Introduction
Rivers around the world and their dependent biodiversity are under threat from dams (Kondolf Reference Kondolf1997, Kingsford Reference Kingsford2000, Dudgeon et al. Reference Dudgeon, Arthington, Gessner, Kawabata, Knowler and Lévêque2006, Agostinho et al. Reference Agostinho, Pelicice and Gomes2008), plastic pollution (Wagner et al. Reference Wagner, Scherer, Alvarez-Muñoz, Brennholt, Bourrain and Buchinger2014, Holland et al. Reference Holland, Mallory and Shutler2016), overfishing (Allan et al. Reference Allan, Abell, Hogan, Revenga, Taylor, Welcomme and Winemiller2005, Dudgeon et al. Reference Dudgeon, Arthington, Gessner, Kawabata, Knowler and Lévêque2006) and sand and gravel mining (Kondolf Reference Kondolf1997, Meador and Layher Reference Meador and Layher1998, Dudgeon et al. Reference Dudgeon, Arthington, Gessner, Kawabata, Knowler and Lévêque2006). These threats become even more alarming in the case of rivers that hold globally threatened species. The White-bellied Heron Ardea insignis is the second largest heron in the world. It is seen along, but not restricted to, braided river systems between 300 m and 1,500 m above mean sea level (asl), occupying a varied range of forests from broadleaved tropical to chir pine Pinus roxburghii forests (Pradhan et al. Reference Pradhan, Norbu and Frederick2007, Stanley Price and Goodman Reference Stanley Price and Goodman2015, BirdLife International 2017). IUCN has classified it as ‘Critically Endangered’ since 2007, facing a projected decline in range and population due to loss and degradation of habitat and anthropogenic threats including hydroelectric projects that modify watercourses and powerlines that pose significant threats to flying birds (Stanley Price and Goodman Reference Stanley Price and Goodman2015, BirdLife International 2017).
White-bellied Heron is currently known to be extant in parts of Bhutan, north-east India, north-west Myanmar, and China. It was likely driven to extinction in Nepal in the 19th century and in Bangladesh (Stanley Price and Goodman Reference Stanley Price and Goodman2015). Habitat loss, forest fires, hydroelectric projects, and hunting are some of the threats faced by the White-bellied Heron across its current range (Stanley Price and Goodman Reference Stanley Price and Goodman2015). Bhutan presently holds the largest known population (25–35 individuals) (Wangdi et al. Reference Wangdi, Dhendup and Tshering2017). However, even in Bhutan, there have been occasional reports of heron deaths, due to herons landing on live pole-tops and being electrocuted, and probable local extirpation from Sunkosh Valley due to hydroelectric projects (BirdLife International 2017). Forest fires have been reported to negatively affect the nesting of these birds in Bhutan (Dendup Reference Dendup2016). In Myanmar, heron populations have been identified in Hkakaborazi, Hponkanrazi and Hukaung Valley Wildlife Sanctuary (Rappole et al. Reference Rappole, Aung, Rasmussen and Renner2011, Stanley Price and Goodman Reference Stanley Price and Goodman2015). The current status of the White-bellied Heron from Hukaung Valley Wildlife Sanctuary in Myanmar remains unknown because of the civil unrest in the region that has hampered the efforts of researchers and conservationists alike (J. W. Duckworth pers. comm.). The bulk of the ecological information on the species is from Bhutan and a single locality in India, the Namdapha Tiger Reserve (Stanley Price and Goodman Reference Stanley Price and Goodman2015).
In India, the White-bellied Heron most likely occurred from West Bengal (22.57°N, 88.37°E), Assam, Mizoram, and Nagaland, to eastern Arunachal Pradesh (27.51°N, 96.61°E) in the past (Stanley Price and Goodman Reference Stanley Price and Goodman2015, Choudhury Reference Choudhury2016). Records from further west in Bihar (George Reference George1967), must be treated with caution because of the large geographic distance from the established range, the atypical habitat of these sightings in paddyfields, the unusually large flock sizes, and the records being from a single observer, all of which suggest that its presence there is unlikely albeit not inconceivable. The bulk of information on the heron from India is in the form of sighting records. The species has been recorded from different areas in north-east India including the Namdapha Tiger Reserve (Maheswaran Reference Maheswaran2007, Srinivasan et al. Reference Srinivasan, Dalvi, Naniwadekar, Anand and Datta2010, Mondal and Maheswaran Reference Mondal and Maheswaran2014), Manas Tiger Reserve (Rabha and Das Reference Rabha and Das2018) and Dibru−Saikhowa National Park (Choudhury Reference Choudhury1998). A juvenile White-bellied Heron was photographed at Maguri Beel, Assam, which is located close to D’Ering Widlife Sanctuary and Dibru−Saikhowa National Park in October 2015 (http://orientalbirdimages.org/search.php?Bird_ID=1113&Bird_Image_ID=114469). Recently, the bird was recorded from the Lohit River valley adjacent to Namdapha and Noa-Dehing River valley in eastern Arunachal Pradesh (https://arunachaltimes.in/index.php/2019/03/23/camera-trap-captures-white-bellied-heron-in-ktr/). In India, the only confirmed breeding record of the bird is from the Namdapha Tiger Reserve (Mondal and Maheswaran Reference Mondal and Maheswaran2014). The White-bellied Heron is classified in Schedule 1 of the Indian Wildlife Protection Act (1972), India, which accord it maximum legal protection (https://indiacode.nic.in/bitstream/123456789/1726/1/197253.pdf) (Anonymous 1972).
Arunachal Pradesh constitutes more than 30% of the heron’s global range. Among all the states in north-east India, Arunachal Pradesh offers the highest proportion of suitable habitats in the form of several braided rivers that originate in the Himalayas and flow down to meet the Brahmaputra in Assam. More than 132 dams have been proposed across the different rivers in Arunachal Pradesh (Vagholikar and Das Reference Vagholikar and Das2010). These dams will permanently change the riverine habitat and pose significant threats to riverine birds in the region, specifically potential detriment to the White-bellied Heron diet as fish populations may be affected and potential deaths caused by transmission lines and poles. In addition, riverine birds in the landscape also face potential threats from hunting and habitat loss (Aiyadurai et al. Reference Aiyadurai, Singh and Milner-Gulland2010).
Given the absence of systematic information on White-bellied Heron from the Indian portions of the Himalayan rivers, which constitute 30% of the global range of the species, we decided to conduct a survey in eight drainages in the region. The main objectives of the survey were: 1) to systematically survey sites (using field and questionnaire surveys) across Arunachal Pradesh state and Manas Tiger Reserve in Assam state to determine the presence of White-bellied Heron, and 2) compare habitat parameters of sites where the White-bellied Heron was detected with sites where the species was not detected. This is the first systematic large-scale survey across the White-bellied Heron’s Indian range.
Methods
Study area
The study was conducted between October 2017 and April 2018 in Arunachal Pradesh (26.28°N–29.30°N and 91.20° E–97.30°E) and in January 2019 in Manas Tiger Reserve in Assam in north-east India. Arunachal Pradesh is part of the Eastern Himalaya and Indo-Myanmar Biodiversity Hotspots (Myers Reference Myers1988). It is the most biodiverse region for riverine birds in the world (Buckton and Ormerod Reference Buckton and Ormerod2002). New species (Athreya Reference Athreya2006, Alström et al. Reference Alström, Rasmussen, Zhao, Xu, Dalvi and Cai2016), rediscoveries (King and Donahue Reference King and Donahue2006), and range expansions of birds (Naniwadekar et al. Reference Naniwadekar and Datta2013, Menzies and Rao Reference Menzies and Rao2018, Reference Menzies and Rao2019, Menzies et al. Reference Menzies, Rao and Kumar2019) continue to be recorded from the region. Almost 80% of the geographic area of the state is under forest cover (http://fsi.nic.in; 2017). The elevation in the state ranges from 100 to 7,000 m. Arunachal Pradesh harbours a diverse array of habitats including the northernmost tropical rainforests in the world (Procter et al. Reference Proctor, Haridasan and Smith1998). The state has among the lowest population densities in India (17 persons per km2; national average = 382 persons per km2) but high decadal population growth rate of 25.9% (natiosnal average = 17.64%) between 2001 and 2011 (http://censusindia.gov.in). Arunachal Pradesh is occupied by 26 different tribes (and 110 sub-tribes), each with unique language and culture (Singh Reference Singh1995), within its relatively small geographic size and low population densities.
More than 132 dams have been proposed in the state that spreads over an area of 83,743 km2 to generate power for the region and the rest of the country (Vagholikar and Das Reference Vagholikar and Das2010). These dams include 92 large dams (> 25 MW), of which 38 are above 100 MW (Vagholikar and Das Reference Vagholikar and Das2010). Eighty percent of the hydroelectric power potential (57,000 MW) in north-east India is in Arunachal Pradesh (Vagholikar and Das Reference Vagholikar and Das2010). These dams will permanently change the habitat (from lotic to lentic) and also result in other disturbance (e.g. changes in food supply, sediment dynamics, human accessibility) thereby posing significant threats to riverine bird diversity in the area (Nilsson and Dynesius Reference Nilsson and Dynesius1994). It must be noted that the mandatory Environmental Impact Assessments for these projects have been criticised for the lack of rigour in sampling and lack of detail in presentation of methods and results (Vagholikar and Das Reference Vagholikar and Das2010). Apart from dams, riverine birds also face threats from hunting. Hunting wild animals for food has strong cultural roots in the state (Aiyadurai et al. Reference Aiyadurai, Singh and Milner-Gulland2010, Naniwadekar et al. Reference Naniwadekar, Mishra, Isvaran, Madhusudan and Datta2015). The literature on riverine birds from the region is scanty and is restricted to a few sites in the area (Maheswaran Reference Maheswaran2007). Systematic surveys of riverine bird diversity, especially endangered riverine birds like the White-bellied Heron have been lacking.
River surveys
We conducted river surveys across eight drainages (Noa Dehing, Kamlang, Lohit, Dibang, Siang, Subansiri, Kameng, and Manas rivers) (Figure 1). In each drainage we aimed to cover a wide elevation gradient and sample representative protected and non-protected areas. However, our selection of sites was constrained by presence of gorges or steep slopes and occasionally other logistical constraints, like the availability of local guides who could help us access different stretches of the rivers. Given these reasons, the site selection was not random. Field sampling was carried out inside and outside Protected Areas (Table S1 in the online supplementary materials) and across the entire elevation gradient (60–2,000 m) where the White-bellied Heron has been reported to occur by Stanley Price and Goodman (Reference Stanley Price and Goodman2015). We sampled inside five Protected Areas including Kamlang, Manas, Namdapha, and Pakke Tiger Reserves and D’Ering Wildlife Sanctuary (Table S1).

Figure 1. A map showing the different river systems with sites sampled (n = 81) with symbols to distinguish Namdapha Tiger Reserve, Protected Areas and Non-protected Areas in Arunachal Pradesh and Assam, north-east India.
In each drainage, we selected accessible stretches of different rivers that were geographically separated (from 0.5 to 95 km between sites). We sampled 81 sites in 23 localities. A site is a continuous stretch of the river that was sampled up to 2 km in length and a locality comprised of one to several sites. While field observations were collected at the level of the site, interviews were collected at the level of the locality since informants generally accessed a larger area than the site. Two to five observers carried out river surveys at each site on foot with at least two observers familiar with spotting the heron. At each site, we aimed to sample a 2-km long stretch of the river. However, this was not always achievable, especially in the higher altitudes where gorges and meandering rivers in the mountains made it difficult to walk 2 km along the riverbank. The mean length of river sampled at each site was 1.25 km (median: 1.2 km; range: 0.3–2 km). The minimum distance between adjacent sites was 500 m and the furthest sites within a drainage were separated by 95 km and across drainages by 500 km. The total distance covered in the entire study area was 101.3 km from 81 sites. Twenty-four of the 81 sites were inside Protected Areas.
At each site, we recorded the number of White-bellied Herons in each 100-m segment. At every 100-m point during the river survey at a site, we recorded elevation (with eTrex® 30, Garmin, USA), river width (with Hawke LRF400, Hawke Optics, UK), substrate of the river bank (sandy/rocky/sandy-rocky), flow rate (pools/flowing water/rapids), and anthropogenic activity (fishing, boulder or sand mining, presence of garbage). We classified substrate of the river bank into sandy or rocky (Figure S1). The classification was carried out on site by MR and RM. We recorded different kinds of anthropogenic activity during the survey, including, the presence of garbage, fishing, mining (Figure S1; we were unable to distinguish between boulder and sand mining). Garbage was mostly in the form of plastic waste seen in the river. We classified each site as Protected Area (PA) and non-Protected Area (NPA). Each site was surveyed once over the 6-month fieldwork period.
Key informant surveys
Given the rarity of the species, we used key informant surveys in conjunction with field surveys. Key informant interviews have been used to ascertain the presence of rare species that may be difficult to detect directly in field surveys (Pillay et al. Reference Pillay, Johnsingh, Raghunath and Madhusudan2011, Naniwadekar et al. Reference Naniwadekar, Mishra, Isvaran, Madhusudan and Datta2015). Given that the White-bellied Heron occurs in relatively low densities and could potentially be missed in field surveys, we conducted structured key informant interviews across 23 localities. The localities were grouped by a combination of geographic proximity and administrative regimes (e.g. sites in a PA were one locality and those in NPA were a different locality). Since it was difficult to get information on heron presence at the scale of the site, we clumped sites into different localities. The number of interviews in each locality and additional details of the localities are outlined in Table S3. We conducted interviews to determine past and present encounters of White-bellied Heron by the local community members and determine potential pressures on White-bellied Heron such as hunting, fishing and river modification. We (MR and RM) conducted the interviews in Hindi, a language which is spoken by most people in Arunachal Pradesh. We did not require a translator for any of the interviews. The key informant interview surveys were conducted in the same period as the field surveys. Knowledgeable key informants for the interview surveys included local community members who were hunters or fishermen (n = 171), Forest Department field personnel (n = 24), wildlife researchers and conservationists (n = 4), and a birdwatcher (n = 1). The local community members belonged to 10 different tribal communities. Care was taken to ensure that the informants were individuals who frequented the rivers to increase the likelihood of accurate and reliable information. At the onset of the interview, we would ask the interviewee about four bird species including one species which does not exist in the region (Greater Flamingo Phoenicopterus roseus), one which is highly common and widespread (White-throated Kingfisher Halcyon smyrnensis), and two other species (Lesser Whistling Duck Dendrocygna javanica and Purple Heron Ardea purpurea). Only if the interviewee was able to recognise the species present in the area (and not recognise species absent in the area) did we proceed with the interview. We then asked the interviewee if they recognised the White-bellied Heron. Apart from the photographic identification, we also requested the informants to provide ecological and behavioural information on the White-bellied Heron to corroborate the identification. From the informants we recorded the date and time of their sightings (if they could remember), number of birds observed, and how frequently they encountered it if they have seen it on multiple occasions. This approach has been used earlier to map the distribution of rare species (White et al. Reference White, Jennings, Renwick and Barker2005, Anadón et al. Reference Anadón, Giménez, Ballestar and Pérez2009, Pillay et al. Reference Pillay, Johnsingh, Raghunath and Madhusudan2011, Vignoli et al. Reference Vignoli, Macale, Luiselli, Lecis and Casula2017). Since fishing has been suggested as a threat to the piscivorous heron (Pradhan et al. Reference Pradhan, Norbu and Frederick2007), we also enquired about the fishing techniques used by the local communities. We classified different fishing techniques—traditional, modern and destructive – post-hoc. Traditional fishing techniques included different designs of fish traps (including aerial traps) made from bamboo, which are placed in the river to trap fish, herbal fishing wherein plant parts are used to poison or stun fish, and hammering (where boulders are hit hard with a hammer or hammer-like objects to stun/injure fishes) (Lalthanzara and Lalthanpuii Reference Lalthanzara and Lalthanpuii2009). Modern techniques included angling, hook and line, cast net, gill net and use of mosquito nets for fishing. Destructive methods included use of industrial poisons (bleaching powder/pesticides) to poison river/stream sections, use of explosives, and electric shocks to stun or injure fishes and use of excavators (to divert river flow). A template of the pictures of bird species that were shown to the informants is shown in Figure S2.
Statistical analyses
We compared covariates between sites where heron was detected and not detected. In the case of continuous covariates such as elevation and stream width, we used mean values. In the case of categorical covariates (substrate, flow, garbage and mining), we treated covariates as relative proportions of 100 m segments in a site where the parameter of interest was recorded (e.g.proportion of 100 m segments were rocky substrate/ rapids/ garbage/ mining was present in a site).
We checked for normality of different covariates using the Shapiro-Wilk test (S-W test). Since none of the covariates was normally distributed (P < 0.05), we used non-parametric Kruskal-Wallis tests for comparisons. We also ran a pairwise Wilcoxon Rank Sum test to determine between-group differences. All analyses were carried out in R v. 3.5.1 (R Development Core Team 2018).
Results
Field surveys
We sighted White-bellied Heron six times in three out of seven sites (total effort = 12.6 km) in Namdapha Tiger Reserve over four days. All the sightings were of adult birds. Five of the six sightings were of individual birds. On one occasion, we saw a pair. The bird was seen along the Namdapha and Noa Dehing River and not along the Deban river which forms the north-western border of the park. The mean (± SE) encounter rate for the species within Namdapha was 0.55 (± 0.24) km-1. We failed to detect the bird at all the other sites in Arunachal Pradesh (PA and NPA) and Assam. Of the six sightings, three were of perched birds and three were of flying birds (flying upstream). Mean stream width where the birds were seen perched was 71.3 m (range: 40–90 m; n = 3). In two of the three sightings, the bird was perched on boulders next to fast flowing sections of the river and in the other it was on a sandy-rocky bank next to a deep section of the river with flowing water. Two of these birds appeared to be feeding while in one it appeared perched. The sightings of the bird were between 07h10 and 12h15h.
Since herons were seen only inside Namdapha we compared seven sites in Namdapha (NAM) to other Protected Area sites (PA) and non-Protected Area sites (NPA). The Kruskal-Wallis tests indicated that the proportion of 100 m segments that had mining present was significantly lower in NAM and PA compared with NPA (χ2 = 12.192, df = 2, P = 0.002) (Figure 2A; Table 1). The proportion of 100 m segments that had the presence of garbage was significantly less in NAM and PA compared with NPA (χ2 = 21.697, df = 2, P < 0.001) (Figure 2B; Table 1). There was a significant difference in the mean elevation of sites in Namdapha from PA and NPA (χ2 = 18.183, df = 2, P < 0.001) (Figure 2C; Table 1). We did not find significant differences, across the three categories, in stream width and relative proportions of 100 m segments with different kinds of substrate and flow (Table S2).

Figure 2. Comparison across sites of mining, garbage, and the elevation for each category: Namdapha (n = 7), Non-protected Areas (n = 57), and Protected Areas (n = 17). Error bars represent SE.
Table 1. The mean values for each covariate along with standard errors in parentheses for each category – Namdapha, Protected Areas (except Namdapha), and Non-protected Areas. The Kruskal-Wallis Test results are shown with the significant values in bold.
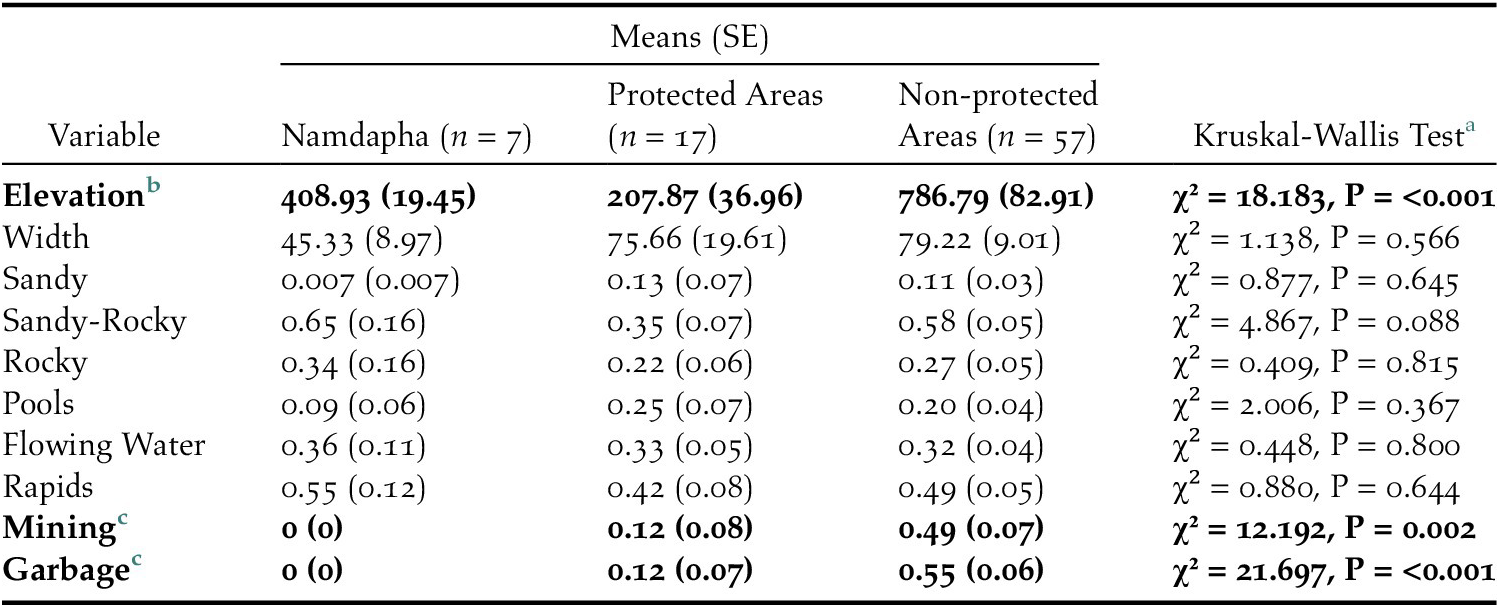
a Significant differences between NAM, PA, and NPA are in bold.
b Pairwise Wilcoxon Rank Sum Test is significant between NAM and PA and PA and NPA.
c Pairwise Wilcoxon Rank Sum Test is significant between both NAM and NPA and PA and NPA.
Key informant surveys
Three to 12 key informants were interviewed in 23 localities. Mean age of the 200 key informant surveys was 40.9 years (SE: ± 1.1; range: 14–85 years). White-bellied Heron was reported as seen in the previous year in only two (Namdapha Tiger Reserve and D’Ering Wildlife Sanctuary) of the 23 localities. All six interviewees (Forest Department personnel: 4; Researcher: 1; Birdwatcher: 1) interviewed in Namdapha reported that they had seen the bird in Namdapha Tiger Reserve and five of them had seen it in the last year. One (local community member) out of the 12 that we interviewed in D’Ering reported sighting two White-bellied Herons in the last year in the southern part of D’Ering Wildlife Sanctuary along the Arunachal Pradesh-Assam border. One bird of the pair was reportedly shot in the first week of February 2018 and was bartered for another hunted bird (a duck) for consumption. In one locality of Siang, one of seven interviewees (local community member) from Ramsing reported the hunting of a White-bellied Heron along the Siring Nala in 2006. He stated that a young boy shot one bird of a pair with a catapult from about 50 m away since the local community had never seen this bird in the area before. The bird was later consumed. In Kane WLS, one of the six interviewees (Forest Department personnel), reported seeing a White-bellied Heron in July 2006 on the Siju River. In no other localities did people seem able to recognise the bird.
At least two key informants per locality, reported fishing in 22 out of the 23 localities. No interviewees reported fishing in Pakke Tiger Reserve. However, we detected evidence of fishing at this site during our field surveys. In 16 localities, at least two key informants reported the use of all forms of fishing techniques, including the destructive techniques. Use of destructive techniques such as explosives was reported by the key informants in 14 localities (by 31 informants), use of electric shocks was reported from 19 localities (by 67 informants), use of poisons was reported from 13 localities (by 34 informants) and use of excavators to modify/alter river flow was reported from one locality (by one informant).
Discussion
Stanley Price and Goodman (Reference Stanley Price and Goodman2015) have identified the lack of precise information on the distribution of the ‘Critically Endangered’ White-bellied Heron as a knowledge gap which requires to be addressed urgently. This is the first systematic survey across the Indian Eastern Himalaya of White-bellied Heron. We had designed the study hoping to detect the species at multiple sites and using an occupancy modelling framework (with 100-m segments within the sites as ‘surveys’) to estimate the occupancy of White-bellied Heron and understand the influence of different covariates in governing occupancy. However, we failed to do that as the species was extremely rarely encountered in our survey. The low encounter rate could be due to non-random site selection which was influenced by accessibility and other logistical constraints. The species appears to be extremely rare in these sites of Arunachal Pradesh which are also habitually visited by local people. It is likely that since White-bellied Herons are threatened by disturbance, the species may avoid areas frequented by people possibly persist in more inaccessible areas. More surveys are required in these areas to ascertain the presence and abundance of the species. We failed to find direct evidence of the White-bellied Heron at any locality except Namdapha Tiger Reserve, which is a known locality for the species. Key informant surveys also indicated possible presence of the species in D’Ering Wildlife Sanctuary. A juvenile bird was photographed at Maguri Beel which is not too far away from this site. Key informants also indicated possible presence of White-bellied Heron in Upper Siang and Kane Wildlife Sanctuary in the past and reported at least two different incidents of hunting the heron. They also indicated the widespread use of destructive methods of fishing in Arunachal Pradesh. Non-protected sites where the heron was not detected had a higher presence of mining and garbage (an indicator of human disturbance).
Namdapha: the heron refuge
This study has reaffirmed the importance of Namdapha Tiger Reserve as a refuge of the White-bellied Heron in the Indian Eastern Himalayas. The bulk of earlier information on the heron from Namdapha has been in the form of species records (Maheswaran Reference Maheswaran2007, Srinivasan et al. Reference Srinivasan, Dalvi, Naniwadekar, Anand and Datta2010, Krishna et al. Reference Krishna, Ray, Sarma and Kumar2012). Mondal and Maheswaran (Reference Mondal and Maheswaran2014) confirmed that the species bred in Namdapha. We have estimated encounter rates of the species for the first time by sampling multiple sections of the Namdapha, Noa Dehing and Deban Rivers. Unlike mammals that face significant hunting pressure in Namdapha (Datta et al. Reference Datta, Anand and Naniwadekar2008), the reserve has a high potential to hold endangered birds such as the White-bellied Heron and other important species such as hornbills (Naniwadekar and Datta Reference Naniwadekar and Datta2013). Despite its immense biodiversity value, Namdapha is not among the best managed Tiger Reserves in the country (Post and Pandav Reference Post and Pandav2013). Currently, there is little evidence of human impacts on the White-bellied Heron in Namdapha, and the heron breeds there despite human presence (in the form of agriculture, camping and passage) along the rivers (Mondal and Maheswaran Reference Mondal and Maheswaran2014). It would be ideal if the Forest Department included systematic long-term monitoring of the White-bellied Heron as part of its wildlife monitoring programme. Further studies are needed to understand species-habitat relationships and potential threats. Stanley Price and Goodman (Reference Stanley Price and Goodman2015) have identified long-term monitoring and in-depth ecological studies as a global priority for the species.
Is hunting and/or fishing responsible for the absence of the White-bellied Heron?
Though we failed to directly detect the species outside Namdapha, there has been recent photographic evidence of the heron from the Lohit Valley which is adjacent to the Noa Dehing Valley (https://www.telegraphindia.com/states/north-east/searching-tiger-spotting-heron/cid/1687584). Outside Namdapha, the bird possibly occurs in extremely low densities and more surveys are required in remote sites to determine its presence and abundance. There have only been a few scattered records and credible reports of the birds (Choudhury Reference Choudhury1998, Rabha and Das Reference Rabha and Das2018, http://orientalbirdimages.org/search.php?Bird_ID=1113&Bird_Image_ID=114469) even from places which are frequently visited by birdwatchers. Outside Namdapha only two out of 194 key informants were able to identify the species, indicating the rarity or absence of the species. Across the whole of Arunachal Pradesh, rivers are frequented by local communities for fishing. It is unlikely that local community members will miss noticing this large heron if it was present. Most of the earlier Lisu (local community living inside and in the vicinity of Namdapha Tiger Reserve) field staff of Nature Conservation Foundation in Namdapha who worked with RN knew about the bird. They even have a unique local name for the bird – Ajye-nye-gaga – which translates to ‘large water bird’ (Srinivasan et al. Reference Srinivasan, Dalvi, Naniwadekar, Anand and Datta2010). Thus, if the heron were present along the rivers, local community members would not fail to notice them and would have reported their presence. Given that only two individuals reported the presence of the species in the past from two sites, it appears that the birds were rare/absent in most of the surveyed areas.
There was no significant difference in any of the parameters (except elevation) between sites in Namdapha (where the heron was detected) and sites in other protected areas where the bird was not detected. White-bellied Heron has been reported to occur from as low as 110 m to above 1,500 m (Stanley Price and Goodman Reference Stanley Price and Goodman2015). Most protected areas in Arunachal Pradesh are at lower elevations. Thus, the difference in elevation between Namdapha and other protected areas merely reflects the lower mean elevations of other protected areas compared to Namdapha. At least two key informants mentioned that the heron was hunted. Given that the species naturally occurs at relatively low densities and with specialized habitat requirements, even low-levels of hunting pressure can potentially wipe out populations of the species, as reported for other large-bodied birds in Arunachal Pradesh (Naniwadekar et al. Reference Naniwadekar, Mishra, Isvaran, Madhusudan and Datta2015).
Pradhan et al. (Reference Pradhan, Norbu and Frederick2007) and Maheswaran (Reference Maheswaran2007) have speculated that fishing was a threat to the persistence of the White-bellied Heron, given its dependence on relatively large fish (Pradhan et al. Reference Pradhan, Norbu and Frederick2011). Key informants reported the presence of fishing in most localities. Use of destructive methods of fishing appears to be pervasive across the state given that they were reported in 70% of localities. Given that we conducted key informant surveys we might have underestimated the prevalence of destructive methods of fishing across the different localities. Unlike some of the traditional fishing methods, the number of fish of all the body sizes killed by destructive methods are likely to be higher and can be expected to have significant negative impacts on fish communities thereby affecting food availability for the heron.
A note on abuse of the riverine habitats in Arunachal Pradesh
Apart from fishing, we documented the prevalence of garbage (mostly plastic waste) and mining pressure extensively in non-protected sites. The impacts of plastic waste on freshwater ecosystems is little studied compared to marine systems though the level of plastic pollution in freshwater ecosystems is comparable to marine ecosystems (Blettler et al. Reference Blettler, Abrial, Khan, Sivri and Espinola2018). Marine fish-eating birds have been documented to swallow plastic with fatal consequences (Gall and Thompson Reference Gall and Thompson2015). No studies on the interactions between birds and plastic waste have been conducted in India. Rivers across north-east India are prime picnic attractions resulting in a lot of plastic waste being left behind as indicated by our data. The rivers are also an important source of boulders and sand which are used in construction, especially of roads. Although licenses for extraction are issued by the relevant local authorities, the impacts on riverine birds and other aquatic fauna, especially fish, are poorly understood. Sand and gravel mining negatively impact crustacean and mollusc populations (Singh and Yadava Reference Singh and Yadava2003, Ranga Reddy Reference Ranga Reddy2014), fish breeding (Kondolf Reference Kondolf1997), and cause permanent damage to the channel morphology, water quality downstream, and river traits such as increased turbidity (Singh and Yadava Reference Singh and Yadava2003, Dudgeon et al. Reference Dudgeon, Arthington, Gessner, Kawabata, Knowler and Lévêque2006, Padmalal and Maya Reference Padmalal and Maya2014), all of which can both directly and indirectly impact the dietary needs of riverine birds, particularly of shy species like the White-bellied Heron. Potential impacts of mining start with disturbance to breeding birds on the banks (noise pollution and human disturbance) and post-mining effects to riverine birds are increased disturbance and increased exposure to predators or possibly hunters (Ashraf et al. Reference Ashraf, Maah, Yusoff, Wajid and Mahmood2011).
Future of White-bellied Heron habitat
Dam construction throughout the state implies a drastic shift in topography and a myriad of negative impacts to riverine bird populations. Apart from the obstruction of the river flow, other impacts will include increased road construction and larger number of electrical wires criss-crossing the river channel. In Bhutan, there have been reports of juvenile White-bellied Heron deaths as a result of perching on live electric support poles placed across rivers (Stanley Price and Goodman Reference Stanley Price and Goodman2015). The recent record of White-bellied Heron from Tawa River (which is the tributary of Lohit) in Kamlang Tiger Reserve is a significant find as areas along the Lohit River have been identified for dam construction. We do not know if the birds from Namdapha are dispersing into the neighbouring Lohit river valley or these rivers are harbouring a resident population of the birds. In both cases, dams will mean that available free-flowing riverine habitats are lost for these habitat specialist, river-dependent birds. It is imperative that a focused survey is carried out for the White-bellied Heron in potential areas north and north-west of Namdapha at least up to Kane Wildlife Sanctuary. These survey efforts should be coupled with raising awareness of the Forest Department staff and the local community members of the global importance and threatened status of the White-bellied Heron. Awareness programmes should be conducted in and around known White-bellied Heron sites in order to protect the species and its habitat. If Namdapha is a source population of the White-bellied Heron, the birds are more likely to occupy the Kamlang and Lohit Rivers. If the habitats along these rivers are altered because of dam construction, there can be potential consequences for the resident/immigrating population of birds. Along with the high density of proposed dams, hunting and other anthropogenic disturbance like mining and garbage are severely impacting riverine ecosystems. Immediate action to raise awareness locally and among the government authorities regarding these impacts should be taken before one of the most biodiverse riverine ecosystems is completely modified with little scope for revival.
Supplementary Material
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0959270920000301.
Acknowledgements
Wildlife Conservation Trust (India), Ravi Sankaran Foundation (India), and Rufford Small Grants for Nature Conservation (23932-1) (United Kingdom) supported this work. We thank the Arunachal Pradesh (CWL/G/13(95)/2011-12/Pt. VI/2531-51) and the Assam Forest Department (WL/FG.31/Pt/Technical Committee/2018) for giving us the necessary research permissions for conducting the study. We are indebted to J. W. Duckworth, Aparajita Datta, Goutam Narayan, Nandita Hazarika, Neelam Datta, Sartaj Ghuman, Gopi Sundar, Abishek Harihar, Kulbhushansingh Suryawanshi, Divya Mudappa and Hari Sridhar for their support during fieldwork, useful discussions and feedback on the manuscript. We thank R. Raghunath for help with the map. Thanks to Japang Pansa for insights and support. We are grateful to the people of Arunachal Pradesh for welcoming us into their homes and for sharing their culture and traditions with us. We obtained the necessary research permits from the Arunachal Pradesh and Assam Forest Departments (Govt. of India). We obtained ethical clearance from the Research Ethics Committee of the Nature Conservation Foundation before the survey. Verbal consent was obtained from the respective village headmen and/or leaders to conduct interviews in the village, and from all key informants before the survey. We explicitly explained the potential impacts of the survey to the respective stakeholders.





