An intra-atrial communication or baffle fenestration has been shown to be able to improve morbidity and mortality in selected Fontan procedures. We report a single case of a failing Fontan circulation that could be relieved using a novel interventional device for the creation of a defined and stable intra-atrial fenestration.
Case history
Patient history
A 25-year-old man with Goldenhar syndrome and tricuspid atresia had undergone a modified Fontan procedure 15 years ago – extracardiac 20-mm Goretex. Over the past 5 years, he developed signs of a failing Fontan physiology despite good ventricular function. He suffered from recurrent ascites, protein losing enteropathy, and impaired excercise capacity, with a distance of <200 m in the 6-min walk test, NYHA III. Catheterisation revealed a transpulmonary gradient of 12 mmHg with a left ventricular end-diastolic pressure of 12 and a mean pressure in the Fontan circulation of 24 mmHg. Despite maximal conservative therapy, the clinical situation deteriorated and the decision was made to create a secondary Fontan fenestration of 6 mm diameter, to alleviate venous congestion. A diameter of 6 mm was chosen based on previous reports about higher re-occlusion rates in late fenestrations <5 mm.Reference Lammers, Derrick, Haworth, Bonhoeffer and Yates 1
On the basis of the patient’s complex habitus with severe scoliosis, no satisfactory trans thoracic echocardiograph nor TEE pictures could be obtained. MRI examination was aborted by the patient owing to intolerable back pain. The underlying anatomy assessed during the first catheterisation revealed a greater distance between the lateral tunnel and the left atrium than expected. Therefore, an alternative approach to create this fenestration was chosen: by puncturing between the pulmonary artery and the pulmonary veinsReference Mehta, Jones and De Giovanni 2 the two circulations could be bridged through native tissue. However, a longer stent would have been required for this “bridging”, so we looked for an opportunity to create a fenestration with a low profile on both sides to reduce the risk of thrombus formation. After consultation with the legal department of the hospital and the local ethics committee and after adequate written informed consent, the option for the clinical use of an Occlutech Atrial Flow Regulator (AFR®, Occlutech Holding AG, Switzerland) device on a compassionate care basis was generated. The device was donated by Occlutech Holding AG, Switzerland.
Device description
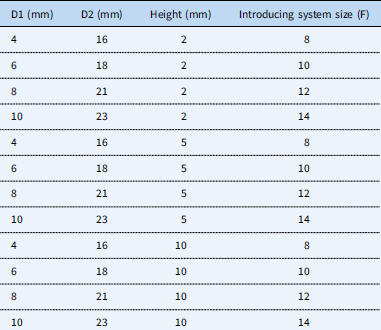
The Occlutech AFR® device is similar to a standardised Occlutech Figulla Flex II® ASD device, Occlutech Holding AG, Switzerland (Fig 1). The implantation procedure is comparable to the implantation of an ASD device.Reference Haas, Happel and Soetemann 3 The connection to the delivery cable allows an angulation of the device to the delivery cable of about 50°, identical to the angulations of other Occlutech devices.Reference Haas, Soetemann and Ates 4 The device comes in different sizes of the hole, 4, 6, 8, and 10 mm, and different diameters of the body, waist 2, 5, and 10 mm (Table 1).

Figure 1 Details of the AFR® device. ( a ) Circular shape of the device with the central hole. ( b ) Connection hub to the delivery cable, which is identical to the delivery set of the Occlutech Flex II® device family. ( c ) Flat profile of the device (here thickness 2 mm). ( d ) Flat profile of an implanted device (5 mm thickness).
Table 1 Occlutech® AFR® device measurements

Procedure
Owing to severe scoliosis, transesophageal echocardiography was not helpful in this patient. After documentation of the exact anatomy by angiography (Fig 2), puncture of the inferior wall of the connection of the pulmonary arteries and conduit area was performed to obtain access to the left atrium (see schematically in Fig 3). Thereafter, a 0.035 extra stiff (COOK®, Amplatz extra stiff, Cook Medical LLC; Bloomington, IN, United States of America) wire was advanced to the left atrium. The perforation was dilated with an 8-mm high-pressure balloon at 12 atm (Savvy®long PTA Catheter, Cordis EMEA; Baar, Switzerland) and a 10 F Mullins long sheath was advanced to the left atrium. According to the patient’s anatomy, a medium-sized Occlutech AFR® device with a 6-mm hole was chosen (D1 6 mm, D2 18 mm, height 5 mm) and deployed analogue to conventional ASD closure. Final angiography revealed an excellent position with right-to-left shunt (Fig 4). Mean pressure within the Fontan tunnel decreased from 24 to 21 mmHg with a transpulmonary gradient of 6 mmHg. The immediate saturation decreased from 96 to 91%. After initial anticoagulation with heparin, the patient got back on warfarin, which he had taken already before the fenestration procedure.

Figure 2 Baseline anatomy. Extracardiac tunnel with a 20-mm conduit (( a ) anteroposterior view, ( b ) lateral view). Visualisation of the right-sided pulmonary veins and the left atrium (( c ) anteroposterior view, ( d ) lateral view). The round tip of the pig-tail catheter inside indicates the area for transseptal puncture.

Figure 3 Schematic illustration of the Occlutech AFR® implantation. ( a ) Classification of the underlying anatomy. ( b ) A transseptal needle is inserted from the jugular vein and the puncture is directed to the left side posteriorally to gain access to the left atrium. ( c ) Balloon dilatation is performed across the puncture side. ( d ) Final position of the Occlutech AFR® device.

Figure 4 Result after implantation of the Occlutech AFR® device. ( a ) Good alignment of the device with flat profile. ( b ) Right-to-left shunting (arrow).
Clinical result
The patient’s clinical condition improved over the following months. Ascites and protein losing enteropathy disappeared, the medication with bosentan could be stopped, and the patient is now clinically in NYHA I, with a distance of 480 m in the 6-min walk test; the saturations drop to 80% during maximal exercise.
Discussion
Fenestration of the Fontan circuit may decrease postoperative morbidity and mortality in selected cases – not only in high-risk patients. The benefits of a fenestration are owing to an increased preload and improved cardiac output resulting from right-to-left shunting. In addition, baffle fenestration can limit the postoperative increase in systemic venous pressure that contributes to postoperative morbidity.Reference Lemler, Scott, Leonard, Stromberg and Ramaciotti 5 , Reference Ono, Boethig, Goerler, Lange, Westhoff-Bleck and Breymann 6
Creation of a fenestration between the venous and arterial side in patients with a failing Fontan circulation has been used by several authors and for many years;Reference Kreutzer, Lock, Jonas and Keane 7 – Reference Rupp, Schieke and Kerst 10 the use of stents in this situation may result in more prolonged patency and less re-occlusion rate compared with balloon dilatation alone.Reference Bar-Cohen, Perry, Keane and Lock 11 Bare-metal and covered stents have been used. The technique is in many cases a snare-controlled, diabolo-shaped stent placement to achieve a bilaterally V-shaped stent protruding in the venous system, as well as in the systemic atrium at various lengths.Reference Casadonte, Wax and Gossett 9 , Reference Rupp, Schieke and Kerst 10 Usually, transseptal puncture is achieved across the Fontan baffle, an extra cardiac conduit or the native atrial septal tissue via the usual transfemoral approach; in rare cases, a transhepatic approach or a transjugular approach may be necessary.Reference Mehta, Jones and De Giovanni 2 , Reference Rupp, Schieke and Kerst 10 , Reference Kenny, McMahon and Walsh 12
However, the overall success rate by using blade/balloon septostomy, stent placement, or Amplatzer fenestrated ASD devices is disappointing; the re-occlusion rate is high when fenestrations are used with a diameter of 5 mm or less and when there is a high amount of surrounding material in place.Reference Vyas, Driscoll, Cabalka, Cetta and Hagler 13
Comparable devices were used by Lammers et alReference Lammers, Derrick, Haworth, Bonhoeffer and Yates 1 in patients with severe pulmonary hypertension (n=7) or to decompress left atrial pressure during extracorporal membrane oxygenation support before heart transplantation. Kretschmar et alReference Kretschmar, Sglimbea, Corti and Knirsch 14 used self-fabricated Amplatzer devices to reduce ASD shunt flow in patients with pulmonary hypertension and/or restrictive left ventricular physiology.
The newly designed Atrial Flow Regulator presented here may have a similar mechanism and functions like the formerly available Amplatzer fenestrated ASD device. This device, however, had an unfavourable device/hole relation with relatively small holes (4–5 mm) compared with the large discs. The Occlutech AFR® device shows a relatively large hole (4–10 mm) and less remaining left- and right-sided discs and no thrombogenic patch material inside the discs; this may prevent an early re-occlusion and potentially decrease the risk of rapid and excessive endothelialisation.
In our case, this device was easy to use after standard transseptal puncture. Balloon dilatation of the achieved fenestration 2 mm larger than the desired final diameter using high-pressure balloons is advised. The implantation process itself is comparable to the implantation of a commonly available ASD device. The final shape of the device shows a flat profile and allows an easy passage across the hole with the potential of subsequent transatrial interventions.
Conclusion
In this single case, transcatheter creation of a Fontan fenestration using the new Occlutech AFR® device was effective. The handling of the device itself was comparable to conventional ASD closure. It creates a relatively flat profile of the fenestration without protrusion into the Fontan circulation or the systemic atrium. This may reduce potential thrombus formation and spontaneous re-occlusion of such an extra-anatomic perforation. The use of this device results in a defined fenestration diameter and may ensure a permanent clinical improvement in these patients. We believe that this device is a very helpful addition to the armentarium of interventional cardiologists treating Fontan patients.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
N.A.H. and I.S.N. are principal investigators for the Prophet trial - Pilot study to assess safety and efficacy of a novel Atrial Flow Regulator (AFR) in patients with pulmonary hypertension (http://clinicaltrials.gov/ct2/show/NCT03022851).
Ethical Standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.