Introduction
Rumen flukes are trematodes that inhabit the reticulum and rumen of several domestic and wild ruminants. These parasites have recently been recognized as a significant threat to livestock, causing significant damage to their ruminant hosts and, consequently, economic losses (Huson et al., Reference Huson, Oliver and Robinson2017). They have a heteroxenous life cycle that involves freshwater snails of various families such as Bulinidae, Lymnaeidae and Planorbidae as intermediate hosts (Rojo-Vázquez et al., Reference Rojo-Vázquez, Meana, Valcárcel and Martínez-Valladares2012). First, the eggs hatch in the environment, and the larvae that emerge (ciliated miracidia) infect snails, where they develop until reaching the cercaria stage. The cercariae then emerge from snails, encyst on vegetation and develop into metacercariae, which can be ingested during grazing by ruminants, which are their definitive hosts. After ingestion, the immature parasites first establish in the small intestine, but later cause significant damage as they move from the small intestine to the reticulum or rumen (Millar et al., Reference Millar, Colloff and Scholes2012; Pavan Kumar et al., Reference Pavan Kumar, Syaama Sundar and Devi Prasad2016).
Infections involving rumen flukes can cause fibrinonecrotic enteritis (Tehrani et al., Reference Tehrani, Javanbakht, Khani, Hassan, Khadivar, Dadashi, Alimohammadi and Amani2015), intestinal haemorrhages, anaemia, recurrent ruminal tympani, diarrhoea, weakness and even mortality in heavy infections, especially in young animals and small ruminants (Millar et al., Reference Millar, Colloff and Scholes2012).
Despite damages caused by rumen flukes in their ruminant hosts, these infections were considered of little importance, especially when compared with Fasciola hepatica infections. Only recently have these flukes been recognized as an important cause of economic losses in ruminant production systems, mainly from the UK (Malrait et al., Reference Malrait, Verschave, Skuce, Van Loo, Vercruysse and Charlier2015; Sargison et al., Reference Sargison, Francis, Davison, Bronsvoort, Handel and Mazeri2016).
The presence of rumen fluke infections in ruminants has been widely reported across several European countries such as the UK (Jones et al., Reference Jones, Brophy, Mitchell and Williams2017), Ireland (Toolan et al., Reference Toolan, Mitchell, Searle, Sheehan, Skuce and Zadoks2015) and the Netherlands (Ploeger et al., Reference Ploeger, Ankum, Moll and Holzhauer2017), and on other continents such as Oceania (Cauquil et al., Reference Cauquil, Hüe, Hurlin, Mitchell, Searle, Skuce and Zadoks2016) and Asia (Ali et al., Reference Ali, Rashid, Shabbir, Akbar, Shahzad, Ashraf and Chaudhry2018). In America, the presence of rumen flukes in ruminants has been reported in Peru (Pinedo et al., Reference Pinedo, Chávez, Casas, Suárez, Sánchez and Huamán2010) and Mexico (Ojeda-Robertos et al., Reference Ojeda-Robertos, Medina-Reynes, Garduza-Arias and Rangel-Ruiz2014).
Despite the widespread infections currently caused by rumen flukes, few studies have examined the efficacy of anthelmintic drugs to control these infections (Fairweather et al., Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012; Huson et al., Reference Huson, Oliver and Robinson2017). On the other hand, a large number of studies have been published on the efficacy of anthelmintic drugs against F. hepatica (Fairweather et al., Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012; Robles-Pérez et al., Reference Robles-Pérez, Martínez-Pérez, Rojo-Vázquez and Martínez-Valladares2014, Reference Robles-Pérez, Martínez-Pérez, Rojo-Vázquez and Martínez-Valladares2015; Novobilský et al., Reference Novobilský, Amaya Solis, Skarin and Höglund2016; Zhang et al., Reference Zhang, Si, Zhou, Shang, Li and Zhang2019). Anthelmintic drugs should be effective against developing eggs and immature and adult rumen flukes. Therefore, the objective of the present study was to evaluate the efficacy of several commonly used anthelmintic drugs against the egg development of rumen flukes recovered from ruminants raised in the humid tropics of Mexico.
Materials and methods
Study area
Rumen flukes were collected from animals slaughtered in a regional abattoir in the municipality of Juarez, Chiapas, Mexico, located at 17°41'N and 93°13'W. Cattle slaughtered in this abattoir were from the municipality of Huimanguillo in the state of Tabasco and the municipalities of Reforma and Juarez in the state of Chiapas. Figure 1 shows the location of these municipalities. Climatic conditions corresponded with those of tropical humid rainforest, with an average temperature of 26°C and more than 2000 mm of annual rainfall (CONAGUA, 2019).

Fig. 1. Localities of origin of the inspected cattle for the collection of rumen fluke eggs.
Collection of rumen flukes and eggs
The rumen and reticulum of the slaughtered cattle were inspected, and the observed flukes were collected. In total, 50 parasites were placed in 50-ml centrifuge tubes containing 10 ml of sterile water. Subsequently, rumen flukes were washed again with sterile water to eliminate cellular detritus. Rumen fluke eggs were obtained placing the parasites in 50-ml centrifuge tubes containing 10 ml of RPMI solutions for five hours at room temperature. Afterwards, a second washing step was carried out with sterile water, and the excreted eggs were screened with a 37-μm sieve. The recovered eggs were transferred to 15-ml tubes with 10 ml of sterile water.
Quantification of rumen fluke eggs
Rumen fluke eggs were counted by adjusting the volume in the centrifuge tubes to 10 ml with sterile water. The tubes were mixed in a vortex to homogenize the eggs throughout the mixture. The egg counts were then performed on a sub-sample of ten aliquots of 10 μl. The average egg count was then extrapolated to the total volume. Subsequently, the volume was diluted to one egg per microliter and streptomycin sulphate (10 mg/l) and benzyl penicillin (9900 units/l) was added at room temperature (27°C) for in vitro assays.
Identification of adult parasites
The flukes were identified based on their morphology (Eduardo, Reference Eduardo1982), and were subsequently fixed in formalin and dehydrated. Ten specimens of each isolate were measured to discriminate species by size (Nikander & Saari, Reference Nikander and Saari2007). Other specimens were hydrated and stained with haematoxylin–eosin and mounted on the slides to differentiate them by their species characteristics.
In vitro assays to evaluate efficacy of flukicidal drugs against egg development
In vitro assays were performed using an established technique to evaluate the development of F. hepatica eggs (Fairweather et al., Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012) with several modifications. Briefly, 96-well polystyrene microtiter plates (NUNC MaxisorbTM Invitrogen by Thermo Fisher Scientific, Waltham, Massachusetts, U.S. ) were used. A single anthelmintic drug was tested in each row, evaluating a total of 12 dilutions. One hundred eggs were placed into the wells, which were filled with 350 μl of sterile water. The following drugs were tested: rafoxanide at a concentration of 0.0015–3.0 mg/ml (RafoxcurTM 200 mg/ml; Riverfarma, Mexico City, Mexico), nitroxinil at a concentration of 0.0025–10.20 mg/ml (Nitroxinil 34%; NitromicTM, Microsules, Canelones, Uruguay) and closantel at a concentration of 0.0015–3 mg/ml (Closantel 10%; Closiver ADETM, Andoci, Mexico City, Mexico). The control group was kept under similar conditions without added anthelmintic drugs. Each assay was replicated four times to ensure the veracity of the results.
The microtiter plates were placed in a dark incubator at a room temperature of 26°C and monitored for 14 days. The plates were exposed to daylight (approximately 12 h) one day before the evaluation to stimulate hatching. The following day, the content of each well was examined under an optic microscope (10×), and the number of eggs in each stage of development was recorded according to Fairweather et al. (Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012).
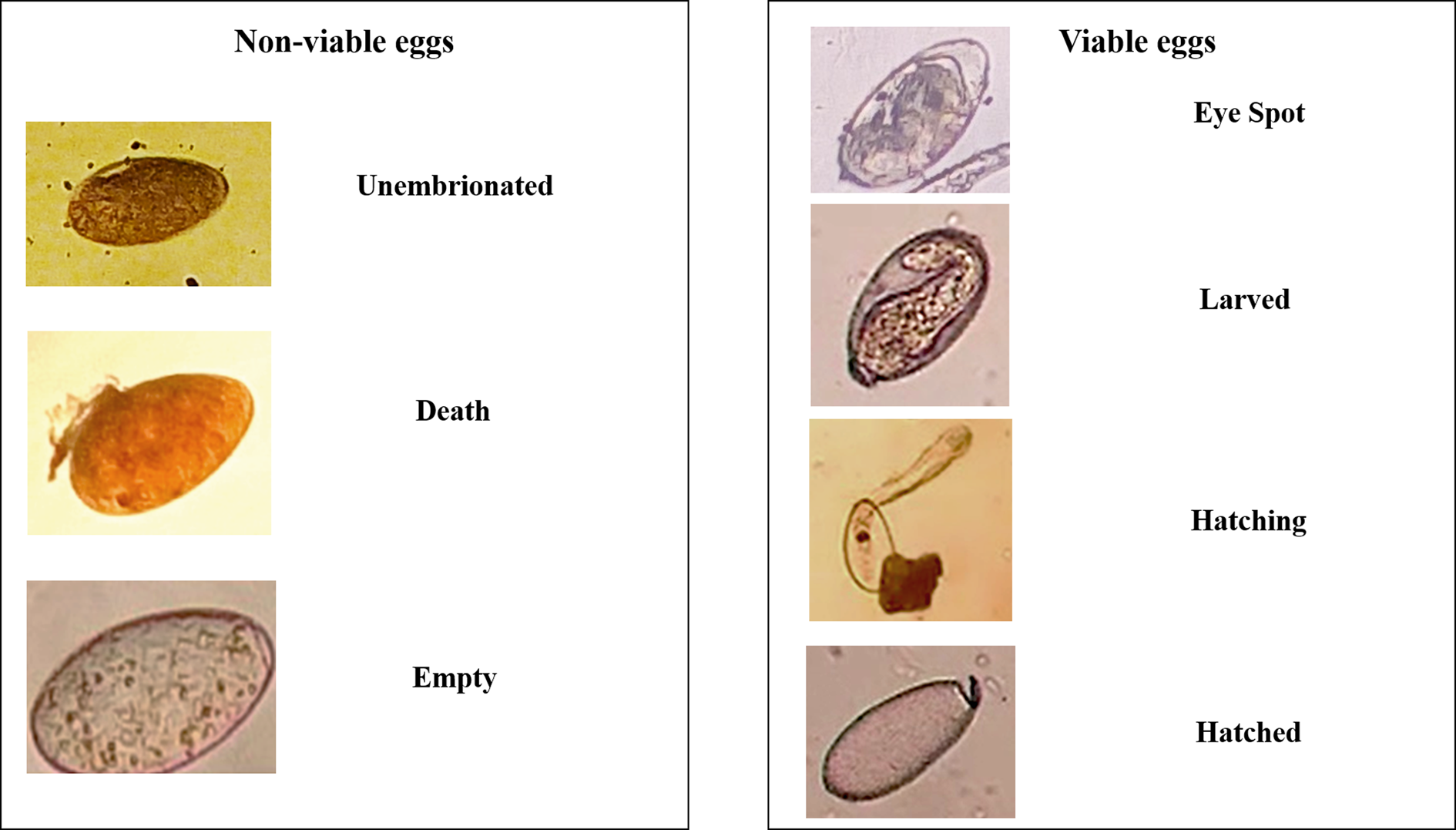
The efficacy of flukicidal drugs was evaluated by probit analysis. Then, rumen fluke eggs were classified as non-viable (dead, empty, unembryonated or under cell division without movement or pulsation after 15 days of incubation) or viable (eye spots, hatching or hatched) (Fairweather et al., Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012).
Statistical analysis
The proportion of non-viable and viable eggs was calculated to obtain the median lethal dose (LD50) and maximum lethal dose (LD99) using a logistic regression model (probit) in the SAS program (SAS, 2017):
where β = vector of parameter estimates, F = cumulative distribution function (normal, logistic or extreme value), x = vector of explanatory variables, Pr = probability of a response, C = natural (threshold) response rate and Ф = the normal cumulative distribution function.
Results
Rumen flukes from a total of 119 animals were evaluated in the abattoir. Three different species of rumen flukes were taxonomically identified: Calicophoron brothriophoron was found in cattle from two localities in Juarez, Chiapas (JC24519 and JRO7619); Calicophoron clavula in cattle from Huimanguillo, Tabasco (HP7619), and Reforma, Chiapas (RM28619); and Paramphistomum cervi in cattle from Juarez, Chiapas (JRJ19719). The number of animals from each locality, number of infected animals and prevalence of rumen flukes in the inspected animals are shown in table 1.
Table 1. Prevalence and coding of paramphistomide strains according to their origin and date of collection.

The lethal doses of the analysed anthelmintic drugs are shown in table 2. Closantel caused the 100% mortality of isolated JRJ19719ϕ at all tested doses. Therefore, no values are reported in the probit analysis. However, closantel resulted in high variability in the LD50 values of the different analysed isolates (17–122 μg/ml). In particular, the isolated RM28619 presented high mortality at all evaluated doses (fig. 2).

Fig. 2. Relationship of the mortality of eggs of four isolates of paramphistomides with respect to the dose used of rafoxanide.
Table 2. Significance and values of the regression parameters (β0 and β1), natural mortality rate (C), mean lethal dose 50 (LD50) and 99 (LD99) in paramphistomide eggs in different strains.

ϕ 100% mortality in all doses; ∞not effective against eggs; ƍhighly effective product. Conc, product concentration; ns, slope was not significant; β0, intercept; *Significant variable with p < 0.005; **Significant variable with p < 0.01.
Rafoxanide showed the lowest efficacy against egg development. The LD50 values for all studied isolates were very high, from 500 to 1713 μg/ml (fig. 3). Moreover, the LD50 and LD99 values for the isolated JRJ19719 were not significant because mortality was similar between them and the control group. Also, it was not effective against eggs of P. cervi.

Fig. 3. Relationship of the mortality of eggs of four isolates of paramphistomides with respect to the dose used of Closantel.
Nitroxinil showed the highest efficacy against egg development. The cases in which the regression slope was not significant occurred due to the high mortality at the lowest doses. The LD50 values of nitroxinil were 0.11–65 μg/ml, and the lethal doses in even the non-significant cases corresponded with high mortality (table 2 and fig. 4).

Fig. 4. Relationship of the mortality of eggs of four isolates of paramphistomides with respect to the dose used of nitroxinil.
The analysis of each developmental category of eggs (viable/non-viable) showed that 20% of eggs died naturally and above the LD50 threshold.
Discussion
The present study is the first to examine the effects of flukicidal drugs against in vitro egg development of common rumen flukes in the humid Mexican tropics. The flukicidal effects of anthelmintic drugs in cattle have generally been tested in vivo (Rolfe & Boray, Reference Rolfe and Boray1987). Rumen flukes can cause significant damage to their ruminant hosts, affecting rumen papillae and causing acanthosis and/or hyperkeratosis of the epithelium. Infiltration of inflammatory cells is often related to epithelial changes in the duodenal mucosa and submucosa (Fuertes et al., Reference Fuertes, Pérez, Benavides, González-Lanza, Mezo, González-Warleta and Ferreras2015). Also, metacercariae from cattle can possibly contaminate vegetables for human consumption, resulting in zoonotic infections (González-Warleta et al., Reference González-Warleta, Lladosa, Castro-Hermida, Martínez-Ibeas, Conesa, Muñoz, López-Quílez, Manga-González and Mezo2013; Sanchís et al., Reference Sanchís, Sánchez-Andrade, Macchi, Piñeiro, Suárez, Cazapal-Monteiro and Arias2013; Ferreras et al., Reference Ferreras, González-lanza and Pérez2014; Khedri et al., Reference Khedri, Radfar, Borji and Mirzaei2015; Huson et al., Reference Huson, Oliver and Robinson2017; Ploeger et al., Reference Ploeger, Ankum, Moll and Holzhauer2017).
The inadequate use of anthelmintic drugs has generated an increase in the resistance of diverse species of parasites in recent times. This can result in high economic losses to livestock production systems, especially considering that flukicidal drugs are expensive (Zhang et al., Reference Zhang, Si, Zhou, Shang, Li and Zhang2019).
An indirect technique to determine the efficacy of flukicidal drugs is through in vitro assays. This technique evaluates the effect of different drugs on the development of fluke eggs, which contain the genetic information on the next generation of flukes. Recently, in vitro protocols to evaluate flukicidal drugs against F. hepatica eggs were developed (Fairweather et al., Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012; Canevari et al., Reference Canevari, Ceballos and Sanabria2014). Subsequently, the methods to determine the viability and development of Calicophoron daubneyi eggs were standardized (Chryssafidis et al., Reference Chryssafidis, Fu, De Waal and Mulcahy2015). The methods of the present study were based on these previous studies.
The results showed high variability in the development and response of rumen fluke eggs following exposure to flukicidal drugs. This could be associated with the frequent use of the same drugs to control rumen flukes, which might result in greater resistance (González-Garduño et al., Reference González-Garduño, Hernández-Hernández, Ortiz-Pérez and Torres-Hernández2019). It is also notable that the number of non-viable eggs was high in all treatments, reflecting the naturally high rate of egg mortality in rumen flukes. Similar results were reported by Chryssafidis et al. (Reference Chryssafidis, Fu, De Waal and Mulcahy2015).
In cases where the probit regression slope was not significant (β1), the LD50 and LD99 values had no biological significance. This implies that there is no dose-dependent effect, and two situations can be occurring: (a) the estimated dose was too high, implying low efficacy, such as occurred with rafoxanide; or (b) the drug was so highly effective that all eggs died at the lowest doses. Therefore, when the mortality was 100% in all wells, the probit analysis could not be performed because no dose-dependent effect was observed, as occurred with closantel. In fact, closantel and nitroxinil presented a good inhibitor effect on egg development that was not observed for rafoxanide.
Rafoxanide and closantel are compounds belong to halogenated salicylanilides, which have similar physicochemical characteristics, such as their molecular weight (rafoxanide 626 and closantel 663 g/mol), their potent inhibitor effect on electron transport associated with mitochondrial phosphorylation and adenosin triphosphate production, are highly lipid-soluble and have low solubility in water (Swan, Reference Swan1999). The availability of closantel depends on binding to plasma proteins (mainly albumin), and it has been suggested that in the digestive tract; it depends on proteins or carrier molecules to reach the target sites (Rothwell et al., Reference Rothwell, Lacey and Sangster2000). Nitroxinil is a halogenated phenol, unlike rafoxanide and closantel; this compound has an adequate solubility in water and its molecular weight (290 g/mol) is less than these compounds (Rahman et al., Reference Rahman, Kabir and Ahmed2017). These nitroxinil characteristics could contribute to passing through the eggshell and affect the viability of the eggs more effectively than rafoxanide and closantel.
The technique to assess anthelmintic resistance based on the classification of seven different categories of eggs previously described by Fairweather et al. (Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012) was not practical and took a long time. Moreover, they described a category corresponding to the ‘cell division stage’, which is reached after approximately seven to eight days of incubation (Fairweather et al., Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012). The proportion of eggs in this category was analysed 15 days after incubation yet did not present movement or pulsation and, therefore, did not reach the following development stage. Accordingly, the eggs that presented these characteristics were considered non-viable. To avoid the use of multiple categories in the egg development and thereby facilitate the interpretation of the results, the probit analyses were performed using only the non-viable and viable egg classifications (fig. 5).
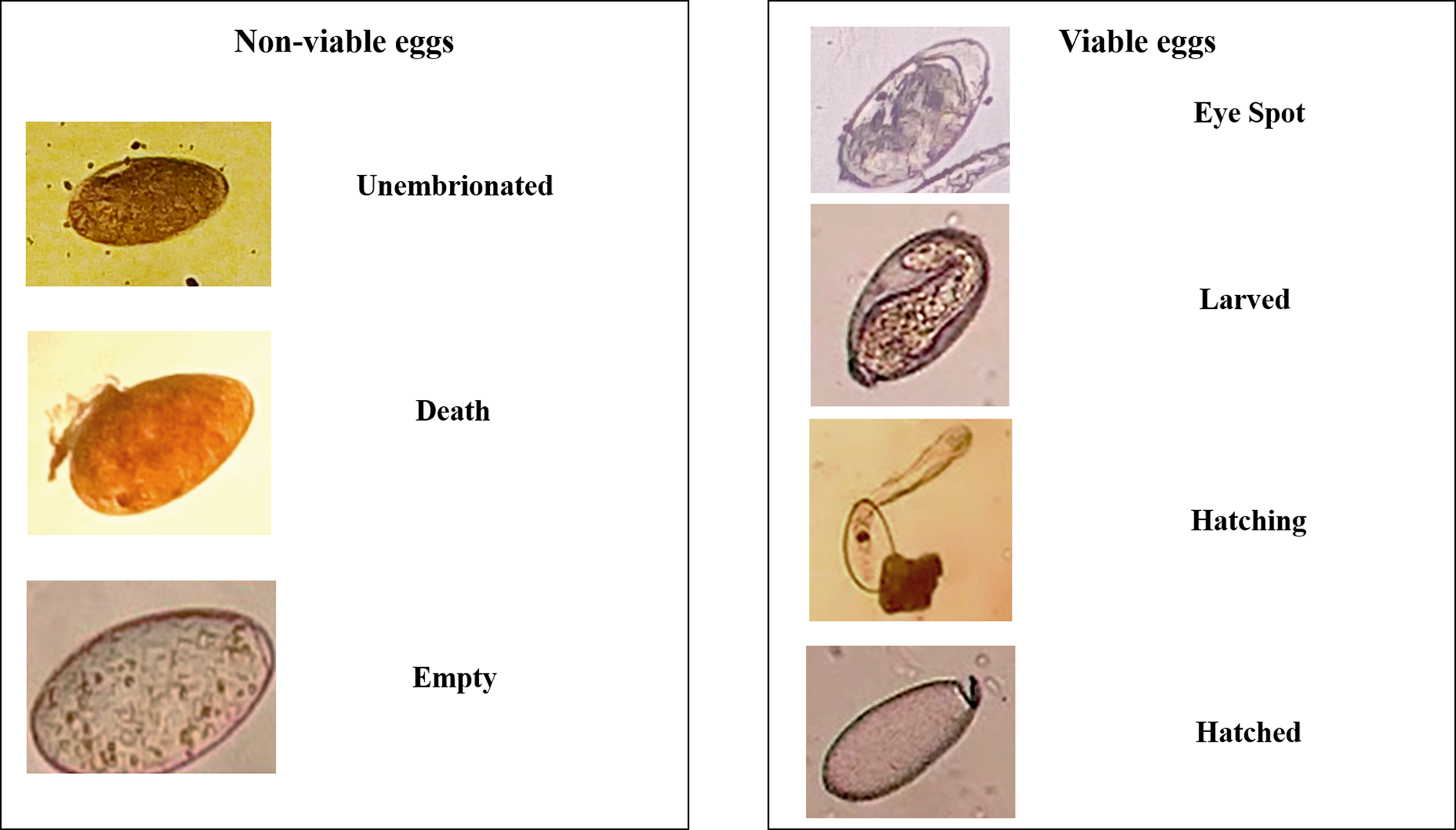
Fig. 5. Classification of stages of development of rumen fluke eggs.
Previous in vivo evaluations of flukicidal drugs have often been based on faecal count reduction tests. These tests suppose that egg production is due to the ineffectiveness of the drugs utilized to eliminate adult flukes. One study carried out in dairy cattle naturally infected by C. daubneyi showed that egg output was not fully suppressed by the studied drugs. A faecal count reduction of 0–26% was found in cows receiving albendazole and netobimin, and 97–99% in cows receiving closantel and oxyclozanide (Arias et al., Reference Arias, Sanchís and Francisco2013). In contrast to the present study, a previous study reported the inefficacy of closantel in treating rumen fluke infections in three different herds (Malrait et al., Reference Malrait, Verschave, Skuce, Van Loo, Vercruysse and Charlier2015). Meanwhile, a study in sheep reported that oxyclozanide was 99% effective against Paramphistomum leydeni adults (Sanabria et al., Reference Sanabria, Moreno, Alvarez, Lanusse and Romero2014).
Compared to in vivo studies, in vitro assays can prevent unnecessary costs through first screening flukicidal drugs and identifying those with little efficacy. It is important to understand which flukicidal drugs affect the viability of eggs and to what extent (Chryssafidis et al., Reference Chryssafidis, Fu, De Waal and Mulcahy2015) to control future infections in herds. Interference in the life cycle of flukes can potentially reduce the risk of infection to final hosts. For example, the production of miracidia can also be delayed by flukicidal drugs (Sanabria & Romero, Reference Sanabria and Romero2008), especially those with a half-life of several days, such as closantel and nitroxinil (Swan, Reference Swan1999).
Given the re-emergence of rumen fluke infections in several countries of the world, it is important to understand the basic biology of flukes in order to develop diagnostic tools. Also, as shown herein, it is important to test the effectiveness of the different flukicidal drugs available for controlling these infections in order to decrease the negative effects on livestock.
Conclusions
The evaluated flukicidal drugs presented differential efficacy against the development of rumen fluke eggs. Nitroxinil presented the highest efficacy in stopping the development of eggs, whereas rafoxanide presented the lowest. Closantel presented variable efficacy depending on the evaluated isolated.
Financial support
This work was supported by the General Department of Research and Postgraduate Studies of the Universidad Autónoma Chapingo (grant number 20010-C-67).
Conflicts of interest
None.
Ethical standards
The procedures were carried out in accordance with the Official Mexican Standard 051-ZOO-1995 for humanitarian treatment of mobilized animals.