Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder associated with the death of dopaminergic neurons in the substantia nigra. Because of strong functional connections between the basal ganglia and neural regions involved in social cognitive processing, individuals with PD experience problems in social cognition, including difficulty recognizing emotions from faces and prosody (Buxton, MacDonald, & Tippett, Reference Buxton, MacDonald and Tippett2013; Clark, Neargarder, & Cronin-Golomb, Reference Clark, Neargarder and Cronin-Golomb2008; Dara, Monetta, & Pell, Reference Dara, Monetta and Pell2008), as well as in theory of mind (Bodden, Dodel, & Kalbe, Reference Bodden, Dodel and Kalbe2010; Freedman & Stuss, Reference Freedman and Stuss2011). Deficits in social cognition in PD are associated with changes in orbitofrontal cortex, cingulate gyrus, temporo-parietal cortex, and the amygdala (Ibarretxe-Bilbao et al., Reference Ibarretxe-Bilbao, Junque, Tolosa, Marti, Valldeoriola, Bargallo and Zarei2009; Péron et al., Reference Péron, Le Jeune, Haegelen, Dondaine, Drapier, Sauleau and Vérin2010), as well as white matter integrity (Baggio et al., Reference Baggio, Segura, Ibarretxe-Bilbao, Valldeoriola, Marti, Compta and Junqué2012). These deficits arise in part from impairments in cognitive function (e.g., working memory, visuospatial skills; Pell et al., Reference Pell, Monetta, Rothermich, Kotz, Cheang and McDonald2014; Yip, Lee, Ho, Tsang, & Li, Reference Yip, Lee, Ho, Tsang and Li2003), but can also manifest independently of cognitive deficits (Bodden, Mollenhauer, et al., Reference Bodden, Mollenhauer, Trenkwalder, Cabanel, Eggert, Unger and Kalbe2010; Gray & Tickle-Degnen, Reference Gray and Tickle-Degnen2010).
A limitation of previous studies in the visual modality is that they have predominantly focused on the perception and recognition of static stimuli such as emotional faces. Few studies have investigated how PD affects the perception of dynamic social information. Human body motion, or biological motion, enables the effective communication of social intentions and emotions (Atkinson, Dittrich, Gemmell, & Young, Reference Atkinson, Dittrich, Gemmell and Young2004; Roether, Omlor, Christensen, & Giese, Reference Roether, Omlor, Christensen and Giese2009). Whether shaking a fist vigorously to convey anger or rubbing one’s stomach to communicate satisfaction following a tasty meal, communicative (also known as intransitive) gestures are a ubiquitous and important part of interpersonal communication. Transitive gestures that are object-oriented (e.g., hammering, throwing a ball) are also important as they allow observers to perceive and understand actions and activities that others are engaged in.
Gesture perception from biological motion may be impaired in PD. A recent investigation from our group found that individuals with PD are less sensitive than healthy adults to biological motion that depicts walking (Jaywant et al., 2016), a deficit that may extend to more socially complex biological motion. Gesture perception activates the superior temporal sulcus (STS) and premotor cortex (Lotze et al., Reference Lotze, Heymans, Birbaumer, Veit, Erb, Flor and Halsband2006; Montgomery, Isenberg, & Haxby, Reference Montgomery, Isenberg and Haxby2007), which are critical regions in biological motion perception (Gilaie-Dotan, Kanai, Bahrami, Rees, & Saygin, Reference Gilaie-Dotan, Kanai, Bahrami, Rees and Saygin2013; Grossman, Battelli, & Pascual-Leone, Reference Grossman, Battelli and Pascual-Leone2005) and have compromised neural integrity in PD (Pereira et al., Reference Pereira, Junqué, Martí, Ramirez-Ruiz, Bargalló and Tolosa2009; Zarei et al., Reference Zarei, Ibarretxe-Bilbao, Compta, Hough, Junque, Bargallo and Martí2013). Individuals with PD may also be impaired in gesture perception because of their own motor difficulty, as there is evidence for a common coding system between perception and action (Bidet-Ildei, Chauvin, & Coello, Reference Bidet-Ildei, Chauvin and Coello2010; Calvo-Merino, Glaser, Grèzes, Passingham, & Haggard, Reference Calvo-Merino, Glaser, Grèzes, Passingham and Haggard2005; Serino et al., Reference Serino, De Filippo, Casavecchia, Coccia, Shiffrar and Làdavas2009).
Only a handful of studies to date have investigated gesture perception and comprehension in PD, with some finding intact performance (Bonivento, Rumiati, Biasutti, & Humphreys, Reference Bonivento, Rumiati, Biasutti and Humphreys2013; Leiguarda et al., Reference Leiguarda, Pramstaller, Merello, Starkstein, Lees and Marsden1997) and one showing impaired implicit processing of non-social gestures (Klooster, Cook, Uc, & Duff, Reference Klooster, Cook, Uc and Duff2015). In a neuroimaging study (Lotze et al., Reference Lotze, Reimold, Heymans, Laihinen, Patt and Halsband2008), PD participants made significantly more errors in recognizing emotional gestures than did healthy control participants, although recognition of non-emotional (object-oriented) gestures was intact. In the PD group, there was reduced activation across several visual-motor regions, such as STS, when contrasting the emotional versus nonemotional gestures. This study provides the strongest evidence to date for impaired gesture perception in PD and its association with neural regions implicated in biological motion perception such as the STS.
One major weakness of these studies is that gesture stimuli are often videos of the hand or full body, which makes it difficult to ascertain to what extent individuals with PD are relying on motion cues to render their decision. The presence of morphological cues from the face and body confounds examination of gesture perception specifically from biological motion, such as in the study by Lotze et al. (Reference Lotze, Reimold, Heymans, Laihinen, Patt and Halsband2008). It is therefore unknown to what extent the PD deficit in this study was specifically related to motion cues. This is an important question because, in visually degraded or noisy conditions when morphological features are obscured or ambiguous, individuals with PD may need to rely predominantly on biological motion for accurate social perception. Even when morphology and motion cues provide social information, understanding potential difficulties in perceiving social information from biological motion could be useful in creating targeted rehabilitation programs for individuals with PD, such as training to improve motion perception in addition to improving other forms of social cognition.
In the present study, we investigated gesture perception and comprehension in PD using point-light stimuli to represent biological motion, where the only visual cues are points of light on the major joints of the body. Such stimuli are commonly used to isolate visual information to motion and reliably convey actions and emotions (Clarke, Bradshaw, Field, Hampson, & Rose, Reference Clarke, Bradshaw, Field, Hampson and Rose2005; Dittrich, Reference Dittrich1993; Johansson, Reference Johansson1973). The use of point-light stimuli allowed us to investigate if individuals with PD are impaired in perceiving gestures specifically from visual motion. We predicted that the PD group would perform more poorly than the control group in discriminating between communicative and non-communicative gestures, and in accurately describing communicative gestures.
We also conducted exploratory analyses to investigate whether gender would affect the performance of PD individuals on gesture perception. Gender differences exist in PD with regard to disease symptoms (Miller & Cronin-Golomb, Reference Miller and Cronin-Golomb2010). Men with PD perform more poorly on tasks of social cognition (e.g., emotion recognition; Clark et al., Reference Clark, Neargarder and Cronin-Golomb2008 and 2010) and have greater difficulty with social communication (Lubomski, Rushworth, Lee, Bertram, & Williams, Reference Lubomski, Rushworth, Lee, Bertram and Williams2014). In healthy adults, women perform better on emotion recognition tasks than men (Thompson & Voyer, Reference Thompson and Voyer2014). When interpreting emotions from ambiguous audiovisual information (facial expressions and speech), men rely more on linguistic cues, whereas women rely more on extra-linguistic, nonverbal cues (Marquardt, Levitt, Sherrard, & Cannito, Reference Marquardt, Levitt, Sherrard and Cannito2014). We therefore predicted that men with PD would have particular difficulty in gesture perception.
Method
Participants
The study included 23 individuals with PD and 24 normal control adults (NC). Participants were recruited through the Parkinson’s Disease and Movement Disorders Clinic at Boston Medical Center, Boston University’s Sargent College of Health and Rehabilitation Sciences, the Michael J. Fox Foundation’s Clinical Trial Finder Web site, and research talks at local PD support groups. PD and NC participants were matched for age, education level, and male:female ratio (see Table 1). All were native speakers of English or completed high school in English, had at least 12 years of education, and lived at home. PD participants were included if they met clinical criteria for mild to moderate idiopathic PD (Hoehn & Yahr Stage 1–3, U.K. Parkinson’s Disease Society Brain Bank diagnostic criteria; Hughes, Daniel, Kilford, & Lees, Reference Hughes, Daniel, Kilford and Lees1992). Motor disability was quantified using the Unified Parkinson’s Disease Rating Scale (UPDRS) Motor subscale (Martinez-Martin et al., Reference Martinez-Martin, Gil-Nagel, Gracia, Gomez, Martinez-Sarries and Bermejo1994). Participants were in the “on” state during testing. Levodopa-equivalent dosage (LED; mg/day) was calculated using a standardized formula (Tomlinson et al., Reference Tomlinson, Stowe, Patel, Rick, Gray and Clarke2010). One individual with PD was not taking any medications.
Table 1 Participant characteristics
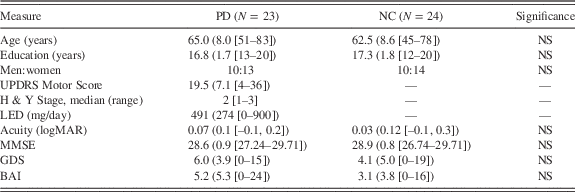
Note. Values presented are means (standard deviations [range]), unless otherwise indicated.
PD=Parkinson’s disease; NC=normal control participants. UPDRS=Unified Parkinson’s Disease Rating Scale; H & Y=Hoehn & Yahr stage; LED=Levodopa-equivalent dosage; logMAR=logarithm of mean angle of resolution; MMSE=Mini-Mental State Examination; GDS=Geriatric Depression Scale; BAI=Beck Anxiety Inventory.
Exclusion criteria for both groups included serious chronic medical, neurologic (other than PD), or psychiatric illness; history of intracranial surgery; history of traumatic brain injury with loss of consciousness greater than a few seconds; current or previous substance or alcohol abuse; and diagnosis of eye disease. Use of antidepressants and anxiolytics was permitted in the PD group only, because of the frequent use of these medications in this population. Use of other psychoactive medications in either group was grounds for exclusion.
All procedures were approved by the Boston University Institutional Review Board in accordance with the Helsinki Declaration. Participants signed an informed consent form before beginning the study.
Screening Measures and Questionnaires
Dementia was screened for using the Columbia-Modified Mini-Mental State Examination (MMSE) and all participants scored above 27/30 (conversion to standard MMSE scores). The Geriatric Depression Scale (GDS) and the Beck Anxiety Inventory (BAI) were used to assess symptoms of depression and anxiety, respectively. Participants had corrected binocular visual acuity equal to or greater than 20/40 (logarithm mean angle of resolution, logMAR ≤ 0.3; Lighthouse Near Visual Acuity Test at 16 inches). Contrast sensitivity was determined using the Functional Acuity Contrast Test (FACT) at a distance of 16 inches.
Gesture Perception Task
Participants viewed point-light human figures that conveyed gestures made with the arms, hands, and fingers. The stimuli were created in a motion capture laboratory at Dalhousie University (Zaini, Fawcett, White, & Newman, Reference Zaini, Fawcett, White and Newman2013) and were composed of 23 white point-lights on a black background (lights placed on a male actor’s head, shoulders, elbows, wrists, all 10 finger tips, hips, knees, and ankles). The actor faced the viewer while performing the gestures. The point-lights depicted gestures that were either (a) communicative – emblems or intransitive gestures that are meant to convey or exchange information with another person and that have commonly accepted meanings (e.g., a thumbs-up gesture to signal “good job”) (McNeill, Reference McNeill1985, Reference McNeill1992); or (b) non-communicative – instrumental pantomimes or transitive gestures of object-oriented actions that do not convey information to another person (e.g., leaning over and moving hands back and forth in a “sweeping the floor” gesture) (examples in Figure 1).

Fig. 1 Example still frames of communicative (a and b) and non-communicative (c and d) point-light gesture videos: (a) “waving hello”; (b) “I’m cold”; (c) “shoveling”; (d) “picking up an object.”
These point-light stimuli were validated on a group of 20 young adults by Zaini et al. (Reference Zaini, Fawcett, White and Newman2013). Briefly, this group of young adults was asked to verbally identify the gestures. The entropy statistic H was calculated to determine participant agreement; stimuli that did not have high agreement were excluded from the corpus. We chose 25 communicative and 25 non-communicative gestures (see Table 2) from the corpus that were highly recognized by the research participants from the validation study and that were suitable to present to older adults in this study. Some stimuli we chose elicited relatively poorer recognition in the validation study, but were selected because they appeared particularly relevant to our sample of older adults (e.g., sewing gesture). We did not include stimuli with an obvious cultural component (e.g., signing the cross).
Table 2 List of communicative and non-communicative gesture stimuli

The 50 point-light gestures were presented to participants in a random order. The stimuli ranged in duration from 1.85 to 5 s. After viewing each stimulus, participants had two tasks: (1) make a forced-choice decision as to whether the stimulus depicted a communicative or non-communicative gesture (Gesture Discrimination Task); and (2) provide a short verbal description of the gesture (Gesture Description Task). Participants were allowed as much time as needed to respond, and encouraged to guess if necessary, but were not allowed to replay a video. Although the instructions stated to first discriminate and then describe the gesture, some participants first described the gesture and then provided their discrimination response.
The Gesture Discrimination Task assessed whether patients could identify communicative and non-communicative gestures in a forced-choice format, while the Gesture Description Task assessed whether patients could infer, retrieve, and verbalize the correct intention of the gesture. A participant’s response to a stimulus on the Gesture Discrimination task was scored independently of his or her response on the Gesture Description task.
At the beginning of the test, participants were provided with detailed instructions as well as definitions and examples of what constituted communicative and non-communicative gestures. They were instructed to respond “communicative” if they believed that the gesture was communicating information to another person who was not shown in the video, and “non-communicative” if the gesture was not communicating information to another person and instead depicted an action involving an object (that was not shown). They were then asked to provide a short verbal description of the action or gesture. Participants answered verbally and the responses were recorded by the examiner. Six practice trials were provided using stimuli that were not included in the experiment. In the practice trials only, participants were given feedback on their responses.
Gesture Perception Task: Scoring
On the Gesture Discrimination task, participants were awarded one point for each stimulus they correctly identified as communicative or non-communicative. Percent correct was calculated for each gesture type. On the Gesture Description task, correct responses were awarded one point, and were defined as follows: (1) exact wording or paraphrasing of the stimulus, based on the responses provided by Zaini et al. (Reference Zaini, Fawcett, White and Newman2013); (2) lengthy descriptions of the correct answer (e.g., “he’s having lunch” was considered a correct response for “eating”); (3) synonyms of the correct answer (e.g., “admonishing” was considered a correct response for “shaming”); (4) for non-communicative gestures that involved an object, replacement with another appropriate object or the generic name of that object (e.g., “drinking a liquid,” “drinking water,” and “drinking a beer” were all acceptable responses for “drinking”). Of participants’ correct responses for communicative gestures, 37% fell under response type (1), 51% fell under response type (2), and 12% fell under response type (3). Of participants’ correct responses for non-communicative gestures, 68% fell under response type (1), 19% fell under response type (2), 7% fell under response type (3), and 6% fell under response type (4). Responses in which the participant only described the action without demonstrating an understanding of the meaning of the gesture were scored as incorrect (e.g., “he raised his hand to his head” was not a correct response for “saluting”).
A trained research assistant who was blind to participants’ group status scored the responses. An additional research staff member checked this initial scoring for accuracy. Discrepancies (i.e., a disagreement in scoring or a scoring error) were resolved by discussion. There was agreement between the two scorers on the majority of responses across participants (communicative gestures: 91% agreement of initial scoring decision; non-communicative gestures: 96% agreement of initial scoring decision). Any response that both scorers deemed ambiguous was further judged for correctness by agreement of six or more of eight independent scorers, who were blind to group. This resulted in an additional 10 responses being marked as correct.
Procedure
Administration of screening measures, questionnaires, vision tests, and perception tasks was completed in a quiet testing room. The gesture stimuli were QuickTime movie files (640×480 pixes, 24 frames per second) that were converted to Audio Video Interleaved (.avi) format and displayed on a CRT monitor (Mitsubishi Diamond Pro 21 inch monitor with 160 Hz max refresh rate). All stimuli were presented using either MatLab 2009a (MathWorks, Natick, MA) and Psychophysics Toolbox version 3.0 (Brainard, Reference Brainard1997; Kleiner, Brainard, & Pelli, Reference Kleiner, Brainard and Pelli2007; Pelli, Reference Pelli1997), or using SuperLab 5.0 Presentation Software (Cedrus, USA). Participants sat in a comfortable, adjustable seat and were given frequent breaks as needed.
Statistical Analyses
Group differences on the Gesture Discrimination Task and Gesture Description Task were analyzed separately using mixed design analysis of variance (ANOVA) with Group (PD, NC) as the between-subjects variable and Gesture Type (communicative, non-communicative) as the within-subjects variable. Results of all ANOVAs are reported in Table 3 and mean (standard deviation) accuracy in Table 4. Post hoc analysis was conducted using independent samples t tests. Percent correct was used as the dependent variable.
Table 3 Results of statistical analyses (ANOVA)

*p<.05.
**p<.01.
Table 4 Mean (standard deviation) accuracy on the Gesture Discrimination and Gesture Description Tasks by Group

For simple main effects and interaction effects in ANOVA, we report effect size using η2, the proportion of the total variance in the data that is accounted for by that effect, where .01 is a small effect, .06 is a medium effect, and .14 is a large effect. For post hoc and planned comparisons of two means, we report effect size using Cohen’s d, where .2 is a small effect, .5 is a medium effect, and .8 is a large effect. The pooled standard deviation was used as the standardizer (denominator) for calculating Cohen’s d.
Results
Demographics and Clinical Measures
PD and NC participants did not differ significantly in age, education, visual acuity, general cognitive status (MMSE), symptoms of depression (GDS), or symptoms of anxiety (BAI) (all Fs<2.52; ps>.05). There was no difference in male:female ratio between groups (χ2=.02; p>.05) nor did men and women differ in age, education, acuity, and symptoms of depression or anxiety (all Fs<2.49; ps>.05).
Group differences in contrast sensitivity (FACT chart) were determined using a 2×5 mixed design ANOVA with a between subjects factor of Group (PD, NC) and within subjects factor of Spatial Frequency (1.5, 3, 6, 12, and 18 cycles/degree). There was a main effect of Spatial Frequency (F(4,172)=236.79; p<.01; η2=.84) and a main effect of Group (F(1,43)=8.58; p<.01; η2=.17). PD had poorer contrast sensitivity than NC regardless of spatial frequency (p<.01; d=.86). There was no Group×Spatial Frequency interaction (F(4,172)=1.71; p>.05;, η2=.01). There was a Group×Gender×Spatial Frequency interaction (F(4,164)=2.64; p<.05; η2=.01). PD men had poorer contrast sensitivity than NC men at 6 and 12 cycles/degree (t(18)s>2.27; ps<.05; ds>1.07). PD women had poorer contrast sensitivity than NC women at 3 and 18 cycles/degree (t(25)s>2.80; ps<.01; ds>1.11). There was no significant difference in contrast sensitivity between PD men and PD women.
Gesture Discrimination Task
Results are displayed in Figure 2. As shown by the 95% confidence intervals (error bars) of the means in Figure 2, both PD and NC performed significantly above chance level (50%). There was a main effect of Gesture Type, where participants demonstrated better accuracy on non-communicative gestures than on communicative gestures (p<.05; d=.67). There was no significant main effect of Group or Group×Gesture Type interaction.

Fig. 2 Performance on the Gesture Discrimination Task by Group (Parkinson’s disease [PD], normal control [NC]) and Gesture Type (communicative, non-communicative). Error bars represent 95% confidence intervals. PD and NC did not differ in performance. Non-communicative gestures were better recognized than communicative gestures, regardless of group.
Gesture Description Task
Results are displayed in Figure 3. There was a main effect of Gesture Type, with all participants performing better on non-communicative gesture trials than on communicative gesture trials (p<.01; d=1.09). There were trends toward a main effect of Group and a Group×Gesture Type interaction. To explore this trend, we conducted an independent samples t test, analyzing PD-NC differences separately for communicative and non-communicative gestures in the Gesture Description Task. Whereas there was no significant PD-NC difference for communicative gestures (t(45)=.59; p>.05; d=.17), PD performed significantly more poorly than NC (large effect) on the non-communicative gesture trials (t(45)=2.82; p<.01; d=.82).

Fig. 3 Performance on the Gesture Description Task by Group (Parkinson’s disease [PD], normal control [NC]) and Gesture Type (communicative, non-communicative). Error bars represent 95% confidence intervals. PD had poorer accuracy than NC for non-communicative gestures, but not communicative gestures. Non-communicative gestures were easier to describe than communicative gestures regardless of group.
Effect of Gender on Gesture Discrimination Task and Gesture Description Task
To investigate the possible effect of gender on performance, we conducted 2×2×2 ANOVAs separately for the Gesture Discrimination Task and the Gesture Description Task. The between-subjects factors were Group (PD, NC) and Gender (men, women) and the within-subjects factor was Gesture Type (communicative, non-communicative). Results on the Gesture Discrimination Task are shown in Figure 4. As shown by the 95% confidence intervals (error bars) of the means in Figure 4, both PD and NC men and women performed significantly above chance (50%). There was a significant Group×Gender interaction. Regardless of gesture type, men with PD had poorer accuracy than women with PD (t(12)=1.97; p=.07; d=.86) and NC men (t(12)=2.15; p=.05; d=.96) at a trend level (large effect). Women with PD did not differ from NC women (t(25)=.59; p>.05; d=.23). NC men and women did not differ (t(22)=.97; p>.05; d=.40). There was no significant Gesture Type × Gender interaction or Group×Gesture Type×Gender interaction.

Fig. 4 Performance on the Gesture Discrimination Task by Group (Parkinson’s disease [PD], normal control [NC]) and Gender (male, female), averaged across Gesture Type (communicative, non-communicative). Error bars represent 95% confidence intervals. Men with PD performed worse than women with PD and NC men at a trend level. Women with PD did not differ from NC women. NC men did not differ from NC women.
On the Gesture Description Task, there was a significant Group×Gender interaction, shown in Figure 5. Regardless of gesture type, men with PD had poorer accuracy than women with PD (t(21)=1.87; p=.08; d=.76) at a trend level (medium effect), and significantly poorer accuracy than NC men (t(18)=2.21; p<.05; d=.99). Women with PD did not differ from NC women (t(25)=.05; p>.05; d=.02). NC men and women did not differ (t(22)=.84; p>.05; d=.34). There was no significant Gesture Type×Gender interaction or Group×Gesture Type×Gender interaction.

Fig. 5 Performance on the Gesture Description Task by Group (Parkinson’s disease [PD], normal control [NC]) and Gender (male, female), averaged across Gesture Type (communicative, non-communicative). Error bars represent 95% confidence intervals. Men with PD performed significantly worse than women with PD and NC men. Women with PD did not differ from NC women. Men and women NC did not differ.
Correlation with Contrast Sensitivity
Because of the main effect of Group and the Group×Gender×Spatial Frequency interaction on contrast sensitivity, we explored the relation between gesture perception and contrast sensitivity using Spearman rank-order correlations, with a conservative alpha of .01. In the PD group, contrast sensitivity at 1.5 cycles/degree correlated with better performance in discriminating (ρ=.64; p=.001) and describing (ρ=.60; p=.002) communicative gestures only. In the NC group, contrast sensitivity at 1.5 cycles/degree correlated with worse performance in describing communicative gestures (ρ=−.49; p=.01), and contrast sensitivity at 12 cycles/degree correlated with better performance in discriminating communicative gestures (ρ=.61; p=.002). In the sample as a whole, no significant correlations were found between contrast sensitivity at any spatial frequency and performance on the gesture discrimination and gesture description tasks (all ρs<.34; ps>.02). Given this latter finding, and our quasi-experimental design that precludes analysis of covariance, contrast sensitivity was not used as a covariate in the analyses.
Discussion
This study investigated whether perception of communicative (intransitive, social) and non-communicative (transitive, object-oriented) gestures was impaired in PD along two dimensions: the ability to identify and describe an observed gesture and the ability to discriminate between communicative and non-communicative gestures. The gestures were perceived from point-light biological motion, which restricted visual information to motion cues alone.
Our results supported the hypothesis that PD would demonstrate a deficit in describing gestures from biological motion, although we found this group difference only for non-communicative gestures. We also discovered that gender affected the relation between PD and gesture perception. Our findings are consistent with those of our recent study, in largely the same sample of PD and NC participants, which showed reduced sensitivity to perceiving point-light walking (Jaywant et al., 2016). Here we show that perception of meaningful object-oriented actions from biological motion, beyond simple walking, is also impaired in PD. Critically, the ability to perceive gestures from biological motion in PD depends on the type of information conveyed by the gesture (communicative or non-communicative) and the gender of the PD observer.
We found that PD participants as a whole had a selective deficit in describing non-communicative gestures. Being able to accurately perceive non-communicative gestures from motion cues is important because, even though some gestures do not have a communicative intent, they allow the observer to determine the action and activity of a potential communication partner, which can guide social interaction. A deficit in non-communicative gesture perception could, therefore, impact an individual with PD’s ability to initiate conversation or accurately and efficiently manage a social interaction. Being able to perceive gestures from motion cues is important in visually degraded conditions where a communication partner’s morphological features are ambiguous or occluded, such as may occur in dim lighting, far away distance, inclement weather, or crowded situations (e.g., public transit).
This finding of a selective deficit in perceiving non-communicative gestures was unexpected given previous research that has shown the opposite pattern (i.e., a PD deficit for communicative/emotional gestures but not non-communicative/non-emotional gestures) using standard (“full-light”) stimuli (Lotze et al., Reference Lotze, Reimold, Heymans, Laihinen, Patt and Halsband2008). One possibility was that our sample had trouble describing object-oriented actions because of their own motor problems induced by PD, as suggested by evidence for a common coding system for perception and action mediated by the mirror neuron system (Iacoboni & Dapretto, Reference Iacoboni and Dapretto2006).
Another possible explanation for the group difference in performance was that the PD group had worse contrast sensitivity than the NC group. We found correlations between contrast sensitivity and perception of communicative gestures in the PD and NC groups, which did not differ on perception of this type of gesture, but no correlations between contrast sensitivity and non-communicative gestures, for which the PD group performed more poorly than the NC group. Furthermore, both communicative and non-communicative point-light stimuli had the same contrast, but the PD group performed more poorly than NC in describing only the non-communicative gestures. It is therefore unlikely that contrast sensitivity was driving our findings.
A deficit in perceiving non-communicative gestures, but not communicative gestures, may be partially accounted for by the type of stimuli presented in our study. We used point-light stimuli, which restrict visual information to motion, and found those with PD to be impaired at describing non-communicative gestures. In other studies that have found differing results, the stimuli were full-light videos that included morphological cues such as facial expression and posture (Leiguarda et al., Reference Leiguarda, Pramstaller, Merello, Starkstein, Lees and Marsden1997; Lotze et al., Reference Lotze, Reimold, Heymans, Laihinen, Patt and Halsband2008). It may be that difficulty perceiving communicative gestures arises primarily from impairment in decoding morphological features, or integrating morphology and motion information. Moreover, the emotional valence of our communicative gestures was unknown and some gestures (e.g., “call me,” “hello”) were not characterized by a specific emotion. Differences in the emotional content of our gestures relative to those in other studies may also explain the differences in results. That is, because PD is associated with deficits in emotional processing, gesture perception from biological motion may be more impaired when an emotion has to be decoded. The ability of individuals with PD to perceive emotional gestures from biological motion is a topic for future study.
Differences between studies may also reflect differences in sample size and characteristics. Our PD group was composed of 23 participants, while the sample of Lotze et al. (Reference Lotze, Reimold, Heymans, Laihinen, Patt and Halsband2008) had only nine participants; hence our study had more power to detect group differences. Relative to their sample, our PD group had shorter disease duration (4.8 years vs. 12.8 years) and a milder range of disease stage (0 out of 23 vs. 2 out of 9 participants at Hoehn & Yahr stage 4). It is possible that our sample was only beginning to show impairments, and that replication of the study with a PD group at more advanced stages of the disease may yield differences in regard to the interaction between PD and gesture description for communicative and non-communicative gestures. Future studies should also investigate gesture perception from point-light and full-light stimuli in the same PD sample, to better understand how motion cues alone and in combination with morphological cues affect perception.
A secondary goal of the current study was to explore the possible moderating effect of gender on PD gesture perception. There is accumulating evidence that disease profiles can differ in men and women with PD, possibly due to a neuro-protective effect of estrogen in women (Miller & Cronin-Golomb, Reference Miller and Cronin-Golomb2010). Moreover, emotion recognition and social communication is worse in men than women with PD (Clark et al., Reference Clark, Neargarder and Cronin-Golomb2008; Lubomski et al., Reference Lubomski, Rushworth, Lee, Bertram and Williams2014). We found that men with PD performed more poorly than women with PD and NC men in discriminating between communicative and non-communicative gestures, and in verbally describing both communicative and non-communicative gestures. This finding is consistent with prior research and underscores the importance of looking at subgroups of individuals with PD.
Replication of this gender effect on biological motion perception with a larger sample size is warranted. Some of the differences we observed between men and women were only at a trend level, and confidence intervals were large (e.g., Figure 5). Additionally, while our PD and NC groups were matched for age, it is possible that deficits in gesture perception may manifest as an interaction between age, gender, and disease burden in PD, as social perception changes with age (Isaacowitz et al., Reference Isaacowitz, Löckenhoff, Lane, Wright, Sechrest, Riedel and Costa2007; Marquardt et al., Reference Marquardt, Levitt, Sherrard and Cannito2014). A larger sample would allow analysis on the interaction of age and gender in gesture perception in PD.
We observed that both PD and NC were able to more accurately identify non-communicative gestures than communicative gestures across tasks. It is possible that participants demonstrated better accuracy on the non-communicative trials because object-oriented gestures such as “throwing a ball” and “casting a fishing rod” may have concrete and relatively unambiguous stored representations that are readily accessed when perceiving the gesture (Bonivento et al., Reference Bonivento, Rumiati, Biasutti and Humphreys2013). By contrast, several social and cultural factors play into how humans interact with and communicate with one another, which may have resulted in greater ambiguity of our communicative gestures. Additionally, the lack of an observed partner for communicative gesture trials may have contributed to the observed lower performance on these trials by removing an important context for decoding the meaning of social gestures.
In summary, the present study showed that as a group, those with PD are impaired relative to NC in inferring and describing the meaning of non-communicative gestures, but are comparable to NC in describing communicative gestures. Men with PD, however, have difficulty in understanding not only the actions of others (non-communicative gestures), but also others’ communicative gestures in interpersonal exchanges. Our results add to a growing body of literature that indicates difficulty in perceiving social cues in PD. We argue for the importance of assessing dynamic visual perception in PD, which may reveal different deficits than those elicited by conventional tasks that use static visual stimuli.
Our results also have implications for rehabilitation in PD. If future research determines that a biological motion perception deficit has functional ramifications in ecologically valid settings for individuals with PD, then improving biological motion would be an important rehabilitation goal. Perceptual training improves biological motion in healthy young and older adults (Grossman, Blake, & Kim, Reference Grossman, Blake and Kim2004; Legault & Faubert, Reference Legault and Faubert2012), and the possibility exists that training may also enhance social perception from biological motion in PD. Because we found that gesture perception was different in men and women, rehabilitation approaches that aim to improve social cognition and social engagement in PD may require tailoring the rehabilitation approach to specific subgroups with the disease.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke, including a Ruth L. Kirschstein National Research Service Award to A.J. (grant number F31 NS078919) and an R01 grant to A.C.G. (grant number R01 NS067128). We thank Terry Ellis, Ph.D., and Serge Roy, Sc.D., for help with experimental design, statistical analysis, and manuscript review, and Marie Saint-Hilaire, M.D., Cathi Thomas, R.N., M.S.N., and the Fox Foundation Trial Finder for participant recruitment. We are grateful to members of the Vision & Cognition Laboratory for their assistance in scoring and especially to Valerie Levit, B.A., who also assisted in data organization. We acknowledge the contribution of Aaron Newman, Ph.D., who created the point-light figures stimulus set. The authors of this study have no conflicts of interest to declare.











