Introduction
In the last few years, several cases of malformations in batoids have been documented. In general, these malformations are related to embryonic development of endogenous origin (genetic or hormonal), pollution by chemical particles, and environmental anomalies caused by the degradation of the environment (Torres-Huerta et al., Reference Torres-Huerta, Meraz, Carrasco-Bautista and Díaz-Carballido2015; Ehemann & González-González, Reference Ehemann and González-González2018; Capape et al., Reference Capapé, Kassar, Reynaud and Hemida2019).
Elasmobranchs with malformations such as ocular malformation in the round stingray Urobatis halleri (Rubio-Rodríguez et al., Reference Rubio-Rodríguez, Navarro-González and Vergara-Solana2010), pelvic fin deformation in the longtail stingray Dasyatis longa (Escobar-Sánchez et al., Reference Escobar-Sánchez, Galván-Magaña, Downton-Hoffmann, Carrera-Fernández and Alatorre-Ramírez2009), and occurrence of a monoclasper in the banded guitarfish Zapteryx exasperata (Escobar-Sánchez et al., Reference Escobar-Sánchez, Galván-Magaña, Downton-Hoffmann, Carrera-Fernández and Alatorre-Ramírez2009) have been documented in the Gulf of California.
Guitarfishes of the genus Pseudobatos are abundant elasmobranch species captured by artisanal fishing in the Gulf of California (Bizzarro et al., Reference Bizzarro, Smith, Hueter, Tyminski, Márquez–Farías, Castillo–Géniz, Cailliet and Villavicencio–Garayzar2007; Smith et al., Reference Smith, Bizzarro and Cailliet2009). These species represent a fishing resource of wide importance for local and national consumption. The spadenose guitarfish, Pseudobatos buthi Rutledge, Reference Rutledge2019, was recently described from the coast of the Gulf of California. It is an aplacental viviparous (yolk sac viviparity, also called lecithotrophic viviparity) ray, where the embryos receive nutrients through the yolk sac (personal observation; Hamlett et al., Reference Hamlett, Kormanik, Storrie, Stevens, Walker and Hamlett2005).
The present study reports for the first time a clasper malformation in Pseudobatos buthi captured in the Gulf of California. To elucidate the causes of the morphological abnormality, histological analysis was made to describe the reproductive system, and concentration levels of heavy elements (cadmium, copper, iron, manganese, silver, lead and zinc) were determined in muscle and liver.
Materials and methods
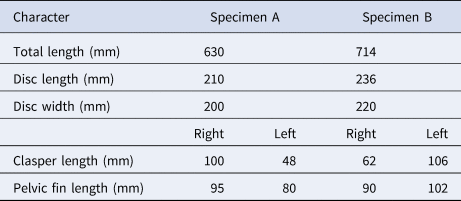
Two specimens of Pseudobatos buthi were collected, the first one on 16 August 2019 (specimen A) and the second one on 19 September 2019 (specimen B) at San Bruno fishing camp, Baja California Sur, Mexico, located in the western coast of the Gulf of California (Figure 1). The specimens were obtained from artisanal fishing of elasmobranchs and were caught with a 6-inch mesh-size gillnet. The total length, disc width, disc length, and the clasper length from base to tip (clasper internal length) of each specimen were measured (Table 1) (Last et al., Reference Last, White, de Carvalho, Séret, Stehmann and Naylor2016).
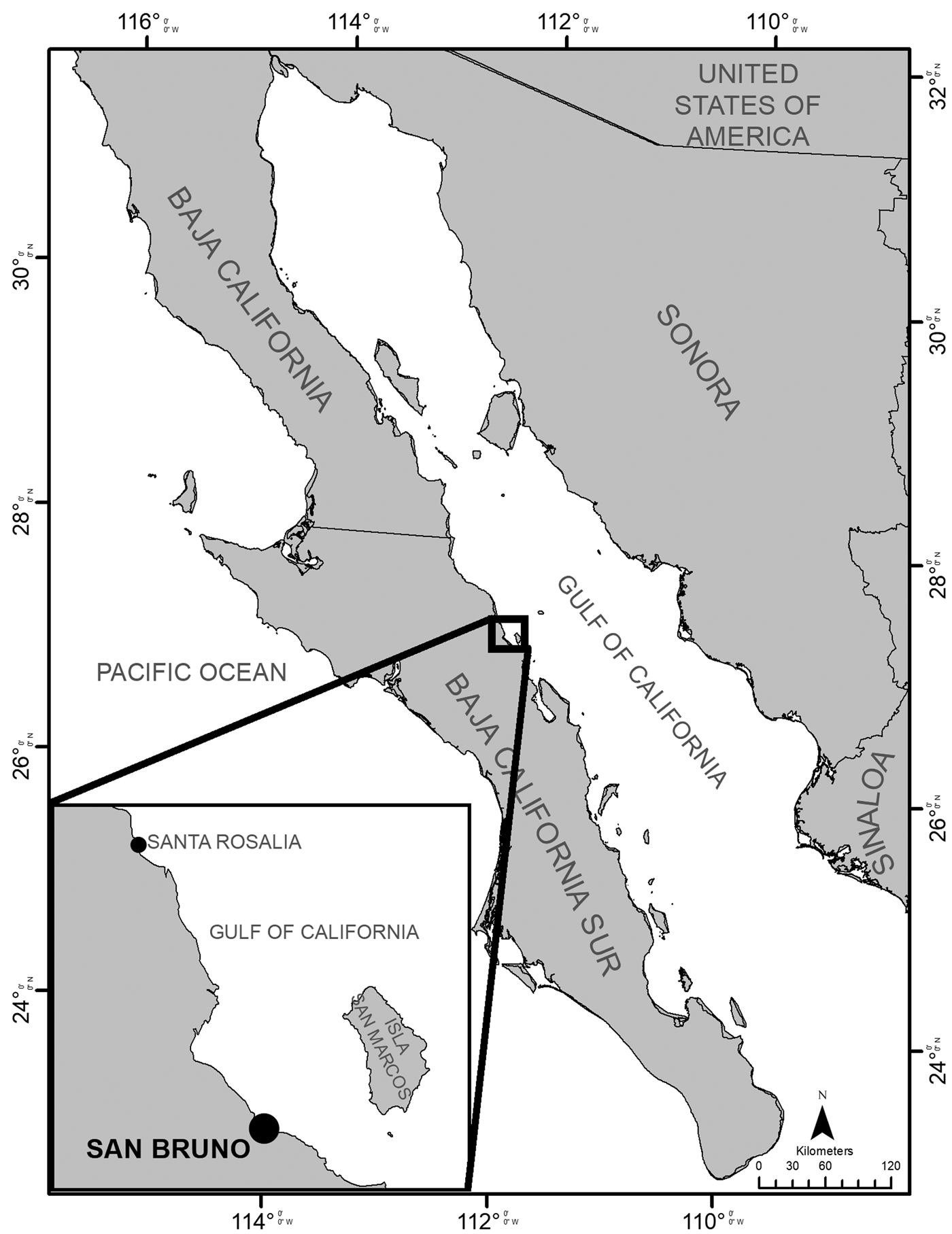
Fig. 1. San Bruno fishing camp in the western coast of the Gulf of California, Mexico.
Table 1. Morphometric data for specimens of Pseudobatos buthi captured in the Gulf of California
The identification of the specimens was based on Rutledge (Reference Rutledge2019) (Table 2). Both specimens were stored frozen in the Laboratorio de Ecología de Peces of CICIMAR, with the catalogue numbers 16082019SR and 19092019SR, respectively. Both organisms were classified as mature males due to maturity characteristics: complete calcification of claspers, articulation at the base with a 180° rotation, and semen in the rhipidion (Hamlett et al., Reference Hamlett, Kormanik, Storrie, Stevens, Walker and Hamlett2005).
Table 2. Morphometric data for specimens of Pseudobatos buthi captured in the Gulf of California.
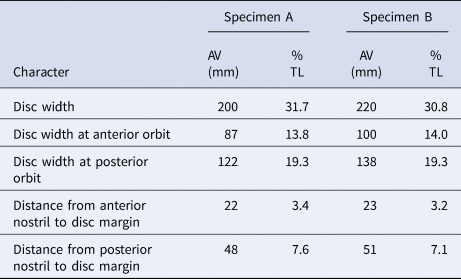
AV, absolute value expressed in mm; TL, percentage of total length.
An abdominal incision was carried out on each specimen to remove the whole reproductive tract; the gonads were dissected and fixed in 10% formaldehyde. Histological analysis was performed using transversal sections of the testis, epididymis, and the seminal vesicle to describe spermatogenesis, the transit of the spermatozoa through the deferent ducts, and the possible sperm storage. Histological sections were stained with periodic acid-Schiff (PAS) to identify polysaccharides, mucopolysaccharides, mucoproteins, glycoproteins and mucins (Humason, Reference Humason1979).
Approximately 10 g of muscle and liver were taken from both specimens for the analysis of the concentration of the heavy metals: cadmium (Cd), copper (Cu), iron (Fe), manganese (Mn), silver (Ag), lead (Pb) and zinc (Zn), with flame absorption atomic spectrometry (FAAS), using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Blanks and standard reference materials (DOLT-5, National Research Council Canada, Ottawa, ON, Canada) were analysed for quality assurance. Per cent recoveries of standard reference materials were within 58–101%. All samples were analysed in duplicate and the coefficient of variation for the samples was less than 10%.
Results
Specimen A exhibited malformation of the left clasper and specimen B of the right clasper (Figure 2; Table 1). The reproductive system of both individuals showed a completely developed and symmetrical testis (Figure 3). The claspers of both specimens (normal and with malformation) had full calcification, with articulation at the base and 180° rotation, and with presence of semen in the rhipidion (Table 1).

Fig. 2. Specimens of Pseudobatos buthi Rutledge, Reference Rutledge2019, captured in the Gulf of California presenting clasper malformation. (A) P. buthi captured in August 2019 with a malformation on the left clasper; (B) P. buthi captured in September 2019 with a malformation on the right clasper.
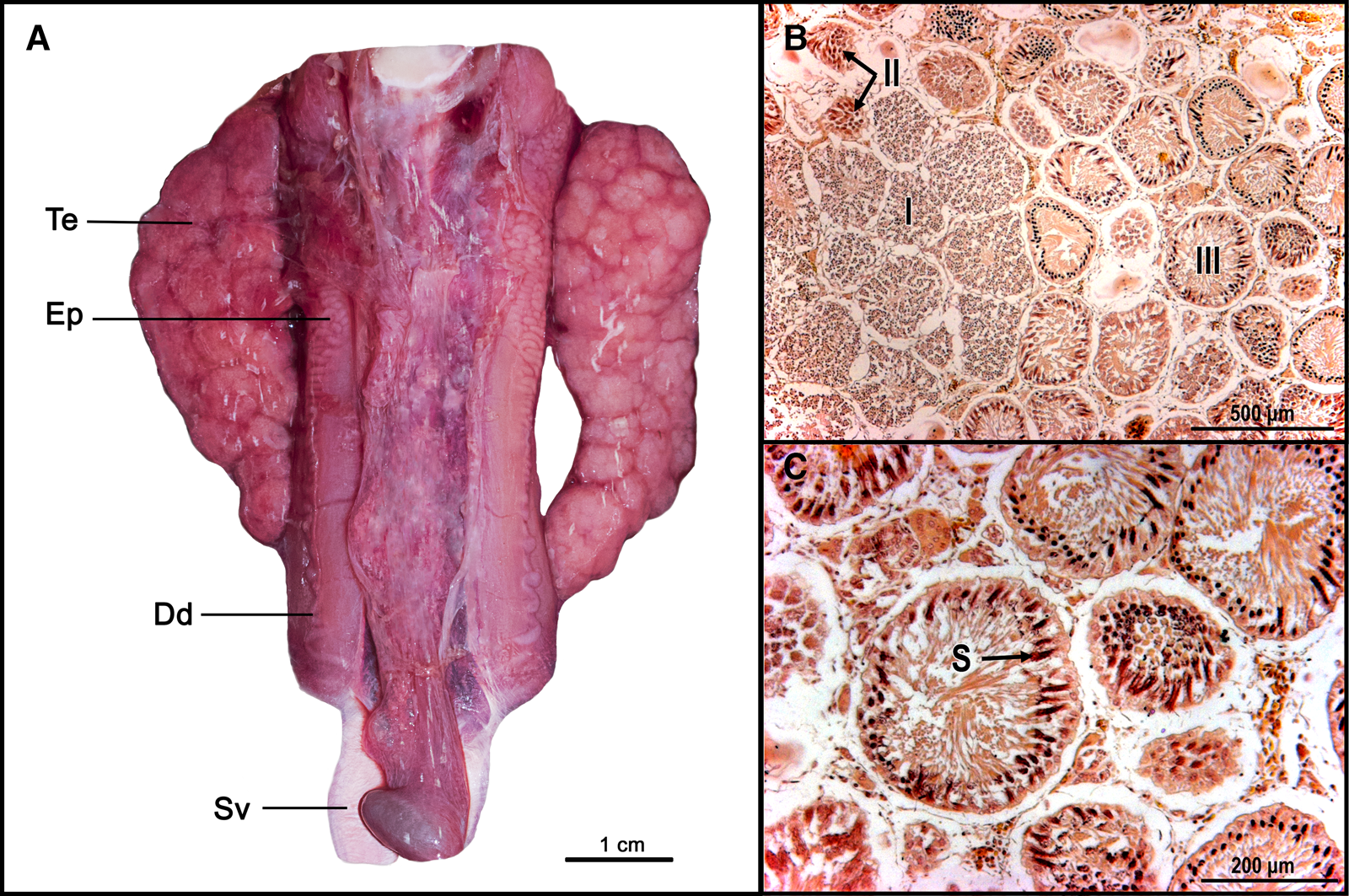
Fig. 3. (A) Pseudobatos buthi reproductive tract with paired testis (Te), epididymis (Ep), ductus deferens (Dd) and seminal vesicle (Sv); (B) Histological analysis of the testis evidencing the progress of spermatogenesis, the formation of spermatocytes (I), spermatid (II) and completely developed spermatozoa (III); (C) Spermatocysts with completely developed spermatozoa (S).
Histological analysis revealed that both testes were functional with compound type development (Pratt, Reference Pratt1988), defined by the origin of the seminal follicles and their progress. All stages of spermatogenesis and compact packages of completely developed spermatozoa were observed (Figure 3). A spermatozoa clump was observed in the anterior zone of the epididymis but was absent in the posterior zone (Figure 4). The seminal vesicle showed solitary or sperm clumps (Figure 5).
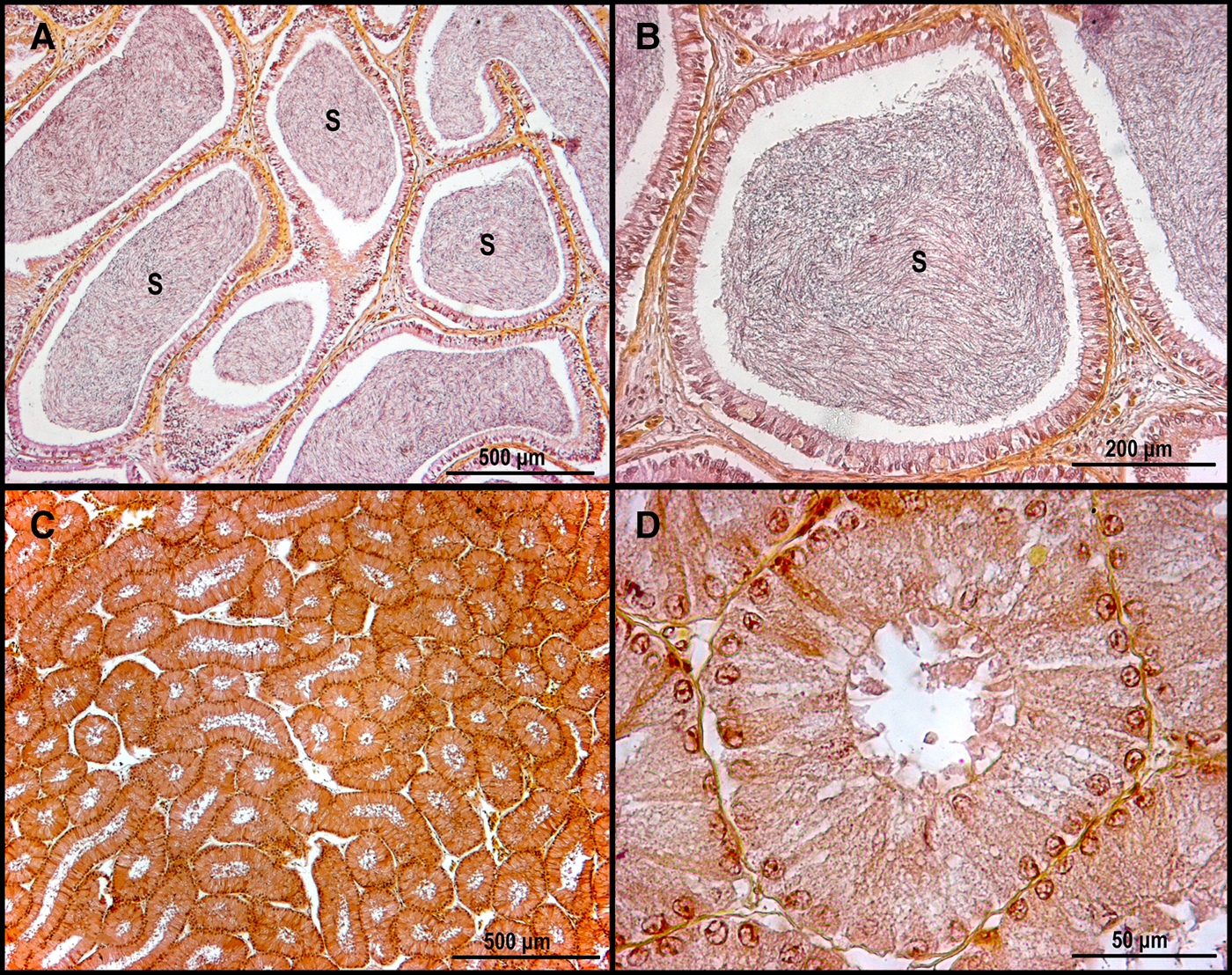
Fig. 4. Epididymis of Pseudobatos buthi. (A, B) Anterior zone of epididymis with sperm; (C, D) Posterior zone of epididymis without sperm. S, Sperm.

Fig. 5. Seminal vesicle of Pseudobatos buthi with sperm remains. S, Sperm.
Lead was the only metal with values below the detection limit (<0.07 mg kg−1) in both tissues, and cadmium presented values below the detection limit (<0.004 mg kg−1) in muscle tissue. Iron and zinc exhibited the highest concentrations in muscle and liver with a wide difference between tissues. Iron concentrations were higher than zinc in both organisms, and specimen B indicated the highest concentration of iron in muscle. Copper, manganese and silver were the metals with the lowest concentrations in the two individuals, and specimen B showed the highest values of these metals. Silver presented higher concentration in muscle tissue than in liver tissue in both specimens; meanwhile, the concentration of manganese was higher in the liver than in muscle. The concentrations of heavy metals in the muscle and liver are shown in Table 3.
Table 3. Concentration of cadmium (Cd), copper (Cu), iron (Fe), manganese (Mn), silver (Ag), lead (Pb) and zinc (Zn) expressed in mg/kg in wet weight present in the muscle and liver of Pseudobatos buthi

DL, Detection limit.
a Maximum permissible limit in Mexico (NOM 242-SSA1, 2009).
b Maximum permissible limit by Canada, Hungary and Australia, respectively (Papagiannis et al., Reference Papagiannis, Kagalou, Leonardos, Petridis and Kalfakakou2004).
c Maximum permissible limit by WHO/FAO (Tiimub & Dzifa-Afua, Reference Tiimub and Dzifa-Afua2013).
Discussion
In recent years, elasmobranch malformations have been more commonly reported in batoids than sharks, and some of these deformations do not influence the complete functionality of the individual. In Pseudobatos buthi, the cellular development of both of the claspers (with malformation and without malformation) were not affected by the differences in size considering that they presented all the distinctive characteristics of an adult individual, exhibited complete spermatogenic development in both testes, and contained sperm in the seminal vesicle. The absence of the right clasper in other species of the same genus, such as Pseudobatos percellens, evidenced a malformation and a poor functionality of the right testis and showed signs of hermaphroditism through the development of an oviducal gland (Ehemann & González-González, Reference Ehemann and González-González2018). A small sized clasper may not be used by the male and may decrease the success of mating, even when a single clasper is inserted into the female (Torres-Huerta et al., Reference Torres-Huerta, Meraz, Carrasco-Bautista and Díaz-Carballido2015; Ehemann & González-González, Reference Ehemann and González-González2018).
Both testes were functional, even with the clasper malformation. The absence of continual sperm flow from the testis to the seminal vesicle was found by histological analysis, contrary to what occurs in other elasmobranchs such as Isurus oxyrinchus (Pratt & Tanaka, Reference Pratt and Tanaka1994), Aetobatus narinari (Schluessel et al., Reference Schluessel, Bennett and Collin2010) and Potamotrygon magdalenae (Del Mar Pedreros-Sierra & Ramírez-Pinilla, Reference Del Mar Pedreros-Sierra and Ramírez-Pinilla2015). The sperm flow could be related to the storage times of the spermatozoa in the seminal vesicle. If we consider that P. buthi is a coastal ray without long migrations, it might not store sperm like other species of the same genus (Romero-Caicedo & Carrera-Fernández, Reference Romero-Caicedo and Carrera-Fernández2015; Martins et al., Reference Martins, Pasquino and Gadig2018).
In elasmobranchs, the strategy of sperm storage is an advantage to nomadic species that mate opportunistically, because it ensures that the investment in sex products (sperm and oocytes) is used most efficiently (Pratt & Tanaka, Reference Pratt and Tanaka1994). Some species' reproductive strategies may not require the capacity for sperm storage. Species with ‘local’ distribution never become appreciably separated from reproductively active conspecifics, and investment in storage to take advantage of rare or opportune encounters is not needed (Pratt & Tanaka, Reference Pratt and Tanaka1994). This is the case of P. buthi.
The sperm remains in the seminal vesicle as a result of sperm release during copulation, which could indicate a mating period for P. buthi in this season. This is similar to the mating period of Pseudobatos productus, which occurs each year and takes place in mid-summer in the Gulf of California (Marquez-Farías, Reference Márquez-Farías2007).
Elasmobranch malformations may happen due to genetic anomalies during embryonic development or contamination of the environment (Torres-Huerta et al., Reference Torres-Huerta, Meraz, Carrasco-Bautista and Díaz-Carballido2015; Ehemann & González-González, Reference Ehemann and González-González2018; Burgos-Vázquez et al., Reference Burgos-Vázquez, González, Falla and Escalon2019). There are reports of malformation in elasmobranchs caused by teratogenic agents, where there is no change in the genetic load (Wosnick et al., Reference Wosnick, Takatsuka, Mello, Dias, Lubitz and Azevedo2019). Considering the values obtained for heavy metals for rays in the present study, it is possible that the bioaccumulation of some metals could have caused malformations, as has been observed in Mustelus mustelus (Zaera & Johnsen, Reference Zaera and Johnsen2011). This shows the need for more studies in order to evaluate the levels of heavy metals in P. buthi and their relationship with morphological and/or physiological damage, as well as the contamination of the environment and concentrations of these heavy metals in the trophic network which they inhabit.
Pseudobatos buthi is a species commercially caught for human consumption. The concentrations of cadmium and lead in the muscle of the specimens analysed by us were below the permissible limit for human consumption (<0.05 mg kg−1) as established by Mexican law (NOM 242-SSA1, 2009); therefore, they do not represent a risk for human consumption and commercialization. Studies of other heavy metals in batoids of the same genus carried out on the Mexican Pacific coast indicated that the concentrations of mercury, cadmium and selenium in the muscle and liver tend to be within the permissible limit for human consumption of <0.05 mg kg−1 (Murillo, Reference Murillo2014). Mercury concentration levels were not analysed in P. buthi, so further studies regarding this metal should be carried out in the Gulf of California to corroborate if the trend observed in their Pacific counterparts is similar, considering that previous studies reported the existence of mercury and cadmium in P. productus from the Gulf of California, with values below the permissible limit for human consumption (García-Hernández et al., Reference García-Hernández, Cadena-Cárdenas, Betancourt-Lozano, García-De-La-Parra, García-Rico and Márquez-Farías2007; Ruelas-Inzunza et al., Reference Ruelas-Inzunza, Escobar-Sánchez, Patrón-Gómez, Moreno-Sánchez, Murillo-Olmeda, Spanopoulos-Hernández and Corro-Espinosa2013; Murillo, Reference Murillo2014).
Studies regarding heavy metals in sediments in the San Bruno area have shown that zinc is the most concentrated metal (570 ppm ± 710), in contrast to other metals such as cobalt, copper and lead (Shumilin et al., Reference Shumilin, Rodríguez-Figueroa, Bermea, Baturina, Hernández and Meza2000). The existence of this element in the environment has been attributed to mining activity carried out in the area since 1896 (Shumilin et al., Reference Shumilin, Rodríguez-Figueroa, Bermea, Baturina, Hernández and Meza2000). Iron and zinc concentrations in the analysed specimens were more evident than other metals. These two elements can bioaccumulate in organisms. Zinc toxicity studies in the small-spotted catshark Scyliorhinus canicula demonstrated the capacity of elasmobranchs to counteract the toxic effects of this element; they also indicated that the accumulation in the body depends on the concentration of the elements in the environment (Crespo & Balasch, Reference Crespo and Balasch1980; Crespo et al., Reference Crespo, Soriano, Sampera and Balasch1981). Moreover, studies of Squalus mitsukurii emphasized the negative relationship between the concentration of zinc and iron with the total length of the organism, where the embryos tend to have a higher concentration than larger sharks (Taguchi et al., Reference Taguchi, Yasuda, Toda and Shimizu1979).
Zinc was the second metal with the highest concentration in both P. buthi specimens; however, the values obtained in the muscle (8.65–17.457 mg kg−1 dry weight) are similar to other elasmobranch species such as Squalus acanthias (12 mg kg−1 dry weight), S. suckleyi (4.2–12.1 mg kg−1 dry weight) or S. mitsukurii (8–15 mg kg−1 dry weight) (Taguchi et al., Reference Taguchi, Yasuda, Toda and Shimizu1979); to date, this metal has not been related to health problems in elasmobranchs.
There are few studies in elasmobranchs regarding the concentration of iron. In P. buthi, iron was the metal with the highest concentration in the muscle (0.455–2.272 mg kg−1 wet weight), which was lower than that reported for Squalus mitsukurii (1.5–5.7 mg kg−1 wet weight) (Taguchi et al., Reference Taguchi, Yasuda, Toda and Shimizu1979).
Iron and zinc are essential metals in metabolism. In some elasmobranch species, iron and zinc showed a positive correlation (Taguchi et al., Reference Taguchi, Yasuda, Toda and Shimizu1979). Our results indicated that both metals exhibited high concentrations, reflecting this relationship. Nonetheless, there are no studies about the absorption and/or elimination of copper, manganese and silver in elasmobranchs; therefore, more studies on this topic are necessary.
In Mexico, there is no permissible limit of these metals for human consumption. However, it is possible to compare the specific values of copper, iron, manganese and zinc with the food standards established by other regions such as Canada (Cu: 100 mg kg−1; Zn: 100 mg kg−1); Hungary (Cu: 60 mg kg−1; Zn: 80 mg kg−1); Australia (Cu: 10 mg kg−1; Zn: 150 mg kg−1); and organizations such as the World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) (Fe: 43 mg kg−1; Mn: 5.5 mg kg−1). Thus, the heavy metal concentrations reported in the study are below the permissible limit for human consumption (Table 3) (Papagiannis et al., Reference Papagiannis, Kagalou, Leonardos, Petridis and Kalfakakou2004; Tiimub & Dzifa-Afua, Reference Tiimub and Dzifa-Afua2013).
The malformations in P. buthi may increase due to the existence of contaminants in the environment. This study might be the first record of these effects due to the mining activity in the area. Nevertheless, the malformations are rare given that elasmobranchs can counteract the effects of contamination. Considering the bioavailability of these metals in the environment in the area, more specialist studies, such as histochemical analyses, are needed. This could help identify the damage at a cellular level that heavy metals with high bioavailability could produce, such as anatomical malformations for this and other species (Jezierska et al., Reference Jezierska, Ługowska and Witeska2009; Zaera & Johnsen, Reference Zaera and Johnsen2011). If this damage is evidenced in seasonal elasmobranch species, such as rays (Bizzarro et al., 2009; Smith et al., Reference Smith, Bizzarro and Cailliet2009; Ramírez-Amaro et al., Reference Ramírez-Amaro, Cartamil, Galván-Magaña, Gonzalez-Barba, Graham, Carrera-Fernandez, Escobar-Sanchez, Sosa-Nishizaki and Rochin-Alamillo2013), they could be considered as biological indicators in highly polluted systems (Horvat et al., Reference Horvat, Degenek, Lipej, Tratnik and Faganeli2014).
Acknowledgements
The authors thank the Morphophysiology laboratory at Centro Interdisciplinario de Ciencias Marinas (CICIMAR-IPN), La Paz, Baja California Sur, for the facilities granted during the histological analysis, and the Instituto de Investigaciones Oceanológicas of the Universidad Autonoma de Baja California (UABC), Ensenada, Baja California, for the support in determining the concentration of heavy metals. FGM and RIOB thanks the Instituto Politécnico Nacional for fellowships offered through the Comisión de Operación y Fomento de Actividades Académicas (COFAA) and Estímulos al Desempeño de los Investigadores (EDI) programmes. We also thank the fisherman Antonio Vásquez for the specimens.
Author contributions
Soto-López Katherin participated in the collection of samples, data analysis, and the preparation of the manuscript. García-Vázquez Gabriela participated in the collection of samples, data analysis, and the preparation of the manuscript. Martínez-Ayala Julio C. participated in the analysis of data and preparation of the manuscript. Ochoa-Báez Rosa I. participated in the analysis of data and preparation of the manuscript. Galván-Magaña Felipe participated in the preparation of the manuscript and in the obtaining of the funding for the project.
Financial support
This study was funded by project: Ecología trófica de los tiburones en la costa occidental del Golfo de California (Trophic ecology of sharks on the western coast of the Gulf of California) (grant number SIP 20196736) and Ecología trófica de las rayas en la costa occidental del Golfo de California (grant number SIP 20201266).










