INTRODUCTION
Although often discounted because they cannot be independently verified, self-reported post-traumatic symptoms (PTS) are commonly the problems that people with traumatic brain injury (TBI) present to health care providers. Post-traumatic symptoms are reported by persons with mild TBI in the absence of obvious physical/neurological findings or findings on formal performance measures. These symptoms are not unique to those with TBI (not specific). This, together with higher rates of self-reported symptoms in cases with potential for secondary gain (e.g., litigation), has cast doubts about the authenticity of the reports. There is no doubt that PTS are common immediately after the injury. The question is for how long they persist, the fraction of cases that will continue to have them, and what predicts who will continue to have them.
The literature on post-traumatic symptoms is extensive. Problems complicating interpretation of the published studies that involve information on rates of symptoms and the nature of symptoms, however, include: a lack of controls, poor definition of the cases included, biased selection such as cases seen in a clinic because of problems, and poor follow-up. Furthermore, various definitions of post-traumatic symptoms have complicated the interpretation of individual studies and accumulation of information needed to move forward in this area of investigation. Additionally, most studies have only looked at cases of mild TBI.
A recent review by the Institute of Medicine highlights the paucity of good information on the rates and types of symptom reporting six or more months after injury in those with TBI compared to controls (2009). This is especially the case in those with more severe TBI. Six of the eight highest quality studies were restricted to mild TBI (Gerber & Schraa, Reference Gerber and Schraa1995; Heitger, Jones, Frampton, Ardagh, & Anderson, Reference Heitger, Jones, Frampton, Ardagh and Anderson2007; Hoge et al., Reference Hoge, McGurk, Thomas, Cox, Engel and Castro2008; Mickeviciene et al., Reference Mickeviciene, Schrader, Nestvold, Surkiene, Kunickas, Stovner and Sand2002; Reference Mickeviciene, Schrader, Obelieniene, Surkiene, Kunickas, Stovner and Sand2004; Stulemeijer et al., Reference Stulemeijer, van der Werf, Bleijenberg, Biert, Brauer and Vos2006). Even within the mild TBI studies, results were quite variable, but the preponderance of evidence indicates that those with mild TBI report significantly more symptoms than people whose injuries spared the head. Two studies included more severe cases (Masson et al., Reference Masson, Maurette, Salmi, Dartigues, Vecsey, Destaillats and Erny1996; McLean, Dikmen, & Temkin, Reference McLean, Dikmen and Temkin1993). Most symptoms were significantly more common in those with TBI, but there are no explicit comparisons of the more severe cases with controls. A more recent population-based large study based on non-hospitalized mild TBI cases indicates one or more post-traumatic symptoms at three months after injury in 44% of the cases and three or more symptoms in 24% of the cases. Unfortunately, this study did not use a control group (Lannsjo, af Geijerstam, Johansson, Bring, & Borg, Reference Lannsjo, af Geijerstam, Johansson, Bring and Borg2009).
An important distinction to make in studies of post-traumatic symptoms is between those addressing the natural history of recovery and rates of PTS versus those addressing factors contributing to symptom endorsement. Most of the literature on PTS has focused on the latter and has identified numerous factors that contribute to symptom endorsements, such as expectation, pain, depression, and financial compensation. An exception is the well-controlled prospective sport concussion studies, which have yielded valuable information about the natural history of recovery from PTS after these very mild concussions in healthy young people (McCrea, Reference McCrea2008). However, as summarized earlier, there is limited information about rates and types of post-traumatic symptoms after civilian injuries for representative patients with a broad spectrum of TBI severity, which is the focus of the present study. This study addresses the following questions:
1. What is the rate and type of new or worse self-reported post-traumatic symptoms 1 month and 1 year after TBI compared to those whose injury spared the head?
2. What is the relationship of pre-injury and injury factors to symptom reporting at 1 year?
METHODS
Participants with Traumatic Brain Injuries
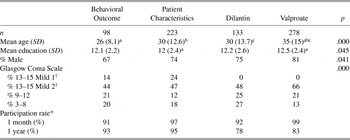
The 829 participants in this study were recruited at the time of injury into one of four prospective longitudinal investigations: the Behavioral Outcome Study, the Patient Characteristics Study, and the studies to prevent post-traumatic seizures, the Dilantin Prophylaxis Study and the Valproate Prophylaxis Study. Please see Supplemental Tables I and II in the Appendix for inclusion/exclusion criteria of the four studies and basic demographic and brain injury severity information, respectively. The studies enrolled participants from 1980 to 1994 among people hospitalized at Harborview Medical Center, Seattle, Washington, the only Level I trauma center for a four-state region. The inclusion criteria varied across the studies, but all participants met the following minimum entrance criteria: positive evidence of TBI (e.g., any period of loss of consciousness, post-traumatic amnesia of at least 1 hour, or computed tomography (CT) evidence of a brain lesion), and willingness to participate in the study. The studies differed on the imposition of additional severity criteria and the exclusion, for some studies, of subjects with preexisting condition (e.g., alcohol problems or hospitalization for prior TBI). The Patient Characteristics Study represents essentially unselected hospitalized cases of TBI. It included all cases of people 16 years of age or older hospitalized with TBI, except those where they or their family did not consent. For determining the overall impact of TBI on symptom endorsement, inverse probability weighting (Horvitz & Thompson, Reference Horvitz and Thompson1952) was used so that the results represent these unselected cases hospitalized with TBI. Study interventions had no effect on symptom endorsements (data not shown). For more detailed selection criteria for these studies and more details about the combining of the samples, see our earlier studies (Dikmen, et al., Reference Dikmen, Machamer, Winn and Temkin1995a; Temkin et al., Reference Temkin, Dikmen, Anderson, Wilensky, Holmes, Cohen, Newell, Nelson, Awan and Winn1999). The 732 participants who provided symptom information at 1 month and/or 1 year comprise the sample for this study.
Demographic information is summarized in Table 1. Subjects were on average young males with a high school education. Post-traumatic symptom information was collected at 1 month post-injury on 603 participants. The severity of the neurologic impairments precluded assessment for 163 subjects. Thirty eight subjects were not approached because 1-month testing was temporarily discontinued due to study budgetary restraints. Symptoms were collected at 1 year on 624 subjects. Twenty subjects died, 35 remained too neurologically impaired to be asked about symptoms, and 57 were not approached because testing was temporarily discontinued due to study budgetary restraints. Thus, of participants who were approached and were neurologically able to respond, symptom information was obtained from 96% at 1 month and 87% at 1 year post-injury. Sixty percent of the TBI group completed both the 1-month and 1-year assessments. This percentage is relatively low because some subjects were neurologically too impaired to provide symptom information, particularly at 1 month, and there were also periods of time when either 1-month or 1-year testing was suspended because of budget constraints as described earlier.
Table 1. Demographics

Note
TBI = Traumatic Brain Injury; TC = Trauma Control; SD = Standard deviation.
† Mild 1 cases have GCS 13–15 with no CT brain abnormalities and PTA under 24 hours; Mild 2 cases had at least one of these two severity features.
General Trauma Comparison Participants
One hundred and twenty comparison participants were enrolled for the Patient Characteristics Study. These subjects sustained traumatic injury to the body, but not the head. The most frequent types of injuries sustained included injury to the skin and muscles (88%) and/or extremity injuries (71%). Demographically, these subjects were similar to the participants with traumatic brain injury (see Table 1). Information about the functional status of the comparison subjects can be found in Dikmen et al. (Reference Dikmen, Ross, Machamer and Temkin1995b). Symptom information was obtained from 99% of the sample at 1 month and from 92% at 1 year. Of the trauma control subjects, 92% were tested at both times. All data were obtained in compliance with ethical regulations of the University of Washington Institutional Review Board.
Measures
Demographics and pre-injury characteristics
Basic demographic information included gender, age, and educational level (dropped out before high school graduation, high school graduate with or without some academic post-secondary education or high school student at the time of the injury, college graduate). Preexisting conditions were defined as a pre-injury history of treatment for alcohol problems including attending meetings of Alcoholics Anonymous, inpatient alcohol treatment program, outpatient structured treatment center, multiple alcohol schools or was in Detox. Preexisting serious psychiatric condition was defined as an inpatient psychiatric hospitalization, and/or major psychiatric diagnosis (e.g., schizophrenia, manic-depressive illness), suicide attempt(s) or long term use of anti-depressant medication). Pre-injury central nervous system disorders included a previous TBI requiring hospitalization, epilepsy, significant learning disability involving many years of special education, or other conditions (e.g., brain tumor).
Injury severity
Brain injury severity was evaluated with the Glasgow Coma Scale (GCS; Teasdale & Jennett, Reference Teasdale and Jennett1974) a measure of coma depth. The mild GCS group (GCS 13–15) was subdivided further into two severity subgroups. The mild 1 subgroup had GCS between 13 to 15, but neither CT abnormality nor post-traumatic amnesia greater than 24 hours. The mild 2 subgroup had some CT brain abnormality (ignoring linear skull fracture or air) or greater than 24 hours of post-traumatic amnesia (PTA). Most of the subjects in this group had both a CT brain abnormality and post-traumatic amnesia lasting more than 24 hours (57%), while 20% had CT abnormality only and 22% had post-traumatic amnesia only. This classification procedure is similar to that used by Williams, Levin, & Eisenberg (Reference Williams, Levin and Eisenberg1990) except for inclusion of PTA in addition to CT abnormality in the classification. The mild 1 group is similar to their simple mild (GCS 13 to 15 and no CT abnormality). Our mild 2 group is similar to their complicated mild group (GCS 13 to 15 with CT abnormality) with the exception of 22% getting into this classification on the basis of PTA greater than 24 hours alone.
Post-traumatic amnesia was assessed retrospectively at 1 month post-injury by asking subjects when they began to remember day-to-day events consistently after their injury (Russell & Smith, Reference Russell and Smith1961). Retrospectively assessed PTA, contrary to commonly held belief, does appear to have good test–retest reliability, correlates well with other indices of brain injury severity, and has a relationship to outcome comparable to GCS and time from injury to consistently following commands (Dikmen, Machamer, & Temkin, Reference Dikmen, Machamer and Temkin2007).
Other system injury severity
Injuries to the body were measured with the Injury Severity Score (ISS) modified to exclude injuries to the head (Baker, O’Neill, Haddon, & Long, Reference Baker, O’Neill, Haddon and Long1974). This score is calculated as the sum of the squares of the three most severely injured body regions on the Abbreviated Injury Scale. ISS was only available for trauma controls and TBI subjects enrolled in the Patient Characteristics Study. Symptom endorsement rates in this subgroup are very similar to those in the entire group (data not shown).
Symptoms
The Symptom Checklist (McLean, Dikmen, Temkin, Wyler, & Gale, Reference McLean, Dikmen, Temkin, Wyler and Gale1984) is a list of 12 symptoms commonly reported following traumatic brain injury. They include headaches, fatigue, dizziness, blurred vision, trouble concentrating, bothered by noise, bothered by light, irritability, temper, memory problems, anxiety, and trouble with sleep. For each symptom, subjects were asked if they had the symptom now, and if yes, whether they had it prior to injury. If they had that symptom prior to injury, they were asked if it was now better, the same, or worse compared to pre-injury. A symptom was counted if it was new or worse since the injury. Total number of symptoms at 1 month and 1 year was calculated by summing the number of new or worse symptoms at each time. The total score was prorated if less than 3 symptoms were missing at a given time. Test–retest reliability for total symptom score over 2 weeks is .85 (Pearson), and Kappa for individual symptoms ranged from moderate to very good, except for problems with sleep (unpublished data from our laboratory).
Data Analysis
To adjust for differences in the four studies’ entrance criteria and to provide results that accurately reflect performance of unselected traumatic brain injury survivors, the symptom variables represent weighted averages of four subgroups using inverse probability weighting and the population of the Patient Characteristics study as the standard. The four subgroups were formed by combinations of the presence or absence of two factors: preexisting conditions and a head injury meeting the Dilantin Prophylaxis study severity entrance criteria. Details of the procedure are in Dikmen et al., Reference Dikmen, Machamer, Winn and Temkin1995a.
The first set of analyses used weighted chi-square tests to compare TBI and trauma controls on symptom reporting at 1 month and 1 year post-injury. The data are summarized by the weighted rate in those with TBI and in those with general trauma sparing the head.
The second set of analyses used unweighted chi-square tests to examine symptom reporting at 1 year post-injury in the TBI group subdivided by demographics, pre-injury characteristics, and injury severity.
A third set of analyses used multiple regression to predict total number of symptoms at 1 year post-injury using ISS and TBI severity as predictors in the subset of subjects with ISS information. ISS was grouped into three categories of severity of other system injuries, ≤ 5, 6–20, and ≥ 21, and subjects were grouped into five severity categories representing trauma controls, mild 1, mild 2, moderate, and severe TBI. ISS severity group was entered first followed by TBI severity. In addition, an unweighted chi-square test was used to examine ISS distribution between trauma controls and those in the mild 1 TBI group.
RESULTS
Demographics and injury characteristics of the people with TBI and controls are given in Table 1. The groups are both predominantly young males with a high school education. The TBIs are mostly in the mild range according to GCS, but all TBI subjects were hospitalized and many of those with GCS 13–15 had CT abnormalities or prolonged PTA. Symptom endorsement was examined by treatment group for the subjects in the clinical trials. There was no difference between treatments in endorsement of individual symptoms or in the number of symptoms endorsed (data not shown).
The percentage of cases endorsing each symptom as new since the injury or more severe since the injury is given in Table 2. Symptoms are commonly reported in both groups at 1 month and are reported by a substantial number of those with TBI at 1 year after injury. Most of the symptoms are significantly more common in those who have had a TBI. Fatigue and cognitive symptoms are prominent at both times, while physical symptoms such as headache, dizziness, blurred vision, and being bothered by light are more common in those with TBI at 1 month after injury. Most of the physical symptoms, although decreased in frequency by 1 year, nevertheless have some of the highest risk ratios. Interestingly, temper, irritability, and anxiety, which were almost equally common among those with TBI and trauma controls at 1 month, are significantly more frequent in those with TBI by 1 year. Although most of the included symptoms are referred to as “post-concussion symptoms,” each is reported by at least some people whose trauma spared the head, and some, such as fatigue, irritability, and trouble sleeping, are still reported frequently by the trauma controls at 1 year after injury.
Table 2. Symptom endorsement (%) at 1 and 12 months after injury. Only symptoms new or worse since the injury are counted

Note
TBI = Traumatic Brain Injury; TC = Trauma Control; SD = Standard Deviation.
Rate of symptom endorsement generally declined between 1 month and 1 year after injury, but this was not uniformly the case on an individual basis. Between 4% and 18% of those with TBI examined at both times endorsed a given symptom at 1 year that they had not endorsed at 1 month after injury. Problems with irritability, memory, and temper were the most likely to be newly reported at 1 year. For trauma controls, the rate of new endorsement was between 4% and 13%, with problems with irritability and memory being most frequently newly endorsed.
Several pre-injury and injury characteristics were examined to determine their relationship to symptom reporting at 1 year after injury. Although women are often said to be much more likely to complain about symptoms after TBI, we found only limited evidence of that at 1 year after injury (see Table 3). Being bothered by noise, headaches, and reporting more than one symptom were significantly more often seen in females after adjusting for the number of symptoms examined. For most symptoms, the rate of endorsement is almost equal.
Table 3. Symptom endorsement (%) at 1 year after TBI for males and females

Note
TBI = Traumatic Brain Injury; SD = Standard Deviation.
People with TBI in their middle years reported more symptoms than older people and young adults (see Table 4). This was significant for anxiety, irritability, and temper, but the pattern was similar for most symptoms.
Table 4. Symptom endorsement (%) at 1 year after TBI for different age groups

Note
Subgroups marked with the same capital letters are significantly different by Tukey’s post hoc test at p < .01. Subgroups marked with the same lower case letters are significantly different by Tukey’s post hoc test at p < .05. TBI = Traumatic Brain Injury; SD = Standard Deviation.
Other factors examined include pre-injury alcohol, psychiatric, and neurological conditions, educational level and brain injury severity (see Tables 5–7). Pre-injury alcohol abuse and psychiatric history were associated with greater symptom endorsements.
Although those who did not complete high school tended to endorse more problems, educational level was not an important factor in symptom reporting.
Comparisons between TBI severity subgroups, adjusting for the multiple comparisons on the 12 symptoms, yielded significant difference on only the memory complaints. Those with GCS ≤ 12 endorsed more memory problems than those in the mild 1 group. Comparing the rate of symptom endorsement in injury severity subgroups to that of trauma controls, those in the mild 2 subgroup or with moderate and severe injuries endorsed more problems, especially with memory and temper than trauma controls. Irritability and trouble concentrating are also endorsed more often by some of the more severe subgroups. Although those in the mild 1 group did not differ significantly from the trauma controls, their symptom endorsement rates were consistently higher. The nonsignificant results could be due to smaller sample size of this severity subgroup. One might question whether the other system injuries were comparable in the severity groups. Those with more severe head injuries tended to have more severe other system injuries than the trauma controls. However, the mild 1 cases have almost an identical other system injury severity distribution as the TCs (p = .810).
Because of the possibility that other system injuries might contribute to symptom endorsement, we examined the contribution of ISS and TBI severity to the number of symptoms endorsed at 1 year. ISS (excluding the head) did not contribute significantly to total symptom endorsement at 1 year post-injury (R 2 = .010, p = .218). However, after other system injury severity was controlled, TBI severity continued to be a significant predictor of total symptoms at 1 year post-injury (R 2 change = .078, p = .0001).
Table 5. Symptom endorsement (%) at 1 year after TBI as a function of preexisting conditions at the time of injury

Note
PE = Preexisting; CNS = Central Nervous System; SD = Standard Deviation.
Table 6. Symptom endorsement (%) as a function of pre-injury level of education

Note
Subgroups marked with the same capital letters are significantly different by Tukey’s post hoc test at p < .01. Subgroups marked with the same lower case letters are significantly different by Tukey’s post hoc test at p < .05. HS = High School; SD = Standard Deviation.
Table 7. Symptom endorsement (%) as a function of TBI severity (GCS)

Note
† Mild 1 cases have GCS 13–15 with no CT brain abnormalities and PTA under 24 hours; Mild 2 cases had at least one of these features. Subgroups marked with the same capital letters are significantly different by Tukey’s post hoc test at p < .01. Subgroups marked with the same lower case letters are significantly different by Tukey’s post hoc test at p < .05. TBI = Traumatic Brain Injury; GCS = Glasgow Coma Scale; TC = Trauma Control; SD = Standard Deviation; ISS = Injury Severity Score (excluding the head); CT = Computed Tomography; PTA = Post-Traumatic Amnesia.
DISCUSSION
This study provides information about rates of post-traumatic symptoms in a sample of hospitalized TBI subjects at 1 and 12 months after injury. This TBI sample should be representative of hospitalized patients with civilian TBI of a broad spectrum of injury severity. Compared to current hospitalization practices, patients with less severe brain injuries are probably over-represented because data were collected between the years of 1980 and 1994, when admission to hospitals were more lenient in the lower severity range compared to now (Thurman, Alverson, Dunn, Guerrero, & Sniezek, Reference Thurman, Alverson, Dunn, Guerrero and Sniezek1999). Subjects were recruited in the acute stage, based on the characteristics of their injury, and were prospectively studied rather than recruited based on outcome (e.g., receiving treatment in a specialty clinic). This protects against potential biases, such as over-representation of cases with complicated recoveries, which would inflate the estimates of poor outcomes. To control for demographics such as age, education, gender, and a trauma (other than TBI), a comparison group consisting of individuals with an injury, but not a TBI, were studied. Thus, difference in the rates between those with TBI and our controls should provide rates more closely associated with TBI. Data were collected for research purposes rather than for clinical or litigation reasons. The subject could release the data with informed consent, but this was rare and only the neuropsychological test results were released. Confidentiality agreements from NIH were in place to even protect against subpoena. Undoubtedly, there were cases with complicated recoveries or litigation (as defendant or plaintiff), but they should be in proportion to what happens naturally. Note that there is not a single true value for how frequently a symptom occurs after TBI. Several common variations in the way the questions are asked influence the rates. Logically, rates are likely to be higher when subjects are given a list of symptoms (which was the method in our study), rather than spontaneously reporting them (Nolin, Villemure, & Heroux, Reference Nolin, Villemure and Heroux2006). Other reasons for higher rates include asking about symptom occurrence any time since the injury rather than recently (we asked for current symptoms), or including symptoms present prior to the injury that were not worse (we counted them only if they were new or had gotten worse compared to pre-injury). Finally, rates are likely to be higher when only the occurrence of the symptom is required rather than its interference with functioning or quality of life (we counted them regardless of impact). In addition, some biases increase rates. The most common bias is choosing respondents from those seeking care for clinical reasons or because they are involved in litigation, rather than from representative cases of TBI (our subjects were selected on the basis of their injury and its characteristics and not on their outcome). These variations are likely causes of differences in the rates of PTS reported in the literature.
With these methodological characteristics in mind, the results indicate high rates of symptom endorsement at 1 month and 1 year after injury in spite of decreased endorsement of some symptoms from 1 to 12 months. General trauma controls also report symptoms, but at a lower rate. Although they are usually called post-concussional symptoms, their endorsement is high with all severities of TBI. Perhaps post-traumatic symptoms would be a more accurate way to refer to them.
Difficulties with memory, concentration, fatigue, anxiety, and irritability are the most frequently reported symptoms 1 year after TBI, and, despite substantial endorsement by TC, have the highest excess endorsements by those with TBI. Between 1 person in 4 and 1 in 7 with TBI endorsed each of these symptoms more than would be expected if their injury had spared their head. Half of the symptoms, including some less commonly endorsed, occur at over double the frequency seen in those without TBI, that is, with a risk ratio over 2. Some symptoms, such as headache, dizziness, blurred vision, and being bothered by light, were more common in those with TBI at 1 month after injury. Physical symptoms, although, decreased in frequency by 1 year, had some of the highest risk ratios. Interestingly, temper, irritability, and anxiety, which were almost equally common among those with TBI and trauma controls at 1 month, were significantly more frequent in those with TBI by 1 year. Our study cannot address the etiology for these symptoms as to whether they have direct neurological underpinnings versus represent emotional reactions to the difficulties associated with their injuries and their circumstances, or exacerbation of pre-injury dispositions. However, clearly, such symptoms are more frequently reported by those subjects with more severe rather than milder injuries, as noted below.
Severity of brain injury had some bearing on symptom endorsement. Those with more severe injuries tended to report higher rates of memory problems, temper, and irritability, and also to have 3 or more symptoms, as compared to controls. Significant differences between the TBI severity subgroups were found only in memory complaints. No significant differences in rates of endorsement were found between the mildest TBI (mild 1) group and trauma controls. With the relatively small sample size, this should not be taken as ruling out an important difference. Seven of the 12 symptoms, as well as three or more symptoms, were endorsed at least 50% more often in the mild 1 group than in the trauma controls.
These rates and their persistence are considerably higher than the findings in sports injuries. The results of well-controlled studies of sports injuries indicate that symptoms subside by about 2 weeks in the majority of the cases after a concussion obtained in play (McCrea, Reference McCrea2008). There are important differences, however, between sports injuries and general civilian injuries. First, severity of sports injuries are probably milder than what would lead to hospitalization. Furthermore, those injured are young and healthy individuals that are motivated to get back into play and perhaps even motivated to withhold information about problems. The population of sports injuries are not representative of those with civilian TBI.
The results have a number of implications. First, while PTS occur more often in those with TBI, they are not specific to TBI. These are common symptoms also reported by people in general (Gouvier, Uddo-Crane, & Brown, Reference Gouvier, Uddo-Crane and Brown1988; Iverson & Lange, Reference Iverson and Lange2003; Machulda, Bergquist, Ito, & Chew, Reference Machulda, Bergquist, Ito and Chew1998; McLean et al., Reference McLean, Dikmen, Temkin, Wyler and Gale1984; Mittenberg, DiGiulio, Perrin, & Bass, Reference Mittenberg, DiGiulio, Perrin and Bass1992; Sawchyn, Brulot, & Strauss, Reference Sawchyn, Brulot and Strauss2000; Trahan, Ross, & Trahan, Reference Trahan, Ross and Trahan2001; Wong, Regennitter, & Barrios, Reference Wong, Regennitter and Barrios1994), those with depression (Iverson, Reference Iverson2006), pain (Gasquoine, Reference Gasquoine2000; Iverson & McCracken, Reference Iverson and McCracken1997; Radanov, Dvorak, & Valach, Reference Radanov, Dvorak and Valach1992; Smith-Seemiller, Fow, Kant, & Franzen, Reference Smith-Seemiller, Fow, Kant and Franzen2003), litigation (Binder & Rohling, Reference Binder and Rohling1996; Dunn, Lees-Haley, Brown, Williams, & English, Reference Dunn, Lees-Haley, Brown, Williams and English1995; Lees-Haley & Brown, Reference Lees-Haley and Brown1993; Paniak et al., Reference Paniak, Reynolds, Toller-Lobe, Melnyk, Nagy and Schmidt2002), patients receiving care, and those with other system injuries, as reported in this study and by others (Mickeviciene et al., Reference Mickeviciene, Schrader, Obelieniene, Surkiene, Kunickas, Stovner and Sand2004). Such symptoms are also reported to a greater extent by persons with preexisting conditions such as prior alcohol abuse, and psychiatric difficulties as seen in the present study. Research also suggests that expectation may also play a role in symptom endorsement. In other words, people may be more likely to report symptoms they expect to occur with the condition they have, in this case, following brain injury (Gunstad & Suhr, Reference Gunstad and Suhr2002). These findings indicate that while those with TBI report greater symptoms, these symptoms are not sufficiently sensitive or specific to be used to diagnose a brain injury.
Patients with TBI typically do not have isolated brain injury. They may sustain injuries to other body systems with associated impairments and disabilities. These combined with preexisting and comorbid conditions, as the literature reviewed here suggests, and post-injury circumstances (e.g., litigation, social support) may contribute to reported post-traumatic symptoms. It is impossible to determine on the basis of our dataset the degree to which post-traumatic symptoms have neurological underpinnings or they are a reaction to the impairments associated with TBI, or represent exacerbation of pre-injury dispositions, or other causes. However, just because they are not specific or sensitive enough for diagnostic purposes, and factors other than the index brain injury can contribute to their endorsement, it does not follow that they should be dismissed as irrelevant outcomes following TBI.
Second, these estimates are considerably higher than the common belief that it is rare to have three or more symptoms for more than 3 months after a concussion or mild TBI. Even a year after the injury, 44% of the unselected hospitalized cases in the mild 1 TBI group reported 3 or more symptoms that were new or worse since the injury. The figure for the general trauma controls without TBI was 24%. In this context it is important to raise the question of whether our mild 1 group is representative of those with “concussion” or mild TBI. It is difficult to answer this question because of the variation in how mild TBI is defined. Our subjects probably are more severe than those not hospitalized and those not reported to the medical care system. However, the rates of symptoms of our mild 1 group are comparable to those of a group of subjects only seen in the emergency department for their brain injuries at 6 months after injury using the same examiner-administered symptom inventory (Bell et al., Reference Bell, Hoffman, Temkin, Powell, Fraser, Esselman, Barber and Dikmen2008). In a large population-based study of very mild non-hospitalized TBI (i.e., all subjects had GCS of 15) without other system injuries, 24% endorsed 3 or more PTS as causing at least a mild problem 3 months after injury (Lannsjo et al., Reference Lannsjo, af Geijerstam, Johansson, Bring and Borg2009). These findings raise questions about the basis of the symptom criteria of the DSM IV (American Psychiatric Association, 1994) and ICD10 (World Health Organization, 1992) diagnosis of post-concussional syndrome. These results suggest that it will be very common for mild TBI cases to meet the ICD-10 symptom criterion for post-concussional syndrome – defined as endorsement of 3 or more symptoms without reference to whether these symptoms began or worsened since the injury.
While the utility, the validity, and the weaknesses of these classification systems are worthy of discussion, they go beyond the scope of this article. For discussion, see other publications (Boake et al., Reference Boake, McCauley, Levin, Contant, Song, Brown, Goodman, Brundage, Diaz-Marchan and Merritt2004; Reference Boake, McCauley, Levin, Pedroza, Contant, Song, Brown, Goodman, Brundage and Diaz-Marchan2005; Kashluba, Casey, & Paniak, Reference Kashluba, Casey and Paniak2006; McCauley et al., Reference McCauley, Boake, Pedroza, Brown, Levin, Goodman and Merritt2005; McCrea, Reference McCrea2008). These classification systems seem to assume that it is normal for those with milder injury to be free from most symptoms by 3 months after injury. The most recent and frequently cited reference to support such recovery is Carroll et al.(Reference Carroll, Cassidy, Peloso, Borg, von Holst, Holm, Paniak and Pepin2004). However, it appears that the conclusions in the abstract of that article are much more definitive and not consistent with the text of the article. The abstract states, “For adults, cognitive deficits and symptoms are common in the acute stage, and the majority of the studies report recovery for most within 3 to 12 months.” However, the body of the article states that for adults and for non-sports injuries, findings on duration of symptoms are mixed.
Some of the limitations of our study need to be acknowledged. First, although we were able to examine various factors that might contribute to symptom endorsements, the dataset did not allow examination of the contribution of other factors, such as the specifics of other system injuries, the circumstances of the accidents, post-injury circumstances, and treatments received. Second, although trauma controls are probably one of the most appropriate control groups in civilian TBI, such a sample may not be able to control entirely for other system injuries, particularly in the more severe TBI cases. Injuries severe enough to cause severe brain injury are likely to also be associated with more severe other system injuries. However, other system injury severity did not seem to account for differences in symptom endorsement between the TBI and control groups. Furthermore, the severity distribution of other system injuries was very comparable for the mild 1 cases and the trauma controls. This sample probably includes some subjects who were suing or receiving compensation for their injuries. Their data on symptom reporting was not used for either purpose, so there was no financial incentive to exaggerate their symptom reports for the study. However, it is possible that some did so nonetheless. Finally, although our sample is not entirely representative of those with mild TBI in its broad sense, the more recent literature indicates that the rates there, too, are higher than what is commonly thought.
The controversy related to post-traumatic symptoms has come from both the clinical and litigation areas. In the clinical area, the major issue is the source of difficulties, which has implications for the approach to take for treatment. This is best handled with a differential diagnosis approach (Iverson, Zasler, & Lange, Reference Iverson, Zasler, Lange, Zasler, Katz and Zafonte2007). The more difficult area is the implications of PTS in litigation. While reports of PTS are understandably influenced by secondary gains, patients who have sustained brain injury indeed do continue to report PTS, more than previously thought, even without the incentive of monetary gain. The symptoms reported are probably the result of a combination of direct and indirect consequences of the brain injury, as well as the personal characteristics of the injured person and their circumstances. In other words, factors other than brain injury are likely to contribute or exacerbate PTS. For litigation purposes, unfortunately, the answer is not a simple one if the issue becomes one of trying to prove whether symptoms are neurologically based. In conclusion, many patients with TBI, including those with mild TBI, do continue to report multiple post-traumatic symptoms through at least one year after injury. The specific causes, however, are not simple to determine.
ACKNOWLEDGMENTS
This study was supported by the following grants: H133A070032 from the National Institutes on Disability and Rehabilitation Research, HS04146 and HS05304 from the Agency for Health Care Research and Quality, and NS-19643 from the National Institutes of Health. The authors have no conflict of interest to disclose.
APPENDIX
Supplemental Table I. Inclusion and exclusion criteria for subjects of the four studies contributing to the dataset

Note
TBI = Traumatic Brain Injury; LOC = Loss of Consciousness; CT = Computed Tomography; PTA = Post-Traumatic Amnesia.
Supplemental Table II. Demographic and severity of injury characteristics of the four studies contributing to the dataset

Note
Age Subgroups marked with the same lower case letters are significantly different by Tukey’s post hoc test at p < .05. SD = Standard Deviation.
†Mild 1 cases had GCS 13–15 with no CT brain abnormalities and PTA under 24 hours; †Mild 2 cases had at least one of these features.
* Participation rate is the percent of subjects that were approached, consented, and neurologically able to respond who provided symptom information. See text for reasons not being approached at 1 month and 1 year.
Note that the whole group analysis presented in Table 2 of the manuscript uses inverse probability weighting to adjust for selection criteria based on injury severity and preexisting conditions.