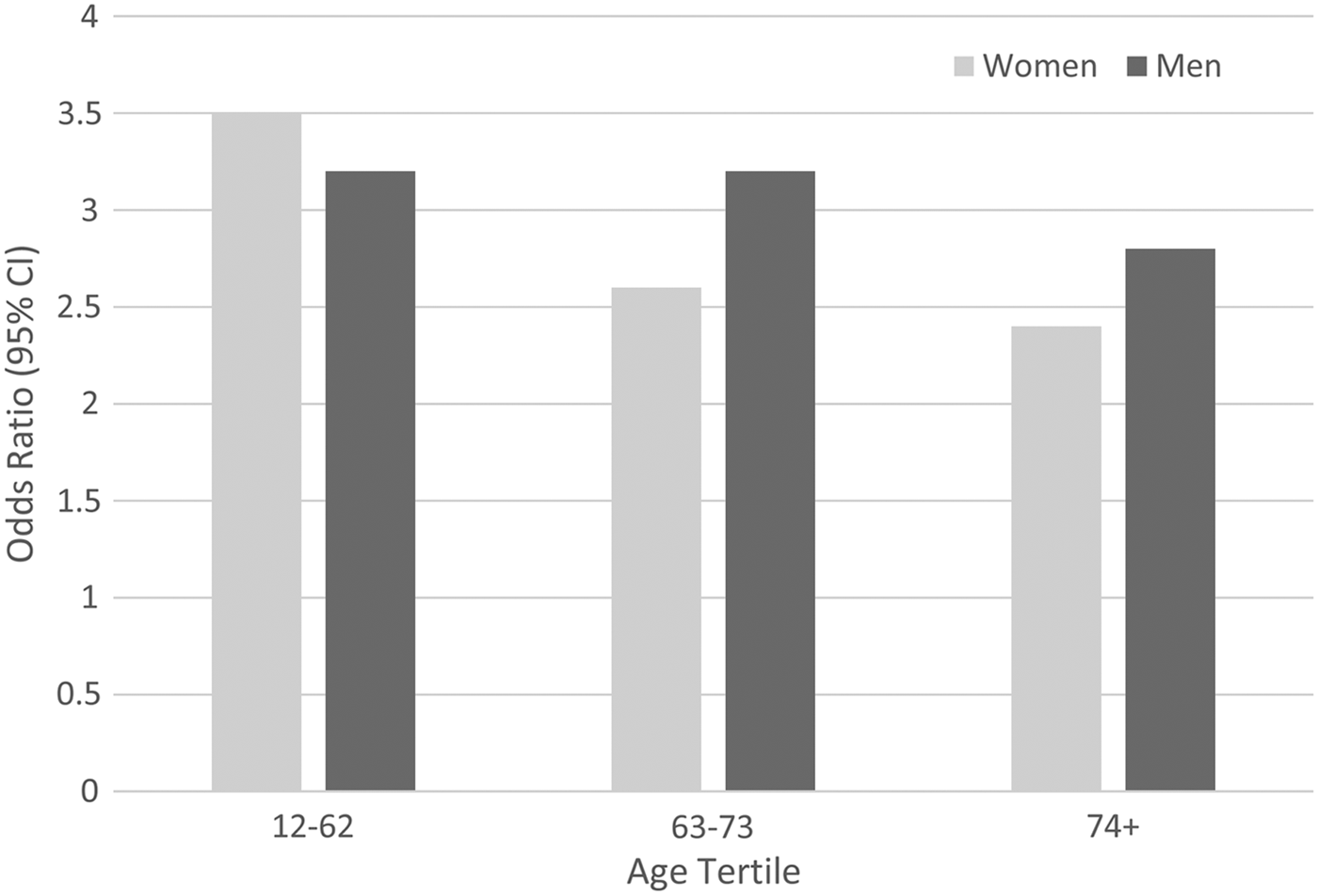
Age by condition interactions characterised by a weakening of the association with age was also observed for arthritis and chronic obstructive pulmonary disease. As the results were similar, only those for chronic obstructive pulmonary disease are presented here. There was a significant 3-way interaction (z = −2.584, p = 0.010), such that the estimates were stratified by three age tertiles and by sex. None of the pooled analyses were characterised by I 2 values >52% nor by significant Q-tests (all p > 0.05). Again, there was a suggestion of declining strength of association with age, see Fig. 2. Odds ratios for men ranged from 3.2 in the lowest age tertile, which represented respondents under the age of 63 (95% CI 2.3–4.3) to 2.8 (95% CI 1.4–5.6) in those ≥74 years old. In women, those under the age of 63 had an OR of 3.5 (95% CI 2.5–4.9) in contrast to 2.4 (95% CI 1.0–5.9) in the ≥74 age group.
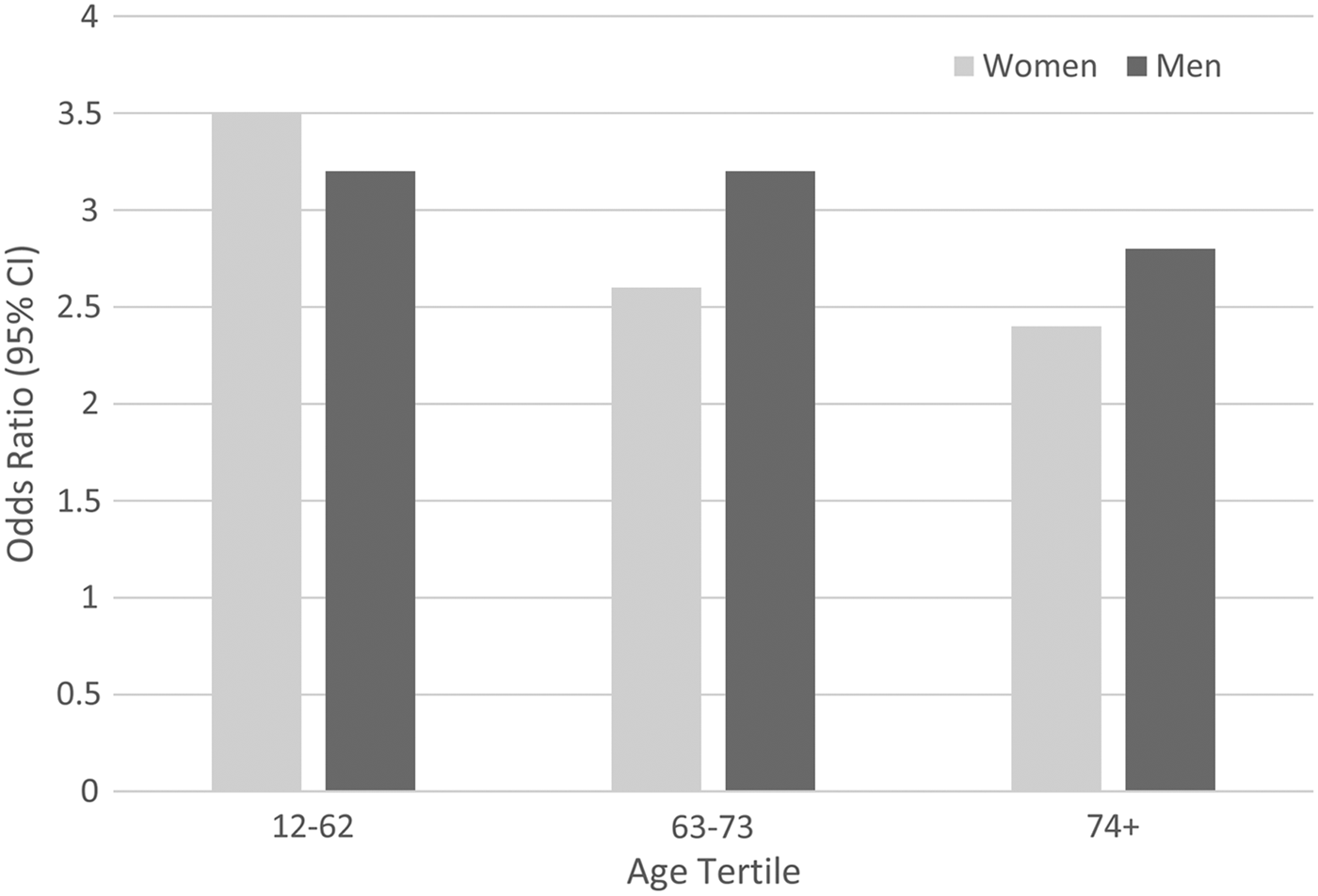
Fig. 2. Association between chronic obstructive pulmonary disease and MDE, by age and sex.
In the migraine analyses there was again a significant 3-way (age by sex by condition) interaction (z = −3.783, p < 0.001), but the effects were in a different direction than for the conditions discussed thus far. Upon simultaneous stratification by age quartile and sex, differences were evident in the stratum-specific estimates. The ORs for women were generally lower than those of men and stratification suggested an increasing strength of association with age in men, but the pattern was less clear in women. In view of this, the results are presented with stratification for age and sex, see Fig. 3. None of these models displayed excessive heterogeneity (all I 2 < 37% and all τ 2 < 0.04, all Q-tests p > 0.1).

Fig. 3. Association between migraine and MDE, by age and sex.
There were no 3-way interactions in the heart disease analysis (z = −0.522 p = 0.602), but 2-way interactions with both age (z = 9.436, p ≤ 0.001) and sex (z = 3.8, p ≤ 0.001) were found. However, with stratification on age and sex all of the ORs fell in the range of 1.5–2.1 with no clearly evident pattern. The stratified estimates, however, were all much higher than the crude OR (1.1), suggesting confounding. Stratification separately by age and sex suggested that the confounding was due to age exclusively. Age adjusted estimates (with age as a continuous variable) are presented separately in view of the significant sex by heart disease interaction: 1.5 for women (95% CI 1.4–1.7) and 1.8 for men (95% CI 1.5–2.1). There was little evidence of heterogeneity in the meta-analyses (all I 2 < 28%, Q-test, both p > p = 0.17, τ 2 both < 0.02).
Age by condition interactions were also significant in the thyroid disease analysis (z = −6.336, p < 0.001). There was no sex by thyroid disease interaction (z = 0.331, p = 0.741). However, ORs stratified by age tertiles were all in the narrow range of 1.4–1.8, suggesting that these interactions, although statistically significant, were small in magnitude. With adjustment for age as a continuous variable there was a significant association of thyroid disease with MDE in women (OR = 1.4, 95% CI 1.2–1.7, p = 0.002) but not in men (OR = 1.1, 95% CI 0.8–1.7, p = 0.44), even though there was no sex by condition interaction in the unadjusted analysis. The I 2 was 33.1% for women and 5.6% for men, in each case the Q-test was not significant (p > 1.8), and τ 2 values were small at 0.008 for women and 0.006 for men.
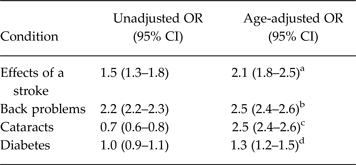
In several of the analyses described above, significant age by condition interactions was observed but, upon stratification, the extent of effect modification by age was found to be small. This same finding emerged for four additional conditions: effects of a stroke, back problems, cataracts and diabetes. All of these conditions are strongly age dependent and confounding by age is expected for this reason. In each case, stratified analysis was consistent with the occurrence of confounding by age, and failed to reveal evidence of confounding by sex. An age-adjusted estimate was then made using logistic regression with age treated as a continuous variable. Table 2 presents unadjusted and age-adjusted estimates for each of these conditions. The most interesting result was seen for cataracts in which the confounding reversed the direction of effect, an example of the so-called Simpson's paradox (Julious & Mullee, Reference Julious and Mullee1994).
Table 2. Unadjusted and age-adjusted odds ratios for four long-term medical conditions
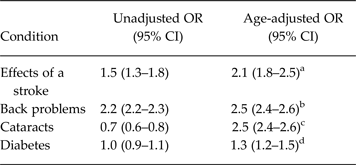
a I 2 and τ 2 were approximately zero, Q-test statistic 8.6, d.f. = 9, p = 0.48.
b I 2 and τ 2 were both approximately zero, Q-test statistic 6.99, d.f. = 10, p = 0.73.
c I 2 and τ 2 were both approximately zero, Q-statistic 4.8, d.f. = 5, p = 0.44.
d I 2 was 16.7%, Q-statistic = 12.0, d.f. = 10, p = 0.29, τ 2 = 0.004.
In the epilepsy analysis no significant interactions were observed in preliminary analyses (all p-values > 0.305) and estimates adjusted for age and sex were all close to the crude value (OR = 1.7, 95% CI 1.3–2.3). The I 2 for the unadjusted estimate was 13.1%, τ 2 = 0.015 and Q = 6.9, d.f. = 6, p = 0.33).
Discussion
Cross-sectional associations between medical conditions and MDE are valuable because they can help to quantify treatment needs in different clinical groups and can generate hypotheses for etiologically-oriented research. The current study provides an advance by examining demographic subgroups within chronic disease categories and finding differences for several conditions. Consistent with prior research, we observed variable strengths of association with relatively strong associations seen for painful conditions and conditions involving inflammation. A strong association was also observed for epilepsy, a condition that is not generally viewed as an inflammatory condition, but where CNS inflammation occurs (Walker & Sills, Reference Walker and Sills2012).
An important general observation is that effect modification by age was present for several conditions (exceptions being epilepsy and thyroid disease), although its magnitude was not always large. Generally, the associations were strongest in younger respondents and weaker in older respondents (a notable exception being migraine, where the opposite was true in men). The most clearly apparent age by condition interactions were observed for high blood pressure and cancer whereas for most other conditions the extent of effect modification was sufficiently small that we considered it reasonable to explore possible confounding by age in spite of statistically significant interactions. While cross-sectional data cannot address causality various hypotheses are raised. For example, chronic medical conditions may be viewed as more ‘trivial’ in older persons (Aldwin et al. Reference Aldwin, Sutton, Chiara and Piro1996) or coping, adaptation and resilience may be especially strong in the elderly (Foster, Reference Foster1997).
There may also be biological underpinnings of the association between medical conditions and depression, potentially these are connected to the interactions observed. These may include age-related and stress-related changes (such as vascular changes and ensuing white matter hyperintensities) that alter brain activity in cognitive and affective circuits (Taylor et al. Reference Taylor, Aizenstein and Alexopoulos2013). Another relevant neurobiological factor is inflammation. Inflammatory mediators have shown a bidirectional relationship with depression in some studies (Stewart et al. Reference Stewart, Rand, Muldoon and Kamarck2009) and may underpin specific depressive symptoms (Jokela et al. Reference Jokela, Virtanen, Batty and Kivimäki2016). Miller & Raison (Reference Miller and Raison2016) speculate that the association between depression and inflammation may have its origins in an adaptive role for inflammation, for example in facilitating wound healing, at times of social disturbance.
The findings reported here have implications for the interpretation of many previously published studies. Studies of the association of depression with cancer, for example, have often been negative even with adjustment for age e.g., see (Pirl et al. Reference Pirl, Greer, Temel, Yeap and Gilman2009), but this may reflect a neglect of effect modification by age in these prior analyses. When effect modifying variables are not accounted for in analyses, study estimates are averaged across different demographic groups. In the case of cancer, weak associations overall may arise by dilution if data from younger and older respondents are inappropriately combined. This problem is not corrected by age adjustment, which only disentangles independent effects of the two variables.
Although effect modification by age has rarely been reported in this literature, there are prior examples. One was a record-linkage study of MDD and hypertension by Wu et al. who reported a prevalence ratio for major depression of 2.3 in the 18–39 age group, 1.5 in the 40–59 group and 1.1 in those 60 or over (Wu et al. Reference Wu, Chien, Lin, Chou and Chou2012). Another was a record-linkage study of mood disorders and breast cancer that reported higher rate ratios in respondents under the age of 40 (incidence rate ratio (IRR) = 1.5) compared with those aged 40–59 (IRR = 1.4) and those 60 or older (IRR = 1.1). No evidence of effect modification by age was reported in an analysis of this association in the National Comorbidity Survey replication (Pirl et al. Reference Pirl, Greer, Temel, Yeap and Gilman2009), but the limited sample size in this study (n = 243 cancer survivors) may have precluded its detection. Another consideration in the cancer literature is that various types of cancer have different ages of onset and survival patterns, such that the interactions do not necessarily reflect effect modification by age but may rather reflect effect modification by cancer type.
The association of MDE with migraine was strong and appeared to get stronger with age in men, whereas in women the association became weaker in the most elderly age quartile. This finding is consistent with recent reports that menstrual-associated migraine may be more severe and disruptive, such that the older quartile women, who would be post-menopausal at this age, may have had a weaker association due to lower severity (Pavlovic et al. Reference Pavlovic, Stewart, Bruce, Gorman, Sun, Buse and Lipton2015). This pattern was not seen with any other condition.
Another distinct pattern was observed for a group of conditions (effects of a stroke, back problems, cataracts and diabetes) in which there was evidence of confounding by age. As MDE is more common in younger respondents and as these medical conditions tend to affect older people, the strength of association is predictably increased when adjustment for age are made. As noted above, the term ‘suppressor effect’ is preferred by some authors to describe this type of confounding. In the current analysis, the strongest confounding was seen with cataracts. An association between cataracts and depressive symptoms has previously been reported in a community study of elderly persons, consistent with the effectiveness of restriction as a strategy for control of confounding (Eramudugolla et al. Reference Eramudugolla, Wood and Anstey2013). Another study using age matching reported higher depression pre- v. post-cataract surgery, also consistent with the current results (Freeman et al. Reference Freeman, Gresset, Djafari, Aubin, Couture, Bruen, Laporte and Boisjoly2009).
While some prior studies have questioned the existence of a thyroid disease-MDE association in modern community populations (Pop et al. Reference Pop, Maartens, Leusink, Van Son, Knottnerus, Ward, Metcalfe and Weetman1998; Engum et al. Reference Engum, Bjoro, Mykletun and Dahl2002; Gussekloo et al. Reference Gussekloo, Van Exel, De Craen, Meinders, Frolich and Westendorp2004; Engum et al. Reference Engum, Bjoro, Mykletun and Dahl2005; Patten et al. Reference Patten, Williams, Esposito and Beck2006; Roberts et al. Reference Roberts, Pattison, Roalfe, Franklyn, Wilson, Hobbs and Parle2006; Almeida et al. Reference Almeida, Alfonso, Flicker, Hankey, Chubb and Yeap2011; de Jongh et al. Reference De Jongh, Lips, Van Schoor, Rijs, Deeg, Comijs, Kramer, Vandenbroucke and Dekkers2011; Saltevo et al. Reference Saltevo, Kautiainen, Mantyselka, Jula, Keinanen-Kiukaanniemi, Korpi-Hyovalti, Oksa, Saaristo and Vanhala2015), the increased power obtained through meta-analysis in this study confirms the existence of an association in women, albeit a weak one compared with some of the other associations observed.
Epilepsy was strongly associated with MDE with no evidence of effect modification or confounding. A potentially explanatory hypothesis is that greater psychosocial disruption among younger people may be offset by a greater neurobiological impact in older respondents. Consistent with this idea, one prior study has reported that most of the variance in depression scores in a sample of people with epilepsy is accounted for by disease-related factors (Gaus et al. Reference Gaus, Kiep, Holtkamp, Burkert and Kendel2015).
Strengths of the study include the methodological similarity of the constituent surveys and the access to large samples. Limitations of the study arise primarily from its reliance on survey data. Detailed clinical information was missing from the datasets. For example, it has been reported that the association of depression with migraine is stronger in migraine with aura (Radat & Swendsen, Reference Radat and Swendsen2005). Breslau et al. reported a sex-adjusted OR of 4.9 for migraine with aura, but only 3.0 in migraine without aura (Breslau et al. Reference Breslau, Schultz, Stewart, Lipton, Lucia and Welch2000). Information about aura was not available to the current analysis. Another limitation is the reliance on cross-sectional data, which cannot identify etiological factors due to an inability to discern the temporal direction of causal effects (Simmonds et al. Reference Simmonds, Kumar and Lechelt1996; Bair et al. Reference Bair, Robinson, Katon and Kroenke2003; Radat & Swendsen, Reference Radat and Swendsen2005; Hare et al. Reference Hare, Toukhsati, Johansson and Jaarsma2014). In some conditions, such as diabetes, there is evidence supporting a bidirectional relationship with depression (Renn et al. Reference Renn, Feliciano and Segal2011), but cross-sectional analyses cannot address issues of temporality. The datasets pooled were largely independent, although the 1998 NPHS included approximately 12 000 people also included in the 1996 NPHS. Removal of the 1998 dataset made no difference to any of the results. Finally, although questions of temporality do not apply to age and sex, these variables are probably markers of underlying biological, psychological or social determinants of depression that were unmeasured. A more detailed exploration of such factors will require more detailed data collection than provided by these epidemiological studies.
Acknowledgements
Dr Patten is a Senior Health Scholar with Alberta Innovates, Health Solutions (AIHS). The analysis was conducted at the Prairie Regional Data Centre, which is part of the Canadian Research Data Centre Network (CRDCN). The services and activities provided by the CRDCN are made possible by the financial or in-kind support of the SSHRC, the CIHR, the CFI, Statistics Canada and participating universities whose support is gratefully acknowledged. The views expressed in this paper do not necessarily represent the CRDCN's or that of its partners’.
Financial Support
This work was supported by an operating grant from the Canadian Institutes of Health Research (MOP-130415) and by the Hotchkiss Brain Institute.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Availability of Data and Materials
The data used in these analyses are available to through the Canadian Research Data Centre Network via a data access approval process. Procedures for accessing the data are available at http://www.rdc-cdr.ca/research.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.