Introduction
Assisted reproductive technology (ART) involves the use of medical methods to artificially manipulate oocytes, sperm, fertilized eggs, and embryos to achieve the goal of conception (Esteves et al., Reference Esteves, Humaidan, Roque and Agarwal2019). Among them, in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) are the core technologies of ART. IVF refers to the natural fertilization of eggs and sperm through artificial methods and the subsequent in vitro culture of early embryos, while ICSI refers to the process of injecting a single sperm into the cytoplasm of an egg using micromanipulation technology to achieve fertilization. In vitro fertilization and embryo transfer (IVF-ET) technology has become an important means of infertility treatment since its discovery, but the risk of poor embryo development with the traditional IVF/ICSI treatment cycle is still unavoidable (Liu et al., Reference Liu, Zhang, Yang, Zhao, Hao, Zhang, Liu, Wu and Wang2016), which leads to pregnancy failure due to poor development of the embryos transferred. Therefore, exploring an efficient technology of embryo culture in vitro to obtain high-quality embryos is one of the key features for improving the effect of ART treatment.
Melatonin (MT) is rhythmically released during the dark hours at night by the pineal gland (Olcese, Reference Olcese2020). The circadian rhythm of MT plays major roles in the secretion of reproductively active hormones (Reiter et al., Reference Reiter, Tan and Galano2014). MT, which is both fat and water soluble, is distinctive in that it can easily pass though cell membranes and easily reaches nuclei and mitochondria, where it accumulates in high concentrations (Acuña-Castroviejo et al., Reference Acuña-Castroviejo, Escames, Venegas, Díaz-Casado, Lima-Cabello, López, Rosales-Corral, Tan and Reiter2014). MT is a direct free radical scavenger with a more powerful antioxidant effect than conventional antioxidants such as vitamins C and E, glutathione (GSH), and mannitol (Reiter, Reference Reiter1995). MT and its metabolites can directly remove reactive oxygen species (ROS) in cells, activate antioxidant enzymes, increase the expression level and activity of GSH, and inhibit pro-oxidant enzymes to reduce cell oxidation injury (Manchester et al., Reference Manchester, Coto-Montes, Boga, Andersen, Zhou, Galano, Vriend, Tan and Reiter2015). Growing evidence of reproductive medicine supports the role of melatonin in human reproduction (Rafael et al., Reference Rafael, Ediane, Allain and Heitor2019). It is possible that in vivo administration may improve the quality of oocytes and in vitro incubation may improve oocytes in vitro maturation (IVM) and embryonic development (Manchester et al., Reference Manchester, Coto-Montes, Boga, Andersen, Zhou, Galano, Vriend, Tan and Reiter2015). It has been reported that adding the appropriate amount of MT to IVM and embryo culture medium can significantly improve the developmental potential of oocytes and the in vitro development of embryos (Lin et al., Reference Lin, Lee, Kang, Oqani, Cho, Kim and Il Jin2018; An et al., Reference An, Peng, Cheng, Lu, Zhou, Zhang and Su2019; Barros et al., Reference Barros, Monte, Santos, Lins, Cavalcante, Gouveia, Müller, Oliveira Junior, Barberino, Donfack, Araújo and Matos2020a). Through research on human IVF and IVM, Li et al. (Reference Li, Mu, Elshewy, Ding, Zou, Chen, Chen, Wei, Cao, Zhou and Zhang2021) found that the addition of MT to the embryo culture medium can improve the clinical outcomes of IVF and IVM. Zou et al. (Reference Zou, Chen, Ding, Gao, Chen, Liu, Hao, Zou, Ji, Zhou, Wei, Cao and Zhang2020) added MT to IVM medium to culture human immature oocytes from a controlled ovarian hyperstimulation (COH) cycle, which significantly reduced the levels of ROS and Ca2+ in oocytes during the IVM process, increased mitochondrial membrane potential, and improved embryo development, eventually resulting in healthy offspring. The present study was the first attempt to use embryo culture medium supplemented with MT to culture embryos of patients undergoing repeated cycles after failed IVF/ICSI cycles and aimed to explore whether the application of MT could improve the embryonic development and clinical outcomes of patients with repeated IVF/ICSI cycles.
Materials and methods
Research patients
In total, 140 patients with failed cycles who visited the Reproductive Medicine Center of the First Affiliated Hospital of Anhui Medical University from June 2020 to November 2020 were selected. The inclusion criteria were as follows: (1) at least one IVF/ICSI failed cycle and (2) patient age ≤ 36 years. After the genetic factors of both the men and women were excluded, the causes of infertility included 18 cases of male-only factors, involving oligoasthenoteratozoospermia; 77 cases of female-only factors, including 41 cases of sequelae of pelvic inflammatory disease, 26 cases of polycystic ovary syndrome, and 10 cases of endometriosis; 40 cases of combined male and female factors; and five cases with unknown causes.
COH regimen
All patients in this study were treated with gonadotropin hormone-releasing hormone (GnRH) antagonist (flexible regimen) for ovarian stimulation. Recombinant human follicle-stimulating hormone (Gn, Gonal F; Serino Barueri, SP, Brazil) was given on the second or third day of the menstrual cycle for ovarian hyperstimulation. After 4–5 days, the dosage of Gn was adjusted according to the follicle size and hormone level. When the dominant follicle reached 12–14 mm, GnRH antagonist (GnRH-A, Cetrotide, Merck Serono SA, Geneva, Switzerland) was added until the day of human chorionic gonadotropin (HCG) injection. When there were two or three follicles with a diameter ≥18 mm, HCG (10,000 U; Pregnyl; AESCA Pharma, Austria) was injected, and oocyte retrieval was completed after 36–38 h under the guidance of a transvaginal ultrasound.
Experimental design
Experiment 1
Immature human oocytes obtained from the COH cycle were collected for IVM to obtain mature oocytes (IVM-MII) in vitro and perform ICSI insemination. Then, embryos were cultured in vitro in medium containing 0, 10–11, 10–9, 10–7 or 10–5 M MT. Ultimately, 10–9 M was determined to be the optimal concentration of MT.
Experiment 2
For the 140 enrolled patients with failed IVF/ICSI cycles, embryo culture medium containing 10–9 M MT was prepared to conduct embryo culture in vitro in the subsequent 140 repeated cycles. High-quality blastocysts were collected and cryopreserved. These 140 MT culture cycles were designated as the experimental group (10–9 M group), and the control group consisted of the previous failed cycles of the same patients (0 M group). In addition, according to the number of failed cycles, the patients were further divided into subgroups of one, two, or at least three failed cycles, and the fertilization and embryo development statuses of each subgroup in the 10–9 M and 0 M groups were compared and analyzed.
Experiment 3
After 3 months, vitrified-warmed embryo transfer was performed. The experimental process is shown in Fig. 1.
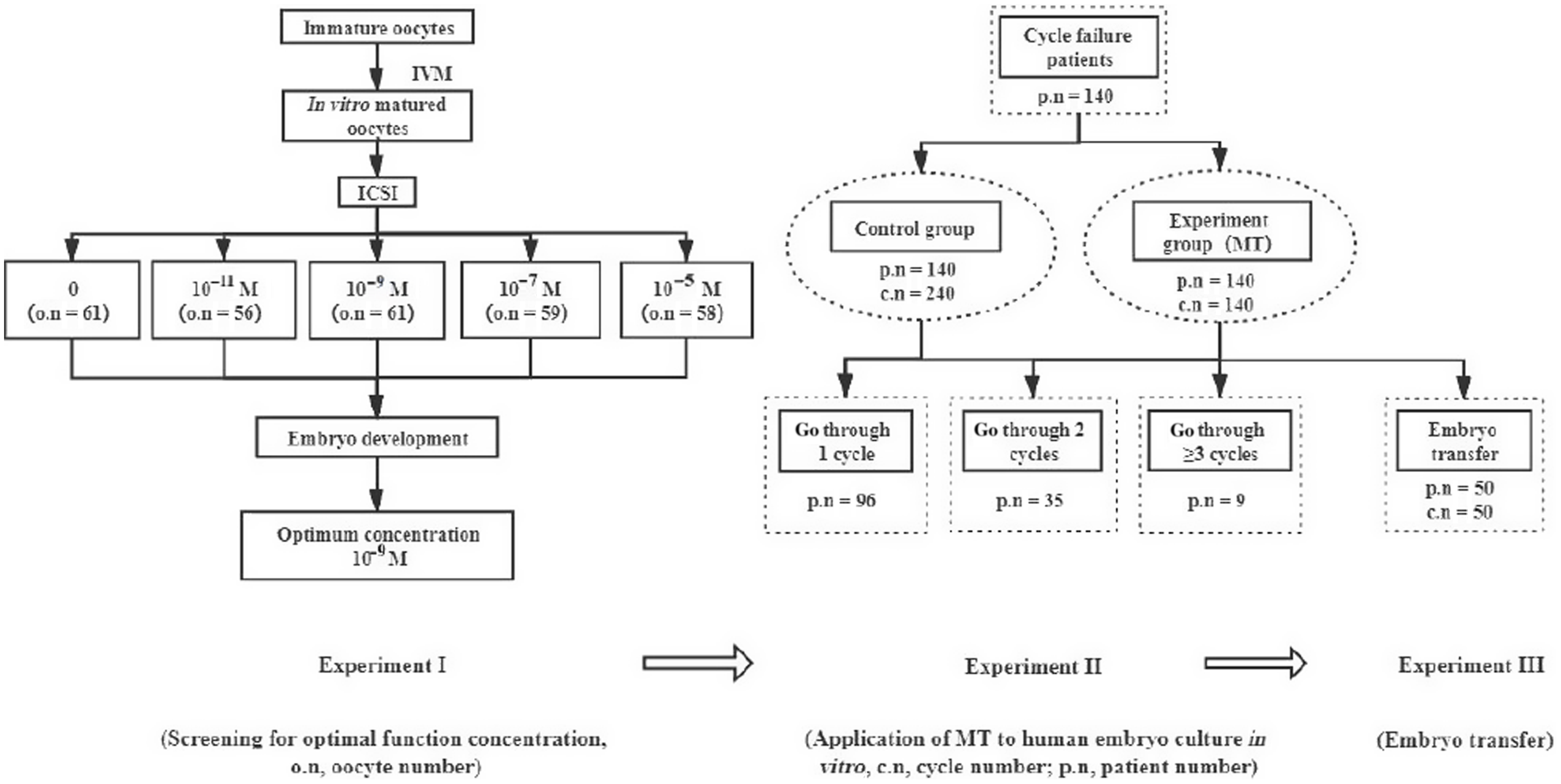
Figure 1. Experimental flow chart. c.n, cycle number; o.n, oocyte number; p.n, patient number.
IVM of oocytes from COH cycles
Human immature oocytes with normal morphology from the COH cycle were collected and placed into IVM medium prepared and balanced overnight to culture for 24 h. Subsequently, the IVM-MII oocytes were selected for ICSI insemination, followed by 5 or 6 days of embryo culture in vitro. The detailed process has been reported previously in the published literature (Sacha et al., Reference Sacha, Kaser, Farland, Srouji, Missmer and Racowsky2018).
Preparation of the embryo culture medium
Firstly, MT (Sigma, USA) was fully dissolved in absolute ethanol and diluted to a MT concentration of 10–2 M (stock solution). Subsequently, the stock solution was distributed into centrifuge tubes, 10–20 μl each tube, and finally frozen at −20°C; On the day before each experiment, one tube or more of the stock solution was warmed and gradually diluted with embryo culture medium (cleavage or blastocyst, Cook, Sydney, Australia) through the dilution multiple method to reach an appropriate MT concentration for the embryo culture medium (10–11, 10–9, 10–7 or 10–5 M). Next, 6–8 droplets (30 μl/drop) were placed in a dish with a diameter of 60 mm and covered with 2 ml tissue culture oil. Finally, the dish was placed at 37°C with 6% CO2 and saturated humidity for 18 h of equilibration.
ICSI/IVF insemination
Under a microscope, the cumulus–oocyte complex (COC) in follicular fluid was picked up and placed into balanced fertilization medium (Cook, Sydney, Australia) at 37°C with 6% CO2 and saturated humidity for 4–6 h of culture in vitro, followed by ICSI or IVF insemination. The process of ICSI insemination has been described in detail in previously publications (Ding et al., Reference Ding, Wang, Li, Chen, Zou, Ji, Hao, Xue, Zou, Wei, Zhou, Cao and Zhang2020). Following ICSI, the inseminated oocytes were transferred directly into cleavage culture medium containing MT for embryo culture in vitro. For IVF insemination, the COC, which underwent 4–6 h of culture in vitro, was transferred into fertilization medium containing 5 × 105/ml grade A and grade B sperm for 6 h of culture at 37°C with 6% CO2 and saturated humidity. Next, the cumulus cells around the oocytes were removed and those with the second polar body, which were considered fertilized oocytes, were selected under an inverted microscope. Finally, all fertilized oocytes were transferred into cleavage culture medium with MT for subsequent embryo culture.
Embryo culture
The oocytes undergoing in vitro culture in the balanced cleavage culture droplets (one oocyte/drop) were observed based on their fertilization status 14–16 h after insemination, and then the culture was continued at 37°C, 6% CO2, and saturated humidity. Two days later, all of the formed cleavage embryos were transferred into balanced blastocyte culture droplets (one to three embryos/drop) for an additional 2 or 3 days of blastocyst culture. Finally, the obtained high-quality blastocysts (refer to Fig. 2) were selected and cryopreserved in −196°C liquid nitrogen using the vitrification method. In this process, all embryos formed were scored according to the Tomás et al. (Reference Tomás, Orava, Tuomivaara and Martikainen1998) and Gardner and Schoolcraft (Reference Gardner and Schoolcraft1999) scoring standards.
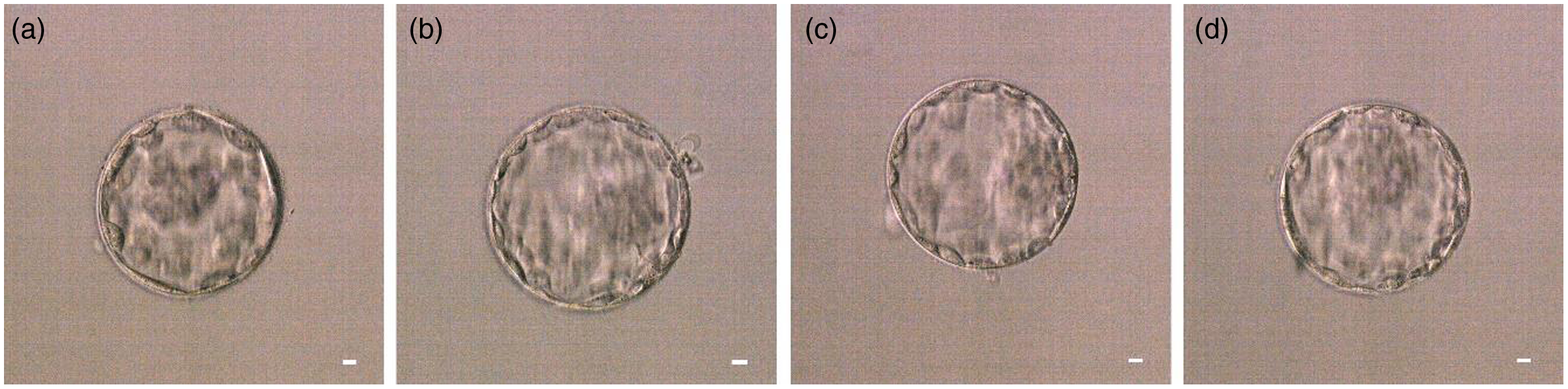
Figure 2. Representative images of the high-quality blastocysts. (a) High-quality blastocyst 4AA. (b) High-quality blastocyst 4AA. (c) High-quality blastocyst 4AB. (d) High-quality blastocyst 4BA. Scale bars, 10 μM.
Embryo transfer and pregnancy determination
Three months later, the cryopreserved blastocyst was warmed. According to the patient’s age, one or two warmed blastocysts were transferred into the uterus under B-ultrasound guidance (≥35 years old, two embryos per transfer cycle, <35 years old, one embryo per transfer cycle). Two weeks after embryo transfer, serum HCG levels were examined and a biochemical pregnancy was defined as a positive HCG value (≥25 IU/l). At 7 weeks following embryo transfer, the presence of a gestational sac identified by an ultrasound scan was referred to as a clinical pregnancy. For the detailed operation process of embryo vitrification and warming, please refer to our previously published literature (Camargos et al., Reference Camargos, Rodrigues, Lobach, El Cury-Silva, Nunes, Camargos and Reis2019).
Statistical analysis
SPSS software version 23.0 was used to perform statistical analysis. The differences in means between continuous variables [female age, male age, basic follicle-stimulating hormone (FSH) level, basic luteinizing hormone (LH) level, basic E2 level, duration infertility, average number of oocytes retrieved and body mass index (BMI)] were analyzed by one-way analysis of variance (ANOVA). Values are presented as mean ± standard deviation. Categorical data on developmental competence (rates of fertilization, cleavage, high-quality cleavage embryo, and high-quality blastocyst) in each group were analyzed using the chi-squared test or Fisher’s exact test. P-values < 0.05 were considered to be statistically significant.
Results
Screening the optimal MT concentration for embryo culture medium
In total, 400 human immature oocytes of 198 COH cycles from 198 patients were collected for IVM culture, and 295 IVM-MII oocytes were obtained. ICSI insemination and embryo culture in vitro were performed, and the detailed results are shown in Table 1. There were no significant differences in age, FSH, LH, oestradiol (E2), BMI, years of infertility, or number of oocytes obtained in each group (refer to Table 2).
Table 1. Effects of different concentrations of MT on embryo development in vitro
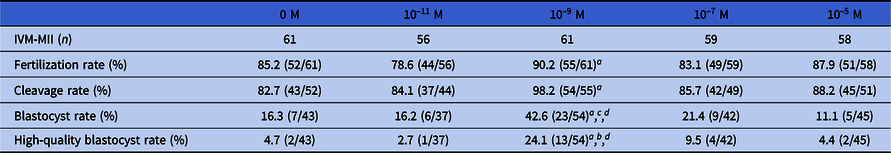
Data were analyzed using chi-squared or Fisher’s exact test. Different symbols within columns and different letters within columns and within rows indicate significant differences.
a P < 0.01, compared with 0 M group;
b P < 0.01, compared with 10–5 M group;
c P < 0.001, compared with 10–5 M group;
d P < 0.01, compared with 10–11 M group.
Table 2. Comparison of baseline data of the five groups of patients
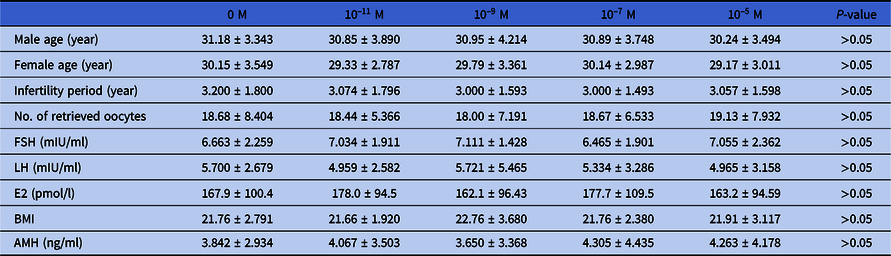
Data were analyzed using one-way analysis of variance (ANOVA). Values are presented as mean ± standard deviation.
As shown in Table 1, after adding 10–9 M MT to the embryo culture medium, the fertilization, cleavage, blastocyst and high-quality blastocyst rates of the 10–9 M group were significantly different from those of the 0 M group (P < 0.01). The blastocyst and high-quality blastocyst rates of the 10–9 M group were significantly different from those of the 10–5 M group (P < 0.01, P < 0.001), and the blastocyst rate of the 10–9 M group was significantly different from that of the 10–11 M group (P < 0.01). The above data revealed that the addition of 10–9 M MT to the embryo culture could significantly improve the fertilization and in vitro development of the fertilized embryo, and that 10–9 M was the optimal concentration of MT.
Fertilization and subsequent embryo development of oocytes in the 10–9 M and 0 M groups
Table 3 shows that in the previous cycle without MT (0 M group), in total, 1838 oocytes were collected, of which 1372 MII oocytes were fertilized by IVF/ICSI, and the fertilization rate was 83.6% (1147/1372). Among the 140 cycles of 140 patients with subsequently added MT (10–9 M group), in total, 1658 oocytes were collected, including 1288 MII oocytes, and the fertilization rate after insemination was 87.7% (1129/1288). The fertilization, cleavage, high-quality embryo, blastocyst, and high-quality blastocyst rates in the 10–9 M group were significantly different from those in the 0 M group (87.7% vs. 83.6%, P < 0.01; 94.1% vs. 90.5%, P < 0.01; 58.3% vs. 43.8%, P < 0.0001; 51.1% vs. 41.8%, P < 0.0001; 43.4% vs. 22.9%, P < 0.0001).
Table 3. Comparison of oocyte fertilization and embryo development between the 10–9 M group and the 0 M group
Data were analyzed using chi-squared test or Fisher’s exact test. Different symbols within columns and different letters within columns and within rows indicate significant difference.
a P < 0.01, compared with the 0 MT group.
b P < 0.0001, compared with the 0 MT group.
Oocyte fertilization and subsequent embryo development in the 10–9 M and 0 M groups of patients who experienced one failed IVF/ICSI cycle
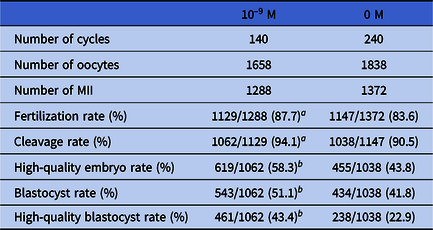
As shown in Fig 3, there were 1116 oocytes in the 10–9 M group and 1009 oocytes in the 0 M group of patients who experienced a single failed IVF/ICSI cycle. In terms of fertilization rate (90.1% vs. 88.2%) and cleavage rate (94.6% vs. 92.9%), the rates of the 10–9 M group were higher than those of the 0 M group, but these differences were not significant. The high-quality embryo rate (56.8% vs. 39.4%) and high-quality blastocyst rate (42.0% vs. 25.3%) of the 10–9 M group were significantly higher than those of the 0 M group (both P < 0.0001).
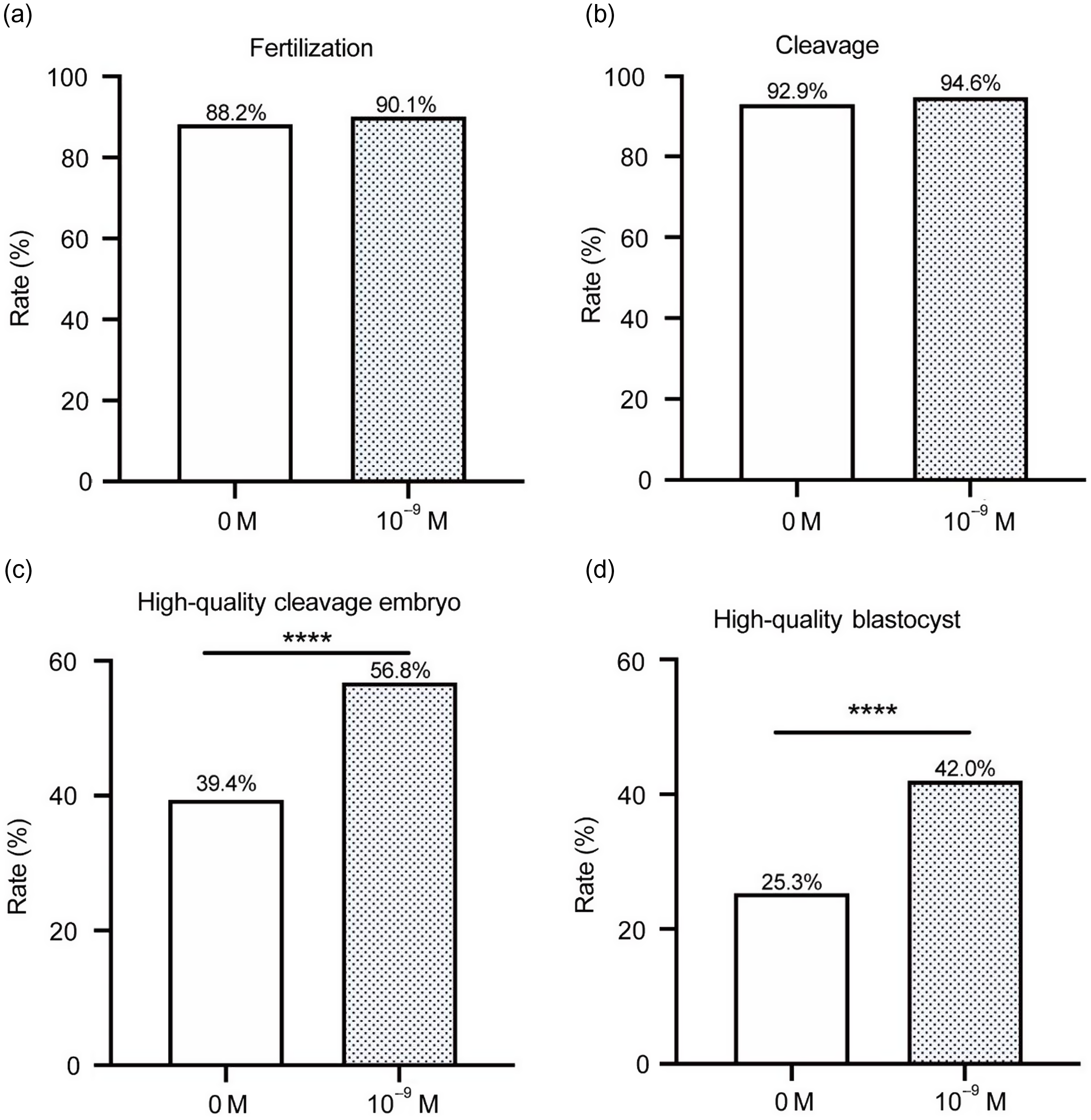
Figure 3. Status of fertilization and early embryo development in patients with one failed IVF/ICSI cycle. (a) fertilization; (b) cleavage; (c) high-quality embryo; and (d) high-quality blastocyst (****P < 0.0001).
Result of oocyte fertilization and subsequent embryo development in the 10–9 M and 0 M groups of patients who experienced two failed IVF/ICSI cycles
As shown in Fig 4, there were, in total, 468 oocytes in the 10–9 M group and 688 oocytes in the 0 M group of patients who experienced two failed IVF/ICSI cycles. The fertilization, cleavage, high-quality embryo and high-quality blastocyst rates of the 10–9 M group were higher than those of the 0 M group. The cleavage, high-quality embryo and high-quality blastocyst rates of the 10–9 M group were significantly different from those of the 0 M group (92.2% vs. 85.6%, P < 0.05; 51.1% vs. 41.3%, P < 0.05; 44.6% vs. 19.9%, P < 0.0001).
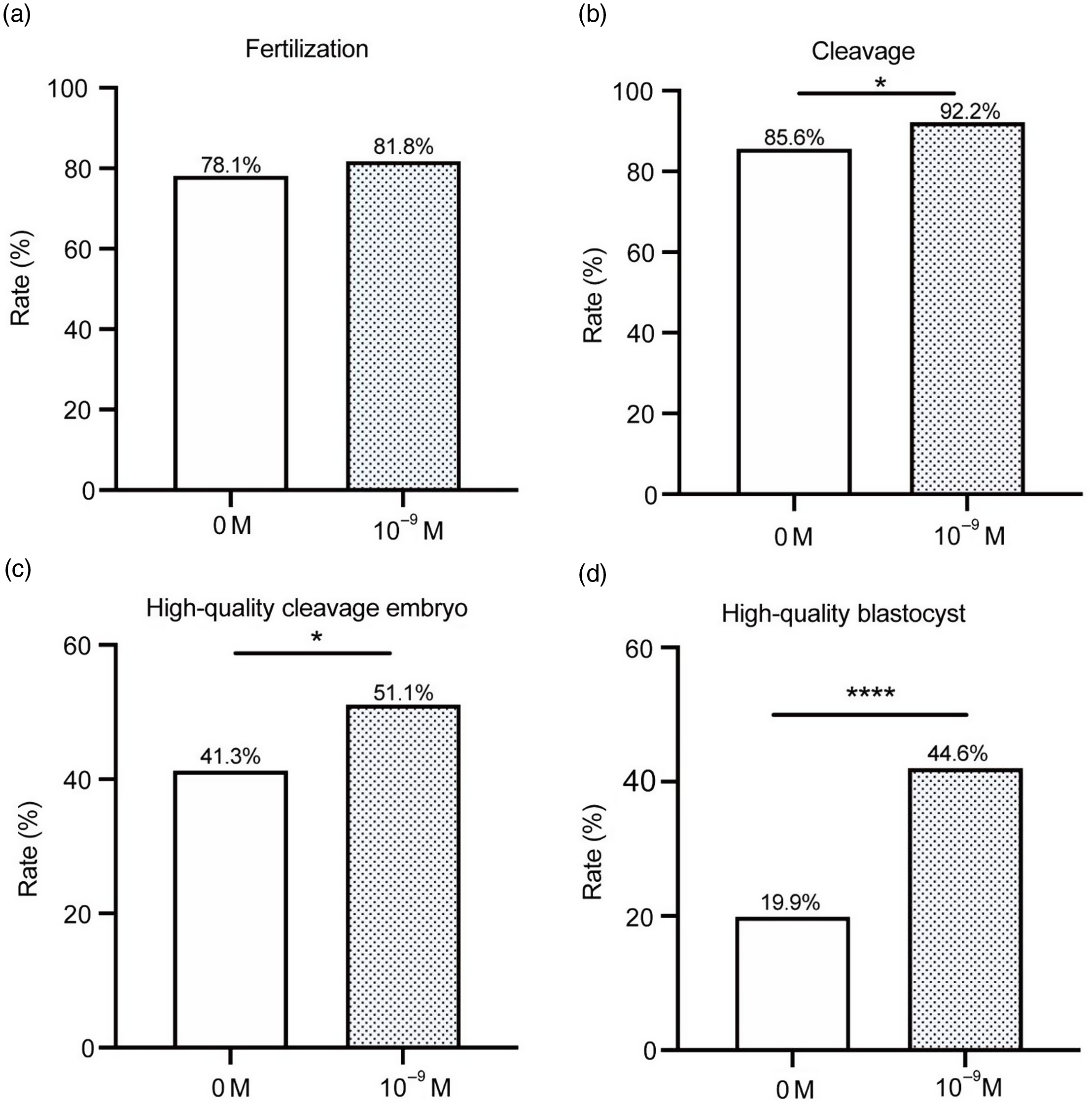
Figure 4. Status of fertilization and early embryo development in patients with two failed IVF/ICSI cycles. (a) fertilization; (b) cleavage; (c) high-quality embryo; and (d) high-quality blastocyst (*P < 0.05; ****P < 0.0001).
Result of oocyte fertilization and subsequent embryo development in the 10–9 M and 0 M groups of patients who experienced ≥ 3 failed IVF/ICSI cycles
As shown in Fig. 5, there were, in total, 74 oocytes in the 10–9 M group and 141 oocytes in the 0 M group of patients who experienced three or more failed IVF/ICSI cycles. The fertilization, cleavage, high-quality embryo and high-quality blastocyst rates of the 10–9 M group were higher than those of the 0 M group. Among these, the high-quality embryo and high-quality blastocyst rates of the 10–9 M group were significantly different from those of the 0 M group (45.8% vs. 34.3%, P < 0.05; 57.1% vs. 20.9%, P < 0.0001).
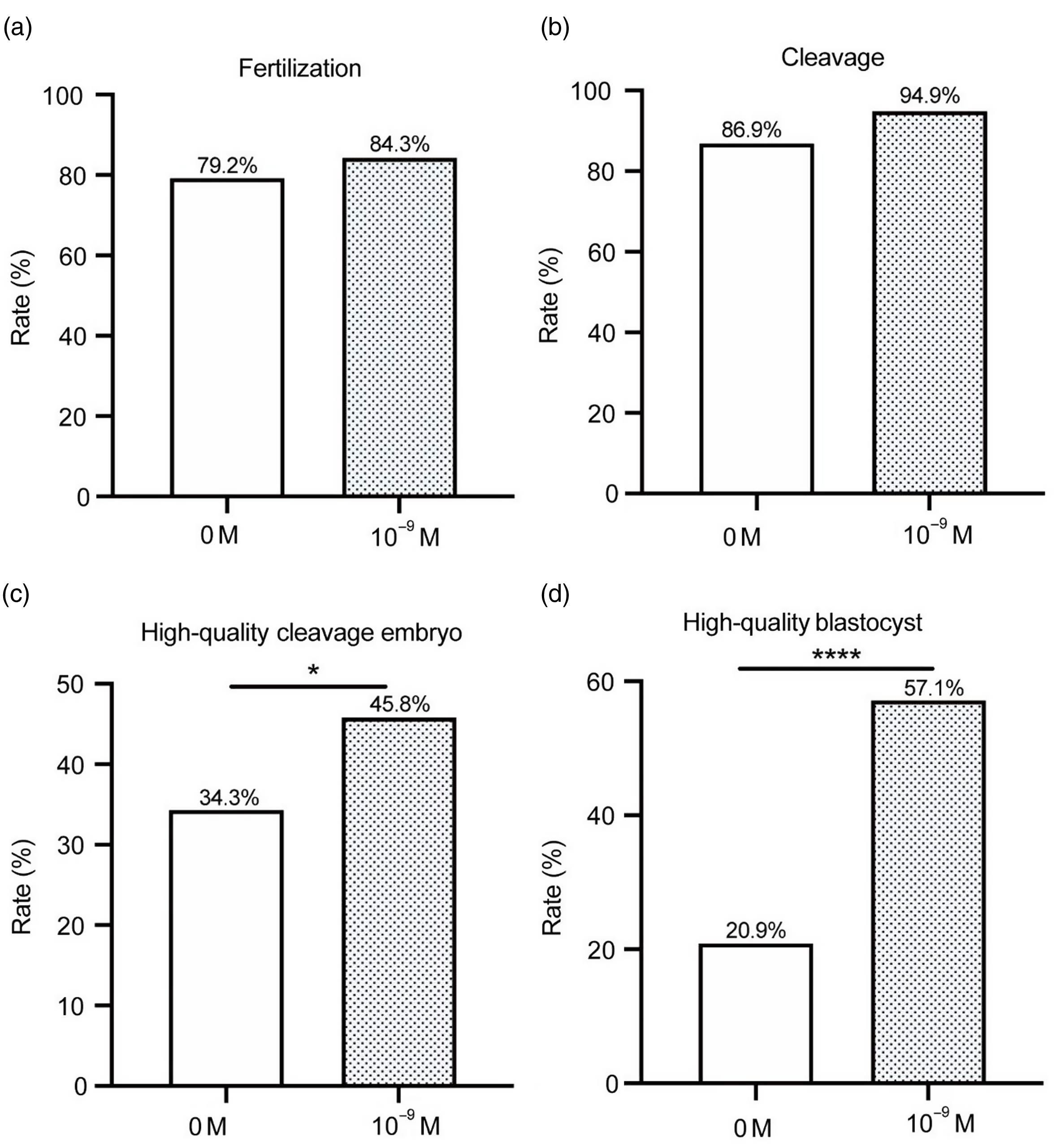
Figure 5. Status of fertilization and early embryo development in patients with three failed IVF/ICSI cycles. (a) fertilization; (b) cleavage; (c) high-quality embryo; and (d) high-quality blastocyst (*P < 0.05; ****P < 0.0001).
Clinical transfer outcomes
By the end of this manuscript submission, there had been 50 cycles of vitrified-warmed embryo transfers conducted the experimental group. In total, 61 warmed blastocysts were transferred, and the average number of transfers was 1.22. Thirty-two cases achieved biochemical pregnancy, and clinical pregnancy was confirmed in 20 cases. The implantation rate, biochemical pregnancy rate and clinical pregnancy rate were significantly higher than those of the 0 M group (65.6% vs. 9.7%, P < 0.0001; 64.0% vs. 12.5%, P < 0.0001; 40.0% vs. 11.7%, P < 0.0001) (see Table 4). Notably, to date, two women delivered two healthy newborns, including a boy and a girl, by caesarean section at 38 weeks of gestational age. Apgar scores were 10 and 9; the body weights were 2.7 and 2.5 kg, and the body lengths were 53 and 51 cm, respectively. The physical and mental development of these infants was normal at their regular postnatal follow-up and the other 18 women who achieved clinical pregnancy also had good examination indexes.
Table 4. Comparison of clinical outcomes between 10–9 M group and 0 M group
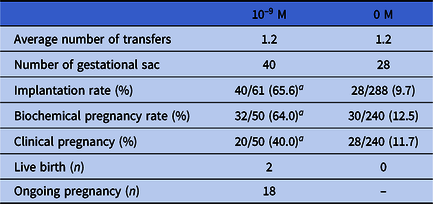
Data analyzed using chi-squared test or Fisher’s exact test. Different symbols within columns and different letters within columns and within rows indicate significant difference.
a P < 0.0001, compared with the 0 MT group.
Discussion
In the process of ART treatment, patients encounter many cycles of treatment failure due to poor embryo quality. There are many factors affecting embryo development, such as culture medium (Tarahomi et al., Reference Tarahomi, Vaz, van Straalen, Schrauwen, van Wely, Hamer, Repping and Mastenbroek2019), CO2 concentration (Brom-De-Luna et al., Reference Brom-De-Luna, Salgado, Canesin, Diaw and Hinrichs2019), temperature (Brom-De-Luna et al., Reference Brom-De-Luna, Salgado, Canesin, Diaw and Hinrichs2019), in vitro operations (Imesch et al., Reference Imesch, Scheiner, Xie, Fink, Macas, Dubey and Imthurn2013; Lu et al., Reference Lu, Liu, Cao, Liu and Dong2019), and sperm and oocyte quality (Keefe et al., Reference Keefe, Kumar and Kalmbach2015; Ribas-Maynou and Benet, Reference Ribas-Maynou and Benet2019). It can be considered that during ART treatment, the quality of oocytes is one of the most critical factors that determine the embryo status and clinical outcome. Any factor affecting the quality of the oocyte will affect its fertilization and subsequent embryo development (Miyara et al., Reference Miyara, Aubriot, Glissant, Nathan, Douard, Stanovici, Herve, Dumont-Hassan, LeMeur, Cohen-Bacrie and Debey2003) and, ultimately, the cycle outcome of ART treatment. Therefore, in this study, an appropriate concentration of MT was added to the embryo culture medium and the embryo development and clinical treatment outcomes of the patients were observed to understand whether the application of MT has a positive effect on embryo development.
According to research reports, in the process of in vitro culture, embryo transfer, fertilization and developmental observation need to be performed in a high-oxygen environment. This inevitable high-oxygen environment will cause a certain degree of oxidative stress damage to embryo development. In addition, frequent opening of the incubator during embryo culture will result in an unstable O2 concentration within the chamber, which will also cause different degrees of oxidative stress damage to embryo development, therefore affecting the quality of oocytes and outcome of embryo development in vitro (An et al., Reference An, Peng, Cheng, Lu, Zhou, Zhang and Su2019; Hiroshi et al., Reference Hiroshi, Mai, Manabu, Yuichiro, Yumiko, Masahiro, Isao, Ryo, Shun, Toshiaki, Akihisa, Russel and Norihiro2020). MT is a highly effective antioxidant with strong antioxidant activity. Animal studies have shown that the addition of MT to embryo culture medium can improve embryo development in vitro, and it has been confirmed that the improvement is concentration dependent (Wang et al., Reference Wang, Tian, Zhang, Gao, He, Fu, Ji, Li, Li and Liu2014). In 2020, we found that adding MT to human IVM medium can improve the IVM outcome of human immature oocytes by promoting mitochondrial function and inhibiting damage due to oxidative stress; 10–5 M was found to be the optimal concentration (Zou et al., Reference Zou, Chen, Ding, Gao, Chen, Liu, Hao, Zou, Ji, Zhou, Wei, Cao and Zhang2020). Based on the conclusions of previous studies, two main questions were posed: (1) Can adding MT to the culture medium of human embryos improve the in vitro development and clinical outcome of embryos? (2) Is its effect also correlated with concentration? Therefore, in this study, immature human oocytes in the COH cycle were collected for IVM to obtain IVM-MII oocytes and ICSI insemination was conducted. Next, the fertilized oocytes were cultured in vitro in culture medium containing 0, 10–11, 10–9, 10–7 or 10–5 M MT. A systematic comparative analysis was performed of the fertilization of oocytes and early embryo development in each group. The fertilization, cleavage, blastocyst and high-quality blastocyst rates of the 10–9 M group were all significantly higher than those of the 0 M group. The blastocyst and high-quality blastocyst rates of the 10–9 M group were significantly different from those of the 10–5 M group, and the blastocyst rate was also significantly different from that of the 10–11 M group. In addition, the fertilization, cleavage, blastocyst and high-quality blastocyst rates of the 10–9 M group were higher than those of the 10–7 M group, but these differences were not significant. It has been reported that the fertilization rate, cleavage rate and total blastocyst cell number of porcine IVF embryos cultured in medium containing 10–9 M MT were significantly increased (Lord et al., Reference Lord, Nixon, Jones and Aitken2013). Wang et al. (Reference Wang, Tian, Zhang, Gao, He, Fu, Ji, Li, Li and Liu2014) added different concentrations of MT to the culture medium of bovine embryos in vitro and found that 10–9 M MT could significantly promote embryo development. These results are consistent with the results of the present study. These results showed that the addition of 10–9 M MT to human embryo culture medium could significantly improve the fertilization of human oocytes and embryo development in vitro obtained after fertilization, and 10–9 M was the optimal concentration.
Next, 10–9 M was selected as the MT concentration to be added to the embryo culture medium of patients with failed IVF/ICSI cycles for embryo culture in vitro. The study found that, in terms of the fertilization, cleavage, high-quality embryo, blastocyst and high-quality blastocyst rates, the values of the 10–9 M group were significantly higher than those of the 0 M group. In this study, further group analysis was also carried out according to the number of failed IVF/ICSI cycles. It was discovered that the application of 10–9 M MT to embryo culture in vitro could significantly improve embryonic development in the repeated cycle of each group. Large numbers of studies have found that adding MT to the culture medium can reduce the levels of ROS in oocytes and promote embryonic development of cattle, mice, sheep, and pigs (Kang et al., Reference Kang, Koo, Kwon, Park, Jang, Kang and Lee2009; Zhao et al., Reference Zhao, Hao, Du, Zhao, Wang, Wang, Wang, Liu, Qin and Zhu2016; Barros et al., Reference Barros, Monte, Santos, Lins, Cavalcante, Gouveia, Müller, Oliveira Junior, Barberino, Donfack, Araújo and Matos2020a, 2020b). Nakano et al. (Reference Nakano, Kato and Tsunoda2012) confirmed that the addition of MT during embryo culture in vitro could reduce the level of ROS in parthenogenetic embryos and promote embryo development. These findings are consistent with the results of the current study, suggesting that the improvement of embryonic development in patients with failed IVF/ICSI cycles by MT may be related to the highly effective antioxidant properties of MT. Oxidative stress inhibits oocyte maturation and embryo development, and MT has a strong antioxidant effect, which can resist oxidative stress, maintain the balance of the antioxidant system, reduce ROS content in the embryo, and promote gamete maturation and embryo development. Moreover, some studies have also demonstrated that MT can improve mitochondrial function (Martín et al., Reference Martín, Macías, Escames, León and Acuña-Castroviejo2000; Rodríguez-Varela and Labarta, Reference Rodríguez-Varela and Labarta2020). Mitochondria are a major source of ROS production and they need additional protection from oxidative stress (Izyumov et al., Reference Izyumov, Domnina, Nepryakhina, Avetisyan, Golyshev, Ivanova, Korotetskaya, Lyamzaev, Pletjushkina, Popova and Chernyak2010). Mitochondria provide ATP for most cellular energy-requiring processes through the oxidative phosphorylation pathway in early embryos. Niu et al. (Reference Niu, Zhou, Nie, Shin and Cui2020) found that MT could compensate for the mitochondrial depletion and energy deficiency due to toxin exposure by reducing ROS production and promoting mitochondrial biogenesis, which rescued impairment of early porcine embryo development. Yang et al. (Reference Yang, Tao, Chai, Wu, Wang, Li, He, Xie, Ji, Dai, Yang and Liu2017) found that, in inferior bovine oocytes, MT treatment significantly normalized the mitochondrial distribution and improved their function to produce more ATP, which promoted the cytoplasmic maturation of oocytes and their embryos development. In our previous research, it has been found that MT can significantly increase the maturation rate and developmental potential of human immature oocytes by directly scavenging ROS and inhibiting oxidative stress (Zou et al., Reference Zou, Chen, Ding, Gao, Chen, Liu, Hao, Zou, Ji, Zhou, Wei, Cao and Zhang2020). Therefore, the above evidence indicated that MT can effectively inhibit oxidative stress not only by scavenging free radical, but also by protecting mitochondrial function, thereby improving gamete quality and embryo development both in human and animals during ART treatment. Further clinical studies indicated that MT greatly ameliorated clinical outcome, and successfully delivered healthy newborns, which strongly proved the feasibility of MT in clinical treatment (Zou et al., Reference Zou, Chen, Ding, Gao, Chen, Liu, Hao, Zou, Ji, Zhou, Wei, Cao and Zhang2020; Li et al., Reference Li, Mu, Elshewy, Ding, Zou, Chen, Chen, Wei, Cao, Zhou and Zhang2021). In this study, to date, 50 cycles of vitrified-warmed embryo transfers with MT intervention have been performed. The implantation rate, biochemical pregnancy rate, and clinical pregnancy rate of the 10–9 M group were significantly higher than those of the 0 M group, and two healthy newborns were successfully delivered. This result shows that the application of 10–9 M MT to embryo culture medium in vitro can significantly improve the clinical treatment effect of patients with failed IVF/ICSI cycles and ultimately achieve the birth of healthy offspring, which greatly enhances confidence in treating such patients and thereby increases the overall success rate of ART treatment.
In conclusion, the addition of MT to embryo culture medium in vitro can improve embryonic development for patients with repeated cycles after failed IVF/ICSI cycles and lead to good clinical outcomes. The optimal concentration of MT was 10–9 M.
Acknowledgements
We gratefully acknowledge all the participants in this study.
Authors’ contributions
Qi Zhu: manuscript writing and sample collection. Kaijuan Wang and Chao Zhang: data collection. Zhaojuan Yu, Bihua Rao and Rufeng Xue: data analysis. Beili Chen, Huijuan Zou and Weiwei Zou: clinical assessments and experiment conducting. Dongmei Ji and Yunxia Cao: manuscript editing; Ding Ding and Zhiguo Zhang: manuscript revising and study design. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Technology R&D Programme of China (No. 2017YFC1002004), General Project of National Natural Science Foundation of China: molecular mechanism of melatonin regulating freezing injury of human oocytes during vitrification (82071724), National Major Scientific Research Instrument and Equipment Development Project (11627803), Natural Science Research Project of Anhui Province Universities: Study on the molecular mechanism of trehalose improving human oocyte development potential after freezing and thawing by regulating DNA methylation (KJ2020A0202) and the Open Project of State Key Laboratory of Reproductive Medicine: mechanism of melatonin promoting maturation of immature oocytes and subsequent embryo development by protecting mitochondrial membrane dynamics (SKLRM-K202005).
Competing interests
The authors declare that there are no conflicts of interest.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Anhui Medical University (2015013). Before conducting the study, the partners of all enrolled patients were interviewed and provided signed informed consent.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.











