Introduction
About half of patients with bipolar disorder (BD) exhibit persistent trait-related cognitive impairments, that present during acute episodes as well as during stable periods of remission (Bourne et al., Reference Bourne, Aydemir, Balanzá-Martínez, Bora, Brissos, Cavanagh and Goodwin2013; Cullen et al., Reference Cullen, Ward, Graham, Deary, Pell, Smith and Evans2016; Torres, Boudreau, & Yatham, Reference Torres, Boudreau and Yatham2007). Similar cognitive deficits are also found in patients’ unaffected relatives – albeit to a lesser degree (Arts, Jabben, Krabbendam, & Van Os, Reference Arts, Jabben, Krabbendam and Van Os2007; Bora, Yucel, & Pantelis, Reference Bora, Yucel and Pantelis2009), suggesting that deficits in cognition are associated with a genetic risk for BD. Nevertheless, the neurodevelopmental trajectory of cognitive impairment in BD is unclear (Goodwin, Martinez-Aran, Glahn, & Vieta, Reference Goodwin, Martinez-Aran, Glahn and Vieta2008) given the paucity of studies on neurocognition in patients at illness onset. Indeed, studies examining cognition in first-episode BD have found widespread neurocognitive deficits on par with chronic, established BD (Bora & Pantelis, Reference Bora and Pantelis2015; Lee et al., Reference Lee, Hermens, Scott, Redoblado-Hodge, Naismith, Lagopoulos and Hickie2014). Further, the identification of neurocognitive endophenotypes (i.e. intermediate phenotypes) may elucidate the pathophysiology of BD by providing insight into the association between cognitive impairments and genetic risk (Kessing & Miskowiak, Reference Kessing and Miskowiak2018). Hence, there is a need for research investigating the pattern of neurocognitive dysfunction both in patients with BD at early illness stages and in their unaffected first-degree relatives.
Meta-analytic findings have consistently shown trait-related deficits in executive function and verbal memory in patients with BD (Bora et al., Reference Bora, Yucel and Pantelis2009; Bourne et al., Reference Bourne, Aydemir, Balanzá-Martínez, Bora, Brissos, Cavanagh and Goodwin2013; Robinson et al., Reference Robinson, Thompson, Gallagher, Goswami, Young, Ferrier and Moore2006; Torres et al., Reference Torres, Boudreau and Yatham2007). However, recent studies indicate that neurocognitive impairments in remitted BD patients are heterogeneous. In fact, studies using data-driven approaches have identified discrete neurocognitive subgroups in remitted patients with BD (Bora et al., Reference Bora, Hıdıroğlu, Özerdem, Kaçar, Sarısoy, Civil Arslan and Tümkaya2016; Burdick et al., Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014; Jensen, Knorr, Vinberg, Kessing, & Miskowiak, Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Lima et al., Reference Lima, Rabelo-da-Ponte, Bücker, Czepielewski, Hasse-Sousa, Telesca and Rosa2019; Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017), comprising a ‘cognitively intact’ subgroup with neurocognitive performance comparable to healthy controls (HCs), one or two subgroups of ‘selectively impaired’ patients exhibiting mild to moderate impairments within the domains of verbal learning and psychomotor speed (although these differ between studies), and a ‘globally impaired’ subgroup with severe impairments across several cognitive domains (Bora et al., Reference Bora, Hıdıroğlu, Özerdem, Kaçar, Sarısoy, Civil Arslan and Tümkaya2016; Burdick et al., Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014; Jensen et al., Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Lewandowski, Sperry, Cohen, & Öngür, Reference Lewandowski, Sperry, Cohen and Öngür2014; Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill and Burdick2017). Compared to patients who are cognitively intact, those with neurocognitive deficits display poorer functional capacity and quality of life in the absence of differences in subsyndromal mood symptoms (Jensen et al., Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Solé et al., Reference Solé, Jiménez, Torrent, del Mar Bonnin, Torres, Reinares and Martínez-Arán2016). Yet it remains unclear whether this cognitive heterogeneity is present already at BD illness onset which would point to a genetic or neurodevelopmental origin. Further, only one study to date has examined cognitive heterogeneity in unaffected relatives of patients with BD (Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017). In this study, relatives of patients with global cognitive impairments presented with deficits in verbal memory and a global measure of cognition albeit less severe compared to their BD relatives (Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017). This provides emerging evidence for global cognitive impairment being associated with genetic risk for BD.
Neurocognitive impairments are not specific to BD; indeed, similar patterns of neurocognitive impairments are found in patients with unipolar disorder and schizophrenia although with varying severity (Kessing & Miskowiak, Reference Kessing and Miskowiak2018). Impairments in affective cognition may therefore constitute a stronger putative illness-specific endophenotype for BD (Miskowiak et al., Reference Miskowiak, Kjærstad, Meluken, Petersen, Maciel, Köhler and Carvalho2017b). Deficits in ‘hot’ (i.e. emotion-laden) cognition, including emotion processing and – regulation, are potential key features of BD, that may also present in patients’ unaffected relatives (Miskowiak et al., Reference Miskowiak, Kjærstad, Meluken, Petersen, Maciel, Köhler and Carvalho2017b). Emotion processing and – regulation tap into neurocognitive processes (Lima, Peckham, & Johnson, Reference Lima, Peckham and Johnson2018). Neuroimaging studies indicate that non-emotional neurocognition and affective cognition involve a shared ‘cognitive control’ neural circuitry including dorsal and medial prefrontal regions (Ochsner, Silvers, & Buhle, Reference Ochsner, Silvers and Buhle2012; Phillips, Ladouceur, & Drevets, Reference Phillips, Ladouceur and Drevets2008). Further, behavioural evidence has shown that neurocognition – particularly performance on processing speed and working memory tests – is closely associated with the performance on emotional face processing tasks (Van Rheenen, Meyer, & Rossell, Reference Van Rheenen, Meyer and Rossell2014; Van Rheenen & Rossell, Reference Van Rheenen and Rossell2016) and emotion regulation in BD (Lima et al., Reference Lima, Peckham and Johnson2018). Emerging evidence also suggests that patients with BD who are neurocognitively impaired experience difficulties with facial expression recognition (Van Rheenen & Rossell, Reference Van Rheenen and Rossell2016) and social cognition (Lima et al., Reference Lima, Rabelo-da-Ponte, Bücker, Czepielewski, Hasse-Sousa, Telesca and Rosa2019). In contrast, patients who are neurocognitively intact display no facial expression recognition difficulties (Van Rheenen & Rossell, Reference Van Rheenen and Rossell2016) but rather superior social cognition relative to HCs (Burdick et al., Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014). Yet, little is known about heterogeneity within affective cognition in BD and whether impairments in this domain are related to the pattern of patients’ non-emotional cognitive deficits. In particular, no study has yet examined affective cognition in cognitive subgroups of unaffected relatives of patients with BD.
The aims of the current study were three-fold: firstly, we aim to assess cognitive heterogeneity in a large sample of remitted/partially remitted patients recently diagnosed with BD using a data-driven approach. We expected to find three district neurocognitive clusters, in accordance with previous research on patients with BD (Burdick et al., Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014; Jensen et al., Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill and Burdick2017). Secondly, we aim to investigate whether patients in the neurocognitive clusters would exhibit differences in affective cognition, including emotion processing and regulation, and functional capacity. Thirdly, we wanted to examine whether neurocognitive function in unaffected first-degree relatives of patients with BD would be intermediate to that of their affected relative and a sample of unrelated HCs across a non-affective (i.e. neurocognitive) and affective cognition.
Methods
Study design
The study is a cross-sectional assessment of baseline data from the ongoing, longitudinal Bipolar Illness Onset (BIO)-study, work-package three, which aims to investigate brain-based biomarkers of BD (Kessing et al., Reference Kessing, Munkholm, Faurholt-Jepsen, Miskowiak, Nielsen, Frikke-Schmidt and Vinberg2017). Recruitment and data collection for the current report was conducted between June 2015 and August 2018. The study protocol was approved by the Committee on Health Research Ethics of the Capital region of Denmark (protocol number: H-7-2014-007) and the Danish Data Protection Agency, Capital Region of Copenhagen (protocol number: RHP-2015-023). All participants provided informed consent prior to inclusion of the study.
Participants
Data were collected from 320 participants; including 158 patients with BD, 52 of their unaffected, first-degree relatives and 110 HCs. Patients were diagnosed with BD within 2 years prior to study inclusion, between 15 and 70 years of age, were consecutively recruited from the Copenhagen Affective Disorder Clinic, Psychiatric Centre Copenhagen, Denmark, where they received treatment as usual throughout study participation. The patients were screened with the semi-structured Schedules for Clinical Assessment in Neuropsychiatry (SCAN; Wing et al., Reference Wing, Babor, Brugha, Burke, Cooper, Giel and Sartorius1990) interview to confirm the ICD-10 (World Health Organisation, 1993) BD diagnosis, and were rated for depression and mania symptoms with the Hamilton Depression Rating Scale-17 (HDRS-17; Hamilton, Reference Hamilton1967) and the Young Mania Rating Scale (YMRS; Young, Biggs, Ziegler, & Meyer, Reference Young, Biggs, Ziegler and Meyer1978) to ensure full or partial remission (HDRS-17 and YMRS-scores ⩽ 14, respectively). Exclusion criteria for the patients were a history of brain injury, severe somatic illness, current substance abuse or neurological illnesses including dementia.
Patients’ unaffected relatives, siblings and/or children between 15 and 40 years of age, were invited to participate in the study with permission from the respective BD patient. Age- and sex-matched controls, 15–70 years of age, were recruited consecutively from the University Hospital Blood Bank, Rigshospitalet, Copenhagen. The relatives and HCs were free of a psychiatric disorder, as confirmed with the SCAN interview. Relatives and HCs were excluded from the study if they had a history of treatment-required psychiatric disorder, current substance abuse disorder or neurological illnesses including dementia. An additional exclusion criterion for the HCs was having a first-degree relative with a history of treatment-required psychiatric illness. All participants were fluent in Danish.
Measures
Measures of neurocognition
Participants were administered a large neurocognitive test battery, comprising the Trail Making Test-A (TMT-A) and the Trail Making Test-B (TMT-B) (Army Individual Test Battery, 1944), the Rey Auditory Verbal Learning Test (RAVLT) (Corwin, Reference Corwin1994; Rey, Reference Rey1958), the Letter-Number-Sequencing subtest from Wechsler's Adult Intelligence Scale 3rd edition (WAIS-III) (Wechsler, Reference Wechsler1997), verbal fluency with letters S and D (Borkowski, Benton, & Spreen, Reference Borkowski, Benton and Spreen1967), Coding and Digit Span Forward from the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph, Tierney, Mohr, & Chase, Reference Randolph, Tierney, Mohr and Chase1998), the Spatial Working Memory (SWM) test and the Rapid Visual Information Processing (RVP) test from the Cambridge Neuropsychological Test Automated Battery (CANTAB). Finally, premorbid verbal IQ was estimated using the National Adult Reading Task, Danish translation (DART) (Nelson & O'Connell, Reference Nelson and O'Connell1978) (premorbid IQ was not calculated for six patients and two HCs due to dyslexia).
Measures of affective cognition
The Social Scenarios Task was used to assess emotion reactivity and -regulation to social scenarios. Short written descriptions of negative or positive social situations and accompanying self-beliefs were presented on a computer screen. Participants were instructed to either naturally react to, or dampen their emotional response, to the social scenarios. The paradigm comprised nine blocks, in which each block consisted of an initial instruction to ‘react’ or ‘dampen’, followed by the presentation of 11 sentences describing the social scenario (3 s each), interleaved with 10 related self-beliefs (3 s each) and finally an emotion rating requiring participants to evaluate their discomfort or pleasure (for the negative and positive scenarios, respectively) on a 100-point visual analogue scale. The first block was a neutral condition, ensued by two negative social scenarios with interspersed react/dampen conditions. Participants were not instructed to use a specific emotion regulation strategy during the ‘dampen’ conditions, with the purpose of eliciting the emotion regulation strategy that would most likely spontaneously be triggered in real-life scenarios (Kjærstad et al., Reference Kjærstad, Vinberg, Goldin, Køster, Støttrup, Knorr and Miskowiak2016). Two social scenarios involved the attraction to, or rejection by, men or women, respectively. Sexual orientation was therefore assessed, and the corresponding version of the task was administered.
Processing of emotional faces was evaluated with the Facial Expression Recognition Task and the Faces Dot-Probe Task and – both from the Emotional Test Battery (P1vital® Oxford Emotional Test Battery, 2017). The Facial Expression Recognition Task assessed facial expression identification. Participants viewed a series of faces depicting anger, disgust, sadness, fear, happiness or surprise on a computer screen, morphed at 10% intensity levels between a neutral face (0%) and full emotion (100%) (Ekman, Reference Ekman1976). Each facial depiction was presented for 500 ms immediately followed by a black screen during which participants were to press a key on the keyboard to indicate which facial expression was shown. A total of 250 pictures of faces were presented, comprising four pictures of each emotion at each of the 10 intensity levels (Harmer, Shelley, Cowen, & Goodwin, Reference Harmer, Shelley, Cowen and Goodwin2004). Accuracy and reaction times were registered.
The Faces Dot-Probe Task assessed attentional vigilance towards emotional faces. Participants were presented with pairs of happy–neutral, fearful–neutral or neutral–neutral faces, in which one of the faces was immediately replaced by either two vertical (⋅⋅) or two horizontal (:) dots. Trials were either unmasked (100 ms) or masked (17 ms). In the masked trial, the pair of faces was replaced by a mask of a jumbled face. Participants were instructed to ascertain the orientation of the dots as rapidly and accurately as possible by pressing the corresponding keys on the laptop keyboard. The paradigm consisted of 16 blocks (eight masked and eight unmasked) in total, and each block included 12 alternately presented trials (192 trials in total) (Murphy, Downham, Cowen, & Harmer, Reference Murphy, Downham, Cowen and Harmer2008).
The Social Scenarios task and the Faces Dot-Probe task were carried out on a Lenovo T450s laptop computer using E-Prime version 2.0, and the Facial Expression Recognition task was completed on a Dell PP181 using Superlab Pro version 1.05.
Measure of functioning
The Functional Assessment Short Test (FAST) is an interviewer-administered interview with high concurrent validity and internal reliability in patients with BD, consisting of 24 items that covers six areas of functioning: autonomy, occupational functioning, cognitive functioning, financial issues, interpersonal relationships and leisure time (Rosa et al., Reference Rosa, Sánchez-Moreno, Martínez-Aran, Salamero, Torrent, Reinares and Vieta2007).
Statistical analysis
Raw test scores were standardised to z-scores (i.e. M = 0, s.d. = 1) based on HCs’ scores using the formula: (test score − HC test M)/HC test s.d. Outlying z-scores of >4 s.d.s below HC mean were then truncated at z = −4.0. The z-scores for Trail-Making Test (A and B) and CANTAB Spatial working memory (‘between errors’ and ‘strategy’) and Rapid visual processing (‘mean latency’) were inverted so that lower scores represented poorer performance. Six neurocognitive domains were calculated from the mean z-scores comprising each domain: Verbal Learning [RAVLT (total correct for trial I-IV, recall following list B, and 30 min recall)]; Processing Speed (TMT-A and RBANS digit-symbol coding); Verbal Fluency (Verbal fluency S and D); Working Memory [SWM (between errors and strategy), RBANS digit-span-forward, WAIS letter-number sequencing]; Sustained Attention (RVP accuracy and mean latency); Executive Control (TMT-B).
To investigate homogeneous subgroups of BD based on neurocognitive performance, we used hierarchal cluster analysis (HCA) with squared Euclidian distance and Ward's linkage as an agglomeration procedure consistent with prior studies on BD (Jensen et al., Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill and Burdick2017). The appropriate number of clusters was determined by conducting visual inspections of the resulting dendrogram and agglomeration schedule (Yim & Ramdeen, Reference Yim and Ramdeen2015). A discriminant function analysis (DFA) was performed to determine the validity of the resulting clusters from the HCA and to examine the predictive power of neurocognitive performance in differentiating patients into discrete neurocognitive groups. Leave-one-out classification was used in the classifications to evaluate the reliability of the classification model generated by the DFA. Only the BD sample was included in the cluster analysis so that the emergent BD clusters could subsequently be compared to the HCs, and the BD sample's relatives could be assigned to the same cluster as their affected relatives.
Demographics and clinical variables were compared between the resulting clusters and HCs with a series of Analysis of variances (ANOVAs) with least significant difference (LSD) correction and χ2. The variables included: sex, age, education, IQ, HDRS-17, YMRS, BD-type, illness duration, untreated BD duration, number of episodes (depressive/manic/hypomanic/mixed/psychotic), number of hospitalisations, medication (yes/no: antidepressants, antipsychotics, anticonvulsants, lithium) and functioning (i.e. FAST domains and total score).
A one-way multivariate analyses of variance (MANOVA) with post-hoc LSD correction was carried out to compare the BD clusters and HCs on neurocognitive functioning. The global cognition composite measure was not included in the MANOVA due to violation of the assumption of multicollinearity. Instead, the BD clusters and HCs were compared on global cognitive functioning using univariate ANOVA. For affective cognition, a series of ANOVAs with post-hoc LSD correction were conducted to compare the BD clusters and HCs on (i) discrimination accuracy and RT during facial expression recognition; (ii) emotional reactivity and down-regulation of emotions to negative and positive social scenarios and (iii) attentional vigilance to happy and fearful faces. The primary analyses were performed unadjusted for mood symptoms and demographic variables. In cases were significant group effects emerged, we performed secondary analyses with adjustment for depressive (HDRS-17) and manic symptoms (YMRS), years of education and IQ.
To examine the association between neurocognitive impairment and genetic risk, the unaffected relatives were assigned groups based on the cognitive cluster of their respective BD relatives. Cognitive performance among relatives of cognitively impaired patients, relatives of cognitively intact patients, and HCs was compared using the same method as above.
Finally, post-hoc exploratory Pearson correlations were conducted to investigate the relationship between global neurocognitive performance and affective cognition. All statistical analyses were conducted using SPSS version 22.
Results
Cognitive clustering
Patients with BD were optimally clustered into three discrete subgroups based on their neurocognitive profiles: 45.6% (n = 72) were cognitively intact; 31.0% (n = 49) selectively impaired and 23.4% (n = 37) globally impaired (see online Supplementary Fig. S1 for agglomeration schedule and dendrogram). Results from the DFA revealed two discriminant functions explaining 62.3% and 37.7% of the variance, respectively (Wilks' λ = 0.180, χ2(12) = 261.54, p < 0.001 and Wilks' λ = 0.490, χ2(5) = 108.90, p ⩽ 0.001; see online Supplementary Fig. S2 for discriminant functions plot). Executive control and verbal fluency contributed most to clustering [highest loading domain for Function 1: executive control (r = 0.86); highest loading domain for Function 2: verbal fluency (r = 0.80)]. The classification results revealed high sensitivity with 91.8% of original grouped cases being correctly classified.
There were only five relatives of BD patients in the ‘globally impaired’ cluster, and we therefore chose to group together relatives who had a BD relative categorised as ‘selectively impaired’ (n = 18) and ‘globally impaired’ (n = 5) into one cluster of relatives with a cognitively impaired BD relative (UR impaired: n = 23; siblings = 20, children = 3; UR intact: n = 25; siblings = 24, children = 1).
Demographic and clinical variables
Comparisons between BD neurocognitive clusters and HCs revealed a significant difference between the groups on HDRS 17 (F 3, 264 = 38.3, p < 0.001, η 2 = 0.30) and YMRS (F 3, 264 = 14.54, p < 0.001, η 2 = 0.14), which reflected more residual depression and mania symptoms in BD patients (irrespective of their neurocognitive cluster) than in HCs (ps ⩽ 0.004), more depression and mania symptoms in the globally impaired group than in the cognitively intact group (p = 0.012 and p < 0.001), and more mania symptoms in the globally impaired group than in the selectively impaired group (ps ⩽ 0.003) (Table 1). The groups also differed in education (F 3, 264 = 6.34, p < 0.001, η 2 = 0.07); the globally and selectively impaired patients had undergone fewer years of education compared to HCs (ps ⩽ 0.007), and the selectively impaired patients had undergone fewer years of education than the cognitively intact patients (p = 0.010). A significant group difference was found for premorbid IQ (F 3, 258 = 8.31, p < 0.001, η 2 = 0.09), driven by patients categorised as globally impaired and selectively impaired having lower premorbid IQ than HCs (ps ⩽ 0.027) and cognitively intact patients (ps ⩽ 0.002).
Table 1. Demographic and clinical variables comparing the three BD cognitive clusters and HCs
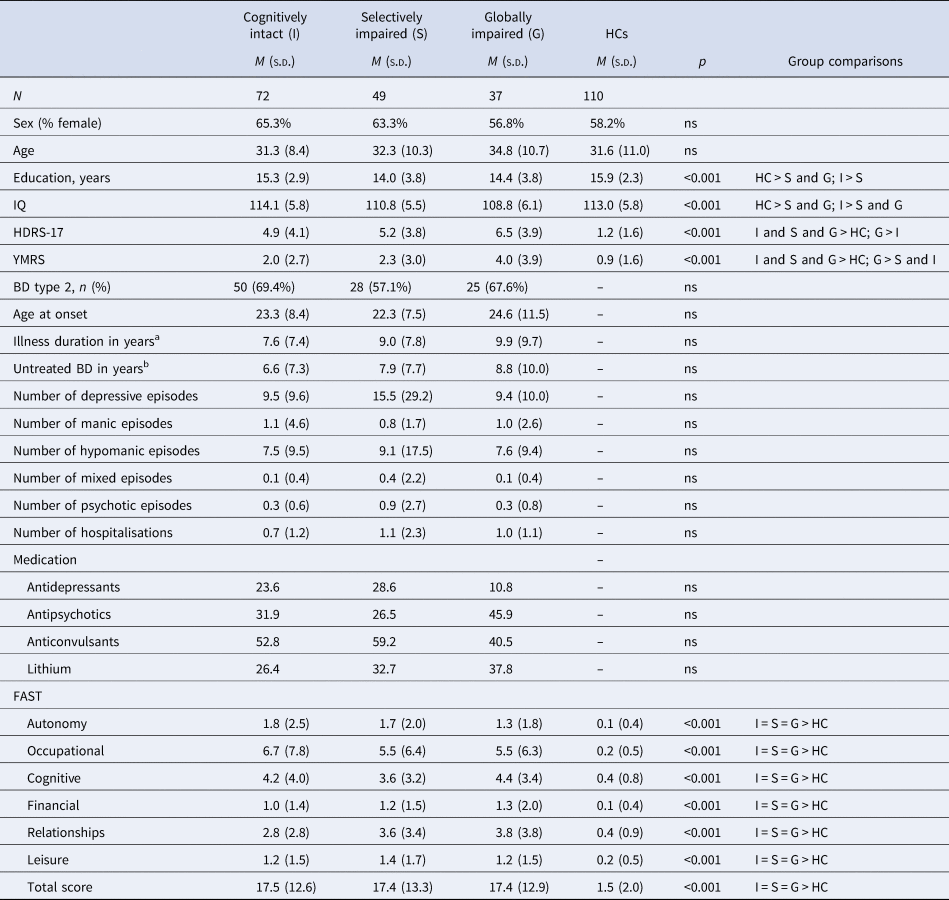
BD, bipolar disorder; HDRS, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; FAST, Functional Assessment Short Test.
a Illness duration was defined as the time from the first mania, hypomania or mixed episode to the time of the first testing in BIO.
b Untreated BD was calculated as time from first mania, hypomania or mixed episode to the time of diagnosis.
There was a significant difference between the relatives grouped according to their affected relatives’ neurocognitive subgroups in age (F 2, 155 = 3.51, p = 0.032, η 2 = 0.043), years of education (F 2, 155 = 3.49, p = 0.033, η 2 = 0.043), IQ (F 2, 153 = 4.66, p = 0.011, η 2 = 0.057) and subsyndromal depression symptoms (F 2, 155 = 6.11, p = 0.003, η 2 = 0.073). This was driven by relatives of cognitively impaired patients being younger, less educated and exhibiting lower IQ compared to HCs (ps ⩽ 0.020). These relatives also displayed more subsyndromal depression compared to HCs and relatives of cognitively intact patients (ps ⩽ 0.031) (Table 2) (for comparisons between patients with BD, relatives and HCs, see online Supplementary Table S1).
Table 2. Comparison of unaffected relatives (URs) of patients with BD and HCs on demographics and functioning

BD, bipolar disorder; HC, healthy control; HDRS-17, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; FAST, Functional Assessment Short Test.
Non-emotional cognition
There was a significant difference between the three neurocognitive subgroups of BD patients and HCs in global neurocognitive functioning (F 3, 264 = 49.03, p < 0.001, η 2 = 0.358) as well as on all individual cognitive domains: verbal learning (F 3, 262 = 7.93, p < 0.001, η 2 = 0.083), processing speed (F 3, 262 = 30.24, p < 0.001, η 2 = 0.257), executive control (F 3, 262 = 62.92, p < 0.001, η 2 = 0.419), working memory (F 3, 262 = 13.21, p < 0.001, η 2 = 0.131), verbal fluency (F 3, 262 = 42.93, p < 0.001, η 2 = 0.330) and sustained attention (F 3, 262 = 7.00, p < 0.001, η 2 = 0.105) (Table 3). LSD analyses showed that patients categorised as globally impaired performed significantly poorer than HCs on the global cognition composite measure, as well as on all individual neurocognitive domains: verbal learning, processing speed, executive control, working memory, verbal fluency and sustained attention. They also performed worse than the cognitively intact patients on all measures, except for sustained attention. The selectively impaired cluster performed worse than HCs on the global cognition measure, working memory and verbal fluency, but not on verbal learning, processing speed, executive control or sustained attention. They also exhibited worse performance than the cognitively intact group on verbal fluency, but superior performance on sustained attention. Finally, the cognitively intact cluster performed comparatively to HCs on global cognition and measures of verbal learning, processing speed and executive control. While they did perform worse than HCs on working memory and sustained attention, their verbal fluency performance was better than in HCs (Fig. 1; Table 3). These differences between groups remained significant after controlling for residual depression and mania symptoms (ps ⩽ 0.001), years of education (ps ⩽ 0.001) and IQ (ps ⩽ 0.001).

Fig. 1. Neurocognitive profiles across the three BD cognitive clusters and HCs.
Table 3. Comparison between the three BD neurocognitive clusters and HCs across neurocognitive domains (z-scores)

BD, bipolar disorder; HC, healthy control.
Among unaffected relatives, there were no difference in global cognitive function between those with cognitively impaired or cognitively intact BD relatives, respectively, and HCs (p = 0.418). However, relatives of the cognitively impaired patients performed worse than HCs on both verbal fluency (F 2, 152 = 3.69, p = 0.027, η 2 = 0.046; LSD: p = 0.007) and working memory (F 2, 152 = 3.50, p = 0.033, η 2 = 0.044; LSD: p = 0.020). They also performed poorer than relatives of cognitively intact patients on working memory (p = 0.015). No other group differences were found for the other cognitive domains (ps ⩾ 0.580). The observed group differences were unaltered after adjustment for subsyndromal depression and mania symptoms and age. However, in secondary analyses controlling for years of education, the differences between relatives of cognitively impaired/intact patients and HCs in working memory were reduced to a trend (F 2, 151 = 2,59, p = 0.078, η 2 = 0.033), and when controlling for IQ, the group differences rendered non-significant (working memory: p = 0.147, verbal fluency: p = 0.155).
Affective cognition
Facial expression recognition
There was a significant difference between the three neurocognitive clusters of patients with BD and HCs in discrimination accuracy to facial expressions (F 3, 247 = 8.55, p < 0.001, η 2 = 0.094); patients categorised as globally impaired displayed lower accuracy during recognition of all facial expressions compared to HCs (p < 0.001), cognitively intact patients (p < 0.001) and selectively impaired patients (p = 0.002) (Fig. 2). Also, there was a strong trend towards differences between the neurocognitive subgroups and HCs in speed of facial expression recognition (F 3, 244 = 2.60, p = 0.053, η 2 = 0.031). This became significant after adjusting for IQ (F 3, 237 = 2.72, p = 0.045, η 2 = 0.033). The group difference was driven by patients categorised as globally impaired being generally slower at recognising facial expressions compared to HCs (p = 0.017), cognitively intact patients (p = 0.050) and selectively impaired patients (p = 0.009). These differences between groups remained statistically significant after adjustment for subsyndromal symptoms, education and IQ.

Fig. 2. Group differences in overall discrimination accuracy across emotions and intensities between neurocognitive clusters in patients with BD, their URs and HCs. BD, bipolar disorder; UR, unaffected relatives; HC, healthy control. Error bars represent standard error of the mean. ***p < 0.001; *p < 0.05.
Among the unaffected relatives, there was a strong trend towards a significant difference between relatives with cognitively impaired and cognitively intact patients and HCs in facial expression recognition accuracy (F 2, 145 = 3.02, p = 0.052, η 2 = 0.040), driven by significantly lower discrimination accuracy in relatives of cognitively impaired patients than in HCs (LSD: p = 0.017) and a trend towards poorer discrimination accuracy in relatives of cognitively impaired patients compared to relatives of cognitively intact patients (LSD: p = 0.057). This main effect of group became statistically significant after adjusting for residual depression and mania symptoms (F 2, 143 = 4.43, p = 0.014, η 2 = 0.058), age (F 2, 144 = 3.93, p = 0.022, η 2 = 0.052) and IQ (F 2, 142 = 3.27, p = 0.041, η 2 = 0.044) (but not education; p = 0.114) (Fig. 2). Results revealed no differences between these groups in speed during facial expression recognition (ps ⩾ 0.110).
Social scenarios task
For the social scenarios task, there was a significant difference between the three neurocognitive subgroups of BD patients and HCs in positive emotion reactivity (F 3, 257 = 3.21, p = 0.024, η 2 = 0.036); driven by patients categorised as selectively impaired reporting lower emotional reactivity to positive scenarios relative to HCs (p = 0.004). There was also a significant difference between the groups for down-regulation of positive emotion (F 3, 257 = 3.07, p = 0.028, η 2 = 0.035); patients categorised as cognitively intact and selectively impaired were less successful at down-regulating their positive emotions than HCs (ps ⩽ 0.027), whereas the globally impaired cluster showed a trend towards being less successful at dampening positive emotion relative to HCs (p = 0.070). In contrast, down-regulation of negative affect revealed no differences between the neurocognitive subgroups in the primary unadjusted analysis (p = 0.098). However, when controlling for subsyndromal depression and mania symptoms, a significant group effect emerged (F 3, 255 = 3.31, p = 0.021, η 2 = 0.037), in which patients categorised as globally impaired were less successful at dampening their emotions to aversive social situations than both HCs (p = 0.006) and patients categorised as selectively impaired (p = 0.011), but not cognitively intact patients (p = 0.147). No significant differences between groups were found for negative emotion reactivity (ps ⩾ 0.808). All differences between the neurocognitive subgroups remained statistically significant when controlling for residual depression and mania symptoms, years of education and IQ.
Among the unaffected relatives, there were no differences in reactivity or down-regulation of emotion in social situations for those with and without cognitively impaired BD relatives or HCs (ps ⩾ 0.401).
Faces dot-probe
No significant differences between the neurocognitive subgroups of BD patients and HCs were found in attentional vigilance to emotional faces as measured with the faces dot-probe task (ps ⩾ 0.233). For the unaffected relatives, there were also no differences between relatives of cognitive impaired patients, relatives of cognitively intact patients and HCs in attentional vigilance to fearful and happy faces (ps ⩾ 0.440).
Functioning
Assessment of functioning in the BD sample showed significant group differences in FAST total score (F 3, 263 = 55.84, p < 0.001, η 2 = 0.39), driven by the three BD clusters having significantly decreased functioning compared to HCs (ps < 0.001), but no differences between the three BD clusters (ps ⩾ 0.778) (Table 1).
For the unaffected relatives, results revealed significant group differences in FAST total score (F 2, 153 = 12.18, p < 0.001, η 2 = 0.137), which was driven by decreased functioning in relatives of cognitively impaired BD patients compared to HCs and to relatives of cognitively intact patients (ps < 0.001) (Table 2).
Exploratory post-hoc correlations
More impairment in the global measure of non-emotional cognition correlated moderately with poorer accuracy of facial expression recognition across the entire cohort (r = 0.39, p < 0.001); significant correlations were also found within the globally impaired patient subgroup (r = 0.43, p = 0.009) and in relatives of cognitively impaired patients (r = 0.64, p = 0.001), and HCs (r = 0.357, p < 0.001). More neurocognitive impairment also correlated with slower speed of facial expression recognition across the entire cohort (r = −0.37, p < 0.001) and within the groups of globally impaired patients (r = −0.59, p < 0.001), selectively impaired patients (r = −0.46, p = 0.003) and HCs (r = −0.41, p < 0.001). Finally, poorer neurocognitive function correlated weakly with less ability to down-regulate positive emotions to social situations across the entire cohort (r = 0.14, p = 0.015) and in the HC group alone (r = 0.20, p = 0.044) (all cognitive clusters: ps ⩾ 0.312). No significant correlations were found between neurocognitive impairment and positive emotion reactivity (ps ⩾ 0.117) (see online Supplementary materials for correlational matrix).
Discussion
The current study explored the pattern of neurocognitive function in a large sample of remitted patients recently diagnosed with BD and their first-degree relatives, as well as the association between neurocognitive function, affective cognition and functioning in these groups. In accordance with our hypothesis, three neurocognitive clusters in patients with BD were detected; a cognitively intact cluster, a selectively impaired cluster and a globally impaired cluster. The three distinct neurocognitive subgroups differed on measures of affective cognition; patients categorised as globally impaired displayed overall poorer facial expression recognition and less successful emotion down-regulation to aversive social situations, selectively impaired patients exhibited less emotional reactivity and difficulties down-regulating emotions in pleasant social scenarios, whereas neurocognitively intact patients were also less successful at down-regulating their emotions in positive social scenarios. Further, not surprisingly, patients with BD (irrespective of neurocognitive subgroup) had decreased functioning compared to HCs. Patients’ unaffected relatives showed no overall impairments in non-emotional cognition. Nevertheless, relatives of cognitively impaired patients exhibited impairments in facial expression recognition and functioning.
The three neurocognitive profiles found in patients recently diagnosed with BD are in line with previous studies in more mixed groups of BD patients with different illness chronicity and levels of mood symptoms (Burdick et al., Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014; Jensen et al., Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Lima et al., Reference Lima, Rabelo-da-Ponte, Bücker, Czepielewski, Hasse-Sousa, Telesca and Rosa2019; Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill and Burdick2017). We identified a subgroup of cognitively intact patients with BD on par with HCs’ performance (global composite z = −0.05); a subgroup of patients with mild global cognitive deficits (global composite z = −0.25) and specific impairment in verbal fluency (z = −0.95); and a subgroup of patients with moderate global cognitive difficulties (global composite z = −1.07) and moderate impairments on all cognitive domains (characterised by scores ⩾0.5 s.d. below the normative mean on all composite measures, consistent with the recommended threshold of cognitive impairment according to the International Society of Bipolar Disorder Cognition Task Force; Miskowiak et al., Reference Miskowiak, Burdick, Martinez-Aran, Bonnin, Bowie, Carvalho and Sumiyoshi2017a). However, the degrees of neurocognitive impairments in the current study were relatively low compared to previous studies of remitted patients; whereas patients categorised as globally impaired in the current study had a global cognition score of −1.07 below the mean, remitted patients with global impairments in previous studies obtained scores ranging between −1.11 and −2.32 below the mean (Burdick et al., Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014; Jensen et al., Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Lima et al., Reference Lima, Rabelo-da-Ponte, Bücker, Czepielewski, Hasse-Sousa, Telesca and Rosa2019; Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017). A possible explanation for the difference in degree of impairments is the lower illness duration of our sample (mean 8.6 years v. mean 15.3 years in previous studies). Indeed, longer illness duration has been suggested to be associated with neurocognitive decline based on evidence from cross-sectional studies (Cardoso, Bauer, Meyer, Kapczinski, & Soares, Reference Cardoso, Bauer, Meyer, Kapczinski and Soares2015). However, recent longitudinal studies with follow-up times of up to 9 years suggest that neurocognitive difficulties remain relatively stable over time and may thus partly have a neurodevelopmental origin (Bora & Özerdem, Reference Bora and Özerdem2017; Demmo et al., Reference Demmo, Lagerberg, Aminoff, Hellvin, Kvitland, Simonsen and Ueland2017). Additionally, it is possible that a greater percentage of patients with BD experience globally impaired neurocognition at later stages of the disorder, since we identified 23.4% of newly diagnosed patients as globally impaired, whereas other studies have identified 21.2–39.7% of remitted patients being globally impaired (Bora et al., Reference Bora, Hıdıroğlu, Özerdem, Kaçar, Sarısoy, Civil Arslan and Tümkaya2016; Burdick et al., Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014; Jensen et al., Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Lima et al., Reference Lima, Rabelo-da-Ponte, Bücker, Czepielewski, Hasse-Sousa, Telesca and Rosa2019; Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017) further indicating that there could be a neurodegenerative component properly associated with the progression of the disorder. However, only a few longitudinal studies on cognition in BD have been conducted and more are warranted to elucidate cognitive trajectories over time (Samamé, Martino, & Strejilevich, Reference Samamé, Martino and Strejilevich2014).
Interestingly, both ‘globally impaired’ patients and relatives of cognitively impaired patients exhibited impairments in working memory (although the difference between relatives and HCs was reduced to trend-level when adjusting for education level). Indeed, meta-analyses have consistently found working memory impairments in remitted patients with BD relative to HCs with medium to large effect sizes (Bora et al., Reference Bora, Yucel and Pantelis2009; Bourne et al., Reference Bourne, Aydemir, Balanzá-Martínez, Bora, Brissos, Cavanagh and Goodwin2013; Mann-Wrobel, Carreno, & Dickinson, Reference Mann-Wrobel, Carreno and Dickinson2011). Studies investigating URs have, however, yielded more inconsistent results, with some studies showing impairments on working memory tasks that require greater cognitive demand (Miskowiak et al., Reference Miskowiak, Kjærstad, Meluken, Petersen, Maciel, Köhler and Carvalho2017b). It is plausible that working memory impairments occur only in relatives of cognitively impaired patients, suggesting shared genetic and/or environmental factors underlying working memory impairments in these patients and their relatives. This would further explain the inconsistencies in the field whereby some studies have failed to identify working memory deficits in URs of patients with BD (e.g. Frantom, Allen, and Cross, Reference Frantom, Allen and Cross2008; Keri, Kelemen, Benedek, and Janka, Reference Keri, Kelemen, Benedek and Janka2001; Pattanayak, Sagar, and Mehta, Reference Pattanayak, Sagar and Mehta2011).
Our finding that neurocognitive subgroups exhibit differential patterns of aberrant affective cognition, of which patients categorised as globally impaired have the greatest impairments, is in line with emerging evidence suggesting that cognitively impaired patients with BD also have difficulties with social cognition (Lima et al., Reference Lima, Rabelo-da-Ponte, Bücker, Czepielewski, Hasse-Sousa, Telesca and Rosa2019) and facial expression recognition (Van Rheenen & Rossell, Reference Van Rheenen and Rossell2016). This association between neurocognitive functioning and affective cognition suggests that pro-cognitive treatments (whether pharmacological or psychological) that improve neurocognitive function in patients with BD who are globally impaired could indirectly ameliorate impairments within emotion regulation and facial expression recognition (Van Rheenen & Rossell, Reference Van Rheenen and Rossell2016), and provide further evidence of directionality between neurocognitive deficits and affective cognition. Moreover, the pattern of neurocognitive deficits in these subgroups can elucidate the neurocognitive mechanisms of affective cognition. Specifically, patients who exhibited selective neurocognitive deficits remained intact in their facial expression recognition abilities, suggesting that the specific neurocognitive domains that are preserved in patients who are selectively impaired (i.e. processing speed, executive control, sustained attention and verbal learning) are those needed for successful emotion recognition. In line with this, lower processing speed, executive function and verbal memory have been associated with selective impairments in the recognition of facial expressions in euthymic BD patients (Martino, Strejilevich, Fassi, Marengo, & Igoa, Reference Martino, Strejilevich, Fassi, Marengo and Igoa2011). Our finding is also in accordance with neuroimaging evidence for disruption in fronto-limbic neural networks in BD (Strakowski, Delbello, & Adler, Reference Strakowski, Delbello and Adler2005; Strakowski et al., Reference Strakowski, Adler, Almeida, Altshuler, Blumberg, Chang and Townsend2012). Indeed, the frontal lobe and its connections with subcortical areas play a key role in executive functions and working memory (Curtis & D'Esposito, Reference Curtis and D'Esposito2003; Drapier et al., Reference Drapier, Surguladze, Marshall, Schulze, Fern, Hall and McDonald2008; Elliott, Reference Elliott2003) as well as social cognition (Townsend & Altshuler, Reference Townsend and Altshuler2012). Patients with BD exhibit decreased prefrontal activity coupled with increased limbic activity during emotion processing (Green, Cahill, & Malhi, Reference Green, Cahill and Malhi2007; Phillips et al., Reference Phillips, Ladouceur and Drevets2008), thus suggesting deficient top-down control of emotions in BD.
The facial expression recognition difficulties in unaffected first-degree relatives of the neurocognitively impaired BD suggests that aberrant affective cognition is associated with genetic risk in patients categorised as globally impaired. As such, it is plausible that the neurocognitively impaired patients are affected by susceptibility genes that are lacking in patients who are neurocognitively intact. This is in line with the RDoC translational approach to psychiatric disorders in which transdiagnostic subgroups of patients are grouped according to dimensional behavioural domains (Cuthbert, Reference Cuthbert2014). As such, cognitive performance is sensitive to individual genetic differences and can be sorted on a normality-pathology continuum (i.e. cognitively intact to globally impaired) (Morris & Cuthbert, Reference Morris and Cuthbert2012). The globally impaired subgroup thus seems to be characterised by a deficit within the RDoC ‘cognitive systems’ domain that has specific genetic and neurobiological underpinning that affect the ‘social processes’ domain via overlapping neural networks. Nevertheless, research has not been able to convincingly qualify impairments within neurocognition as an endophenotype of BD, mostly due to similar patterns of non-specific neurocognitive impairments that are comparable across schizophrenia, unipolar disorder and BD, despite some differences in their severity between the disorders (Kessing & Miskowiak, Reference Kessing and Miskowiak2018). This may be explained by diagnostic group comparisons that assess the average cognitive performance between patients and controls, thereby obscuring the demonstration of distinct neurocognitive subgroups among patients. The present data-driven approach thus seems superior in identifying distinct genetically related neurocognitive subgroups that may also occur transdiagnostically (Clementz et al., Reference Clementz, Sweeney, Hamm, Ivleva, Ethridge, Pearlson and Tamminga2016; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill and Burdick2017). The demonstration of facial expression recognition impairments in both globally impaired patients and their relatives point to this aspect of cognition representing a putative illness endophenotype that may be more specific for BD compared to neurocognitive endophenotypes. The longitudinal part of the study will clarify whether this is indeed a risk marker of BD. If so, future assessment of facial emotion recognition in individuals at familial risk could help identify and target those at particularly high risk of developing BD with preventive strategies (Miskowiak et al., Reference Miskowiak, Kjærstad, Meluken, Petersen, Maciel, Köhler and Carvalho2017b).
Finally, we found no differences between the three neurocognitive subgroups in observer-rated functioning (i.e. FAST scores). This was surprising given that one would assume that BD patients who exhibit more persistent cognitive impairments, within both ‘cold’ and ‘hot’ facets of cognition, would also experience greater functional difficulties in everyday life. Yet, functioning correlates only mildly to moderately with neurocognitive function (Martínez-Arán et al., Reference Martínez-Arán, Torrent, Solé, Bonnín, Rosa, Sánchez-Moreno and Vieta2011; Träger et al., Reference Träger, Decker, Wæhrens, Knorr, Miskowiak and Vinberg2017). This result is in line with a recent study on cognitive heterogeneity in BD that also found no differences between neurocognitive clusters of patients with BD on functioning (Lima et al., Reference Lima, Rabelo-da-Ponte, Bücker, Czepielewski, Hasse-Sousa, Telesca and Rosa2019), suggesting that neurocognitive impairments are not directly linked with everyday functioning.
The current results could indicate distinct pathophysiology of cognitive impairment in different groups of patients. That is, patients categorised as globally impaired exhibit cognitive impairments at early stages of the illness, that are likely to persist over time (Demmo et al., Reference Demmo, Lagerberg, Aminoff, Hellvin, Kvitland, Simonsen and Ueland2017; Samamé et al., Reference Samamé, Martino and Strejilevich2014), and are associated with genetic liability for BD, implying a neurodevelopmental or genetic origin. However, due to the cross-sectional nature of our study we cannot rule out and additional neurodegenerative component. Indeed, there is a general lack of longitudinal studies with long-term follow-up (nine longitudinal studies with an average follow-up duration of 5.5 years; Bora and Özerdem, Reference Bora and Özerdem2017). Moreover, the poorer functional capacity in the neurocognitive subgroups and their first-degree relatives contributes to the growing evidence that neurocognitive function is of key importance for patients’ daily function and quality of life (Baune & Malhi, Reference Baune and Malhi2015). Trait-related deficits in neurocognition and facial expression recognition should therefore represent key treatment targets in otherwise remitted patients in line with the recent recommendations by the ISBD targeting cognition task force (Miskowiak et al., Reference Miskowiak, Burdick, Martinez-Aran, Bonnin, Bowie, Carvalho and Sumiyoshi2017a).
Strengths of the study were the comprehensive assessment of non-emotional and affective cognition, functioning and clinical symptom severity. The large homogeneous sample of remitted patients was representative of newly diagnosed BD with a median age of illness onset of 21 years and a median delay in diagnosis of 5 years (Baldessarini, Tondo, Baethge, Lepri, & Bratti, Reference Baldessarini, Tondo, Baethge, Lepri and Bratti2007; Kessler et al., Reference Kessler, Angermeyer, Anthony, De Graaf, Demyttenaere, Gasquet and Haro2007). A limitation was that the BD sample had longstanding psychiatric histories despite being recently diagnosed (i.e. a substantial mean illness duration, years of untreated BD and number of mood episodes). Indeed, BD is often under- and misdiagnosed causing a delayed diagnosis of approximately 10 years (Baldessarini et al., Reference Baldessarini, Tondo, Baethge, Lepri and Bratti2007; Drancourt et al., Reference Drancourt, Etain, Lajnef, Henry, Raust, Cochet and Zanouy2013). This demonstrates the difficulty in recruiting patients early in the course of illness. Also, the patients with BD had to reach clinical remission before being included in the study. Consequently, 84% of the patients with BD in this study were diagnosed within 12 months prior to inclusion, while 94% of patients were diagnosed within 24 months before inclusion. Lastly, five patients received the BD diagnosis 3–7 years before being included in the study. A limitation was the only moderate sample of unaffected first-degree relatives (n = 52), which impeded the analysis of group differences across relatives of patients who were selectively and globally impaired separately (instead of grouping these together). The limited sample of first-degree relatives was primarily due to many relatives having a psychiatric illness, and hence being excluded from the current study, or due to the lack of consent from patients with BD to contact their relatives. In this regard it is interesting to note that we detected only five relatives of the globally cognitively impaired patients, which could suggest that the relatives of this group were either ill themselves or otherwise unable to take part. This may have resulted in the inclusion of a relatively well-functioning group of relatives. Therefore, we cannot exclude that the findings for relatives represent a type-II error. Importantly, we did not correct for multiple comparisons, which could have introduced type-I error. Instead, in keeping with previous studies of cognitive heterogeneity in BD (Burdick et al., Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014; Jensen et al., Reference Jensen, Knorr, Vinberg, Kessing and Miskowiak2016; Russo et al., Reference Russo, Van Rheenen, Shanahan, Mahon, Perez-Rodriguez, Cuesta-Diaz and Burdick2017), group comparisons were conducted using Fisher's least significant distance (i.e. not correcting for multiple comparisons) in order to facilitate comparisons between the results of the present sample of recently diagnosed patients v. patients at more progressed stages of the illness. If correcting for multiple comparisons, then all group differences comparing the respective clusters with HCs on the individual neurocognitive domains would remain significant, with the exception of working memory for which Bonferroni correction would render group differences between selectively impaired and cognitively intact patients, respectively, non-significant (ps ⩾ 0.11) and difference between relatives of cognitively impaired and HCs reduced to a trend (p = 0.06). For affective cognition, the slower recognition of facial expressions in globally impaired patients compared to HCs would be non-significant (p = 0.10), the decreased ability to down-regulate positive emotions in the selectively impaired and cognitively intact patients relative to HCs would render non-significant (p = 0.16) and be reduced to a trend (p = 0.065), respectively. Group differences for facial expression recognition accuracy, emotion reactivity in positive social scenarios and down-regulation of emotions in negative social scenarios would remain significant. Finally, the cross-sectional design limits the investigation of illness-progression on cognitive heterogeneity in patients with BD, and further causal inferences of the relationship between neurocognition and affective cognition in BD cannot be drawn. Nevertheless, the current study is a part of the ongoing, longitudinal BIO-study (Kessing et al., Reference Kessing, Munkholm, Faurholt-Jepsen, Miskowiak, Nielsen, Frikke-Schmidt and Vinberg2017), in which follow-up assessment are currently being conducted.
In summary, three distinct neurocognitive subgroups of patients recently diagnosed with BD were identified: one that was cognitively intact (45.6%), one that was selectively impaired (31.0%) and one that was globally impaired (23.4%). These subgroups presented distinct performance on affective cognition tests; the neurocognitively impaired patients exhibited most difficulties with facial expression recognition and emotion regulation in social situations. Relatives of patients who were neurocognitively impaired also displayed impaired facial expression recognition and functioning. Longitudinal studies of neurocognitive subgroups are warranted to further investigate the causal relationship between impairments in neurocognition and affective cognition, to elucidate the developmental trajectory of impairments in these domains and to elucidate whether facial expression recognition in first-degree relatives are associated with increased risk of illness onset.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719003738.
Conflict of interest
MV has received consultancy fees from Lundbeck in the past 3 years. LVK has within the preceding 3 years been a consultant for Sunovion and Lundbeck. KWM acknowledges the Lundbeck Foundation and Weiman Foundation for their contribution to her salary. KWM has received consultancy fees from Lundbeck, Allergan and Janssen in the past 3 years. The remaining authors declare no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Committee on Health Research Ethics of the Capital Region of Denmark (protocol no. H-7-2014-007) and the Danish Data Protection Agency, Capital Region of Copenhagen (protocol no. RHP-2015-023) and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








