Introduction
In mammals, spermatozoa, originating in the testes, are transported to the epididymis, where they undergo a series of morpho-biochemical modifications known as sperm maturation (Bedford, Reference Bedford1963, Reference Bedford1966; Bedford et al., Reference Bedford, Calvin and Cooper1973). The functional maturation of spermatozoa results from their exposure to the luminal environment of the epididymis, and is a fundamental step in the acquisition of their fertilizing ability (Bedford, Reference Bedford1965; Orgebin-Crist, Reference Orgebin-Crist1968).
Numerous studies have shown that this organ is not only a place for sperm storage and transit (Yoshinaga & Toshimori, Reference Yoshinaga and Toshimori2003) but also plays an active role in sperm remodelling (Axnér, Reference Axnér2006). This action of the epididymis is performed in a region-dependent manner (Dacheux et al., Reference Dacheux, Gatti and Dacheux2003) in which the different regions sequentially provide the components necessary for successful sperm–oocyte interaction.
Chinchilla lanigera is a rodent with seasonal reproductive cycles, the reproductive period being autumn–winter. Classified as an endangered species (Thornback & Jenkis, Reference Thornback and Jenkis1982; Glade, Reference Glade1988), at present it exists almost exclusively in captivity for commercial purposes, with a low reproductive rate in these conditions. In later years the species acquired great economic importance with the commercialization of its fur and of breeding specimens. However, very little information is known about its reproductive biology, so the study of this animal is of great interest, particularly with respect to design of work techniques and protocols for the obtainment, handling and conservation of gametes.
With the progress of assisted reproduction techniques, knowledge of the processes involved in gamete formation and of the changes they experience during their preparation for fertilization and development becomes imperative as it will make possible successful in vitro fertilization. In this sense the study of the anatomy of the organs of the reproductive system is a necessary step for a better understanding of the biological mechanisms involved in the formation of a new individual.
The aim of our work is the microscopic analysis of the epididymis of Chinchilla lanigera Grey and its sectorization based on a histomorphological study under controlled temperature and photoperiod conditions.
Materials and methods
Animals
The animals used in this study were provided by a commercial hatchery. We selected 12 sexually mature male specimens from 11–12 months of age with a body mass of 600–650 g. All animals were housed under a 12 h dark–light cycle at a constant temperature of 22 ± 1°C and received water and food ad libitum. The samples were collected in April (autumn) or in October (spring) according to the time when specimens were slaughtered.
Tissue preparation
Epididymes were collected immediately after slaughter and fixed for 24 h in Bouin's solution and buffered formaldehyde at 4°C. After fixation, samples were washed in 70% alcohol and subjected to routine histological processing for embedding in Paraplast (Oxford Labware, St Louis, MO, USA). Sections (4 μm thick) were stained with hematoxylin–eosin, toluidine blue pH 7 and Mallory's trichrome stain.
Morphometric analysis and photomicrography
The samples from each period of the reproductive cycle were sectioned in different regions for morphometric analysis. Ten preparations of each segment with two slices each per epididymis were analyzed.
Measurements were performed with an inverted Olympus CKX41 microscope at ×200 and ×400 magnifications. Photographs were analyzed with TSView 5.0 CMOS software.
The relative distribution of the different cell types in all segments (initial, caput, corpus and cauda) of the epididymis was estimated by cell count in different sections of the duct. Only transverse epididymal sections were included in this study. Cells were counted in 10 sections per epididymis from each animal. The results of the cell count were corrected by applying the formula of Amann (Reference Amann1962) shown below, which provides a corrected value of the number of cells counted in histological sections:
 \begin{eqnarray*}
{\rm N} &=& {\rm total\ number\ of\ cell\ types }\\
&& \times\, \frac{{{\rm section\ thickness}}}{{{\rm section\ thickness}\,{\rm + }\,\sqrt {\left( {AD/2} \right)^2 - \left( {AD/4} \right)^2 } }}
\end{eqnarray*}
\begin{eqnarray*}
{\rm N} &=& {\rm total\ number\ of\ cell\ types }\\
&& \times\, \frac{{{\rm section\ thickness}}}{{{\rm section\ thickness}\,{\rm + }\,\sqrt {\left( {AD/2} \right)^2 - \left( {AD/4} \right)^2 } }}
\end{eqnarray*}
where AD is the average diameter of 10 nuclei from each cell type and N is the corrected number of cell types.
Statistical analysis
Means and standard errors were calculated for all data sets. Differences between groups were evaluated using one-way analysis of variance (ANOVA). A P-value of <0.05 was accepted as statistically significant.
Results
The epididymis is an organ formed by a single highly folded seminiferous tube that can be divided into four regions or segments: initial segment, caput, corpus and cauda. Based on the measurements of the morphological parameters, which are cited in Table 1, we determined five zones: zone I (initial segment), II (caput), III (corpus), IV and V (cauda), the last two corresponding to a proximal and distal localization respectively from the caudal region.
Table 1 Structural parameters in each epididymal region (mean values ± SEM)

SEM, standard error of the mean.
The epididymis is a highly vascularized tissue with medium caliber vessels and dense connective tissue in the intertubular space. The tubules are surrounded by layers of smooth muscle with varying thickness according to the region analyzed that become more prominent toward the caudal region.
As shown in Fig. 1, the epididymis is 4–5 cm long. The morphometric differences observed in the height of the epithelium, the length of stereocilia, the tubular and luminal diameter and the thickness of the muscle layer in all the histological regions studied are summarized in Table 1.
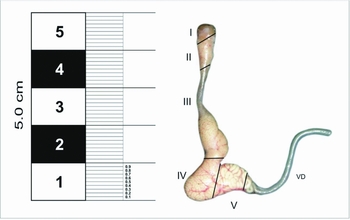
Figure 1 Macroscopic image of epididymis of Chinchilla lanigera in five differentiated zones: I (initial segment); II (caput); III (corpus); and IV, V (cauda). VD: vas deferens.
Histomorphometric evaluation
The epithelium lining the seminiferous tubules is pseudostratified, with principal cells (PCs) with stereocilia, basal (B), clear (C), apical (A), narrow (N) and halo (H) cells. Figure 2 shows sections of all epididymal regions from samples collected in April (autumn) and October (spring). No significant differences among them were found in either the morphometric measurements or the histological evaluation (data not shown).

Figure 2 Microphotographs of the different regions of the epididymis of Chinchilla lanigera from samples collected in April and October. Hematoxylin–eosin stain. Initial segment, caput and corpus: scale bar, 22 μm; cauda: scale bar, 82 μm.
As shown in Fig. 3, in all samples analyzed there were formations in the apical membrane of the cells corresponding to the apocrina secretion process in which epididymosomes are formed and later released into the tubular lumen. These membranous structures have been described in other mammalian species such as human (Frenette et al., Reference Frenette, Legare, Saez and Sullivan2005), mouse (Rejraji et al., Reference Rejraji, Sion, Prensier, Carreras, Motta, Frenoux, Vericel, Grizard, Vernet and Drevet2006), ram (Gatti et al., Reference Gatti, Metayer, Belghazi, Dacheux and Dacheux2005), chimpanzee (Frohlich & Young, Reference Frohlich and Young1996), hamster (Yanagimachi et al., Reference Yanagimachi, Kamiguchi, Mikamo, Suzuki and Yanagimachi1985; Legare et al., Reference Legare, Berube, Boue, Lefievre, Morales, El-Alfy and Sullivan1999) and bull (Frenette & Sullivan, Reference Frenette and Sullivan2001).

Figure 3 Microphotographs of regions of the epididymis showing apocrine secretory activity. (A) Caput: apical membrane of the cells with formation of epididymosomes (arrows) and vesicles released into the lumen (asterisks), H-E stain. (B) Cauda: the epithelium shows numerous forming vesicles (asterisks), Mallory's trichrome stain. A: apical cells; B: basal cells; PC: principal cells; Sp: spermatozoa; arrow: basal layer; white star: muscle layer. Scale bars, 8.2 µm.
Initial segment
In this segment there are tubules of smaller tubular and luminal size than in the rest of the epididymal duct (Table 1). A thin basal layer surrounded by myoid cells can be observed. The ducts are lined by a high columnar epithelium with different cell types: PC, B, N, A and H. The PCs have a higher percentage of relative distribution (Fig. 4), the spherical nuclei are located at the bottom of the epithelium. Vacuoles in their apical cytoplasm can also be seen. B cells, located on the basal layer, have an elongated nucleus parallel to it and scarce cytoplasm. N cells, found only in this segment, appear as thin intensely stained cells, extending from the basal layer to the tubular lumen, with scarce development of the cytoplasm and with a nucleus in the middle part of the cell.
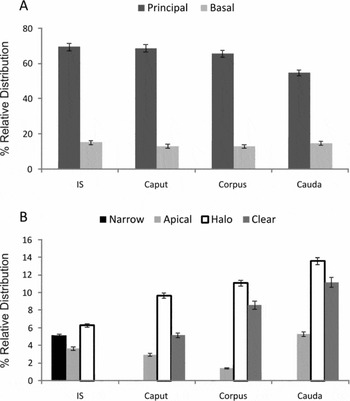
Figure 4 Relative distribution of the cell types present in each region of the epididymis. The values expressed represent the mean ± SEM of 12 animals. IS, inital segment.
Caput
In the April and October period samples analyzed, transverse sections of the caput region showed a high columnar epithelium with the prevalence of PC (68.81%) and B (13.33%) over other cell types. The percentage of relative distribution is similar to the segment described above and there is also a thin basal layer. The stereocilia on the apical surface of the PC in this region are still long compared with the rest of the epididymal duct. In contrast with the previous region, the PC has an elongated nucleus with a visible nucleolus but still retains its basal localization. The presence of numerous vacuoles in the apical cytoplasm is a characteristic that is still present in the epididymal head (Fig. 4).
B cell nuclei are perpendicularly oriented with respect to those of the PC. Muscle fibres surrounding the tubules are not prominent in this region. The tubular lumen increases its size and has a greater number of sperm.
Corpus
In this segment there is a significant increase in the percentage of relative distribution of the halo cells, which are intraepithelial lymphocytes (11.1%), compared with the percentages found along the epididymis. As shown in Fig. 4B , there is an increase in clear cells together with a significant decrease in the percentage of relative distribution in apical cells (1.42%). The apical cell nucleus is located at half-height of the epithelium but, unlike N cells, this cell type is not in contact with the basal layer. The lining epithelium is shorter, the same as in the stereocilia. There is a concomitant increase in lumen diameter. The presence of sperm is more noticeable in this region and, from this segment onwards, contact of sperm with the lining of the epithelium becomes more frequent.
Cauda
The caudal region of the epididymis has a low pseudostratified cuboidal or cylindrical epithelium with very short stereocilia (Fig. 5). Principal and basal cells show no significant morphological changes and there is an increase in clear cells. Measurements at the height of the epithelium show a significant decrease compared with other zones of the epididymis. The basal layer shows no changes with respect to the previous segments analyzed. In this portion of the epididymis we can see an increase in peritubular smooth musculature.

Figure 5 (A, B) Initial segment pseudostratified columnar epithelium. Intensely stained narrow cells (arrows) and numerous vacuoles (asterisks) in the apical cytoplasm. (A) Toluidine blue stain (TB). (B) Hematoxylin–eosin stain (H-E). (C, D) Caput: vacuoles and stereocilia are still noticeable, clear cells appear. (C) H-E stain. (D) Trichrome stain. (E, F) Corpus: numerous sperm appear in the tubular lumen and apical cells. (E) Trichrome stain. (F) H-E stain. (G, H) Cauda: cuboidal pseudostratified epithelium, halo cells and tubular interstitium with medium caliber vessels. (G) H-E stain. (H) Trichrome stain. A: apical cells; B: basal cells; C: clear cells; H: halo cells; PC: principal cells; Sp: sperm; ST: stereocilia; V: vessels. Scale bars: 22.01 μm (E); 35.4 μm (C); 82 μm (A, B, D, F–H).
In this region there is a remarkable increase in the luminal and tubular diameters correlated with the presence of a dense mass of sperm. In addition, the data from the morphometric study show two well defined zones (IV and V), in which the zonal difference lies in the size variation of the tubular and luminal diameters and in the height of the epithelium (Table 1).
Discussion
The histology of the epididymis has been extensively studied in various mammalian species. The epididymis presents variations in its structure and function throughout the duct. However, the number of regions and their zoning depend on the species and on the classification criteria (Goyal, Reference Goyal1985).
On the basis of the results of the morphological study analyzed above and on the cell types identified and their relative distribution, the epididymis of Chinchilla lanigera shows a clear segmentation of the organ into four regions: initial, caput, corpus and cauda. Similar results were obtained by Aguilera-Merlo et al. (Reference Aguilera-Merlo, Muñoz, Dominguez, Scardapane and Piezzi2005) for viscacha, by Serre & Robaire (Reference Serre and Robaire1998) for Brown Norman rat, by Schön & Blottner (Reference Schön and Blottner2009) for roe deer and for hamster by Vicentini & Orsi (Reference Vicentini and Orsi1987).
In general terms, the histoarchitecture of the lining epithelium of the epididymis conserves the characteristics of the cell type described by other authors for other mammalian species. However, the presence of an initial segment has been described only for other rodents. In agreement with the findings in another hystricomorph rodent (Aguilera-Merlo et al., Reference Aguilera-Merlo, Muñoz, Dominguez, Scardapane and Piezzi2005), our observations reveal the presence of narrow cells and the absence of clear cells only in the initial segment, in agreement with studies in adult rats (Adamali & Hermo, Reference Hermo, Adamali, Mahuran, Gravel and Trasler1996).
These results suggest that the functions of this region would be protein degradation and the eventual internalization of other proteins for their later degradation (Hubbard, Reference Hubbard1989; Murphy, Reference Murphy1991; Hermo et al., Reference Hermo, Adamali, Mahuran, Gravel and Trasler1996). Previous investigations confirm that narrow cells are in charge of acidifying the luminal environment, thus contributing to sperm quiescence (Cohen et al., Reference Cohen, Hoffer and Rosen1976; Au & Wong, Reference Au and Wong1980).
Principal and basal cells are the prevailing populations in all regions analyzed. The greatest length of stereocilia was found in the PCs of the head, thus contributing to luminal transit. They become shorter toward the caudal region, with the consequent increase in luminal content, both fluid and sperm, in a caudal direction. In caput and corpus, the cytoplasm of the PC is highly vacuolized, suggesting an absorption function. In contrast, epididymosomes release in the PC apical membrane makes these very special cells, where the secretion/absorption interplay is perfectly regulated.
In 1975 it was postulated that basal cells serve as stem cells of the epididymal epithelium (Hamilton, Reference Hamilton, Greep and Astwood1975). They were later considered as precursors to the PCs (Sun & Flickinger, Reference Sun and Flickinger1982), but at present numerous gap junctions between basal cells and principal and clear cells are known to exist (Hejmej et al., Reference Hejmej, Kotula-Balak, Sadowska and Bilinska2007; Alkafafy et al., Reference Alkafafy, Elnasharty, Sayed-Ahmed and Abdrabou2011). The important point here is the degree of conservation among species, indicating the relevance of this intercellular junction for epididymal functioning.
Another cell type found from the caput to the cauda is the clear cell, similar findings have been reported for rats (Serre & Robaire, Reference Serre and Robaire1998). What is interesting about these cells is that they are species specific, as in gerbils they are found in the initial segment and in the cauda while in Golden hamster they appear only in the epididymal cauda (Beu et al., Reference Beu, Orsi, Stefanini and Cruz2001), no traces of them having been found in either pig (Orsi et al., Reference Orsi, Mello Dias, Valente and Vicentini1985) or monkey (Ramos & Dym, Reference Ramos and Dym1977). They have been implicated in the acidification of the luminal fluid by the secretion of protons, thus contributing to the quiescence of sperm motility in this region (Breton et al., Reference Breton, Smith, Lui and Brown1996; Blomqvist et al., Reference Blomqvist, Vidarsson, Soder and Enerback2006; Pastor-Soler et al., Reference Pastor-Soler, Hallows, Smolak, Gong, Brown and Breton2008).
Halo cells, proposed as lymphocytes or monocytes (Wang & Holstein, Reference Wang and Holstein1983), would play an immunological role in the epididymus. Later studies have demonstrated their active participation in the prevention of an autoimmune response (Beagley et al., Reference Beagley, Wu, Pomering and Jones1998; Serre & Robaire, Reference Serre and Robaire1999). In this work an increase is shown in the number of halo cells in the epididymal cauda compared with the initial segment. It seems likely that the increase in luminal content induces the increase in halo cell population.
Chinchilla lanigera is a species with seasonal reproductive cycles in wild life, in which the fall–winter period corresponds to the months of reproductive activity followed by gonadal quiescence in the summer (Weir, Reference Weir1972; Neira, Reference Neira1987; Rodriguez, Reference Rodriguez1988; Garcia et al., Reference Garcia, Neira and Scheu1989; Neira et al., Reference Neira, Garcia and Scheu1989).
In previous studies conducted with this species in captivity in conditions of natural ambient temperature and natural photoperiods, specimens showed noticeable morphometric changes in seminal vesicles (Orostegui et al., Reference Orostegui, Parraguez, Adaro, Peñailillo and Cepeda2000), bulbourethral glands and prostate (Cepeda et al., Reference Cepeda, Peñailillo, Urquieta and Orostegui1999, Reference Cepeda, Adaro and Peñailillo2006). These data are closely correlated with important decreases in serum testosterone levels during gonadal quiescence (Adaro et al., Reference Adaro, Parraguez, Orostegui, Urquieta and Cepeda2002).
In our laboratory, however, in conditions of captivity in which external factors such as temperature, humidity and light/dark cycles were controlled all year round, we found no significant changes in epididymal histomorphology.
This is the first report of a histomorphometric study in this species with an assessment of the impact of certain controlled environmental factors on the epithelial lining.
In conclusion, each segment shows different histological and morphometric characteristics, which supports the hypothesis of a specific behaviour for each region, giving a segment-specific characteristic to the sperm maturation process in this species.
Moreover, the fact that no differences were found among the samples collected at two different periods when reproductive activity in nature is different suggests the importance of external factors in the control of the reproductive cycle in Chinchilla lanigera. Although this subject requires further study, it should be taken into consideration when analyzing the variables or factors that may have an influence on the control of the reproductive cycle in this species.
Acknowledgements
This work was supported by a grant from the Science Council of the National University of Tucumán (CIUNT).








