Introduction
The use of metformin in pregnancy for management of women with diabetes is increasing Reference Cesta, Cohen and Pazzagli1 despite growing concern about the lack of long-term safety data of metformin exposure in utero for children. Reference Barbour, Scifres and Valent2,Reference Nguyen, Chan and Teo3 Cumulating evidence suggests the potential for adverse cardiometabolic risk. For example, a meta-analysis of childhood outcomes of exposure to metformin versus insulin for the treatment of gestational diabetes found that metformin-exposed children had lower average birthweights compared to children exposed to insulin but were significantly heavier in infancy and had higher body mass index (BMI) in early childhood (aged 5–9 years). Reference Tarry-Adkins, Aiken and Ozanne4 Likewise, in 5–10 year follow-up of 141 children exposed to metformin versus placebo for the treatment of polycystic ovary syndrome, children exposed to metformin had a higher BMI compared to the placebo group. Reference Hanem, Salvesen and Juliusson5
Together these findings are consistent with metabolically disadvantageous childhood growth patterns among children exposed to metformin in utero, but these longitudinal data need cautious interpretation, as the original studies were not powered to look at childhood outcomes and are subject to recall bias and small sample sizes. Indeed, recent follow-up of children from the Metformin in Obese Pregnant Women (MOP) trial (n = 115, aged 3.9 ± 1.0 years, n = 77(66.96%) in metformin-exposed group) reported no significant difference in body composition between metformin or placebo groups, apart from lower gluteal and triceps circumferences in the metformin group. Reference Panagiotopoulou, Syngelaki and Georgiopoulos6 The authors also observed lower central haemodynamics (central blood pressure (BP) and augmentation index) and improved left ventricular diastolic function in metformin compared to placebo-exposed groups, findings suggesting a potential cardioprotective effect of metformin. The clinical significance of these small changes is unclear but highlights a need for more data on follow-up of children exposed to metformin in utero.
We hypothesised that maternal metformin treatment during obese pregnancy would have adverse effects on childhood cardiovascular risk. We aimed to test this hypothesis by follow-up of children born to mothers who participated in ‘EMPOWaR’ (Effect of metformin on maternal and fetal outcomes in obese pregnant women), a randomised double-blind, placebo-controlled trial of the effects of treatment with metformin versus placebo in obese pregnant women without diabetes. Reference Chiswick, Reynolds and Denison7
Method
Participants
Participants in the EMPOWaR trial included pregnant women (BMI ≥ 30 kg/m2; aged ≥16 years) with normal glucose tolerance who were randomised to receive oral metformin 500 mg daily (up to 2500 mg) or matched placebo between 12 and 16 weeks’ gestation until delivery. Between October 2017 and October 2019, we approached women who had participated in the two largest recruitment sites for EMPOWaR, and had given us permission for future contact, and asked if they would be willing for their child to take part in a follow-up study. Clinical studies were conducted at the Royal Edinburgh Hospital for Sick Children Children’s Clinical Research Facility, Edinburgh and at the University Hospitals Coventry and Warwickshire NHS Trust, Coventry. Ethical and management approval were obtained (References 17/SS/0065 and LL447019) and all participants gave written informed consent. Both participants and researchers were blinded to the original treatment allocation.
Clinical protocol
Anthropometry
Children’s height, weight and head circumference were measured with the child clothed but not wearing shoes. Height and BMI were calculated as height centile and BMI centile (adjusted with age, gender and measuring date) according to UK-WHO standard (www.who.int/childgrowth/en). Body fat composition was assessed by measuring subscapular, triceps and biceps skin-fold thicknesses (mm, Harpenden caliper, Baty International, West Sussex, UK) or mid-arm circumference (cm).
Vascular assessment
BP and vascular stiffness were measured as the average of three seated measurements over half an hour with an automated oscillometric device (IEM Mobil-O-Graph 24 Hour Pulse Wave Analysis System, IEM, Stolberg, Germany), in the lesser preferred arm using an extra small cuff, after 5 min rest. The device is validated against invasive and non-invasive gold standards, including in children. Reference Wei, Tölle, Zidek and van der Giet8 In-built pulse wave analysis algorithms (HMS-Client Server Hypertension Management Software, IEM, Stolberg, Germany) generate measurements including central measures of systolic BP (mmHg), diastolic BP (mmHg), mean arterial pressure (mmHg), heart rate (bpm), pulse pressure (mmHg), pulse wave velocity (PWV, m/s) and augmentation index (Alx). Three children found the cuff too uncomfortable to collect good quality data, leaving data suitable for analyses from 37 (92.5%) children.
Questionnaires
While the child was undergoing the clinical assessments, mothers completed the Strengths and Difficulties Questionnaire (SDQ), Reference Goodman9 Children’s Dietary Questionnaire (CDQ) Reference Magarey, Golley, Spurrier, Goodwin and Ong10 and Family Eating and Activity Habits Questionnaire (FEAHQ) Reference Golan11 to rate their children’s behaviour and diet, and their child’s and family eating and physical activity, respectively.
SDQ is designed to screen the behaviour of children aged 4–17 years old, and the scoring system was validated in a UK population. It includes 25 items which are divided by five scales: emotional symptoms (five items), conduct problems (five items), hyperactivity/inattention (five items), peer relationship problems (five items) and prosocial behaviour (five items). CDQ is a 28-item semi-quantitative food-frequency questionnaire developed to assess the dietary intakes of 4–16-year-old children. It was developed and is recommended for Western country populations. It includes four sections: intake of fruit and vegetables, fat from dairy products, non-core foods (such as peanut butter, pie and hotdog) and sweetened beverages. FEAHQ is designed to evaluate the influence of eating and physical activity on the child’s (aged 6–11 years old) obesity risk and to investigate the association of family behaviours and child weight. It has four sections: activity level, stimulus exposure, eating related to hunger and eating style (e.g., eating while standing at the open refrigerator or from the pot; while watching TV or doing homework or reading; following stress (anger, frustration, boredom), and between meals; second helpings; parental presence when the child is eating).
Statistical analyses
Data were analysed using SPSS version 22 (IBM, New York, USA). Data distribution was determined by Q–Q plot and histogram visualisation. Differences between metformin versus placebo-exposed groups were tested using the Student t-test for continuous variables and chi-square test for categorical variables. Kruskal–Wallis test was used for non-parametric data. Multiple regression was used to test whether there were any differences between vascular outcomes according to metformin exposure after adjustment for potential confounders including questionnaire data, maternal age at delivery, BMI and parity, and offspring sex and age. A P-value <0.05 was considered statistically significant.
Results
Fig. 1 shows that out of a potential 170 (n = 102 Edinburgh, n = 68 Coventry) participants for recruitment, 112 (65.9%) were uncontactable, including one woman who had died. From the remaining 58 women who were directly contacted by the research team, the final sample size included 40 children (n = 17 (42.5%) boys, n = 23 (57.5%) girls), representing 69.0% of the contactable women but only 23.5% of the overall sample.

Fig. 1. Flow chart.
Characteristics of mothers and children and potential confounders
Table 1 shows the characteristics of the mothers and their children. There were no differences in any of the baseline characteristics between metformin and placebo groups or between participants and non-participants.
Table 1. Maternal and child characteristics at baseline

GA, gestational age.
Data are mean (SD) or N (%). (*P-value for differences between metformin vs. placebo participants, all tests are from t-test or Mann–Whitney U test apart from †P value from Chi square; **P-value for comparison with the rest of the cohort, all tests are from ANOVA or Kruskal–Wallis test apart from † P value from Chi square).
At follow-up, the children were of mean (SD) age 5.78 (0.93) years. There were no differences in age between children followed up in Edinburgh or Coventry (5.70 (0.53) vs. 5.92 (0.75), P = 0.26 years). Children in the metformin- and placebo-exposed groups were of similar age and sex.
There were no differences in children’s behaviour, diet, own activity levels nor family activity levels between metformin- and placebo-exposed groups (Supplementary Table S1).
Anthropometry and vascular assessment at follow-up according to metformin exposure
The majority of children were of normal weight 28 (70%). Ten (25%) were overweight or obese (n = 4, 10% overweight, n = 6, 15% obese) and 2 (5%) were underweight. Table 2 shows there were no differences between metformin and placebo groups in any of the children’s anthropometric or body composition (biceps, triceps, subscapular skinfold and mid-arm circumference) measurements.
Table 2. Demographics of children at follow-up
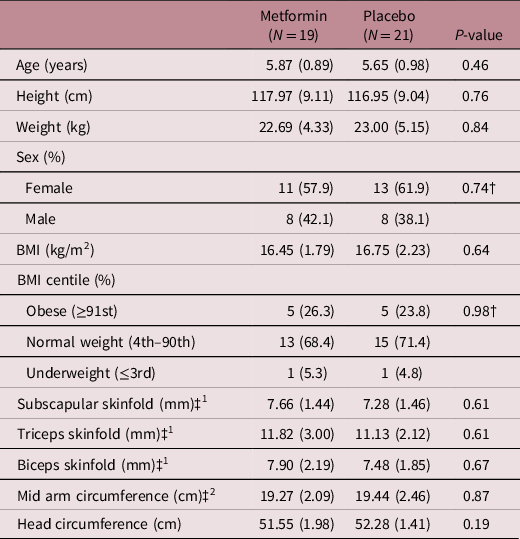
Data are mean (SD) or N (%). All tests are from t-test or Kruskal–Wallis test apart from † P value from Chi square; ‡1Data obtained from Edinburgh site only; ‡2Data obtained from Coventry site only.
Table 3 shows there were no differences between metformin and placebo groups in BP or any of the vascular measurements in univariate or adjusted analyses.
Table 3. Vascular assessments of children at follow-up

Alx, augmentation index; cDia, central diastolic BP; cSys, central systolic BP; PWV, pulse wave velocity; All tests are from t-test or Kruskal–Wallis. Adj-p-value – adjusted for confounders including questionnaire data, maternal age at delivery, BMI and parity, and offspring sex and age.
Using these data in a post-hoc sample size calculation (OpenEpi Version 3.0), we calculated that with 80% power and alpha 0.05 we would have detected 0.8 SD difference between means, for example, 1.78 kg/m2 difference in BMI, 6.3 mmHg difference in systolic BP, but a sample size of 714 per group for BMI and 832 per group for systolic BP would be required to detect possible subtle differences.
Discussion
In this follow-up study of children born to obese pregnant women who had participated in a randomised controlled trial, we found no evidence that metformin exposure in utero influences cardiovascular risk at age 4–7 years, with no differences in anthropometric or vascular measures between metformin- and placebo-exposed groups.
To our knowledge, the only other available data reporting childhood outcomes of exposure to metformin in obese women are from the MOP trial, which reported no differences in body composition other than lower gluteal and triceps circumferences in the metformin group in children at a younger age of 3.9 years. Reference Panagiotopoulou, Syngelaki and Georgiopoulos6 There are important differences to consider between the two samples with diverse ethnicity, higher rates of gestational diabetes and higher dose of metformin used in MOP Reference Syngelaki, Nicolaides and Balani12 compared with EMPOWaR Reference Chiswick, Reynolds and Denison7 meaning that the two studies may not be directly comparable. Of note, there were also marked differences in the maternal characteristics and pregnancy outcomes of the Adelaide and Auckland populations of children followed up in the Metformin in Gestational Diabetes trial (MiG-TOFU) at age 7–9 years. Reference Rowan, Rush and Plank13 In the Adelaide subgroup, women randomised to metformin versus insulin were similar at baseline but had higher glucose levels and more infants with birthweight >90th percentile. At 7 years, there were no differences in offspring obesity measures. In Auckland, at enrollment, women randomised to metformin had a higher BMI but gained less weight during treatment and offspring birth measures were similar. At 9 years, metformin offspring were larger by all obesity measures. Reference Rowan, Rush and Plank13 The authors speculated that infants in the Adelaide group that were exposed to a higher nutrient load in utero (as measured by higher maternal glucose) were ‘protected’ by metformin, so that they were not more obese as they grew. In contrast, the Auckland group and follow-up studies of children exposed to metformin in the context of treatment for gestational diabetes have demonstrated an adverse effect of metformin exposure on childhood growth patterns, with evidence of fetal growth restriction (low birthweight) but subsequent increased weight and BMI in infancy and in early childhood. Reference Tarry-Adkins, Aiken and Ozanne4 No differences in birthweight between metformin and placebo groups were observed in EMPOWaR.
We found no differences according to metformin exposure in vascular measures including central and peripheral BP, arterial stiffness, PWV and augmentation index. Our findings are consistent MOP follow-up except for the central haemodynamics, which were supplemented in MOP by echocardiography data. Reference Panagiotopoulou, Syngelaki and Georgiopoulos6 No BP differences were found in the MiG-TOFU follow-up studies at age 2 years. Reference Battin, Obolonkin, Rush, Hague, Coat and Rowan14 In follow-up at age 8 years of a trial of metformin treatment for polycystic ovary syndrome, a trend towards higher systolic BP in the metformin group than placebo was noted, but the overall sample size was very small (n = 25). Reference Rø, Ludvigsen, Carlsen and Vanky15 As animal models of metformin exposure in pregnancy suggest a potentially adverse cardiovascular phenotype, Reference Menting, Mintjens and van de Beek16 additional human follow-up studies are needed.
The strengths of our study include the original double-blinded randomised controlled trial design. In this follow-up study, the participants and researchers remained blinded to treatment group during the recruitment, data collection and data cleaning prior to analysis. Though we recruited a small sample, the participants were representative of the overall trial participants. We conducted the studies in a clinical research facility environment with highly trained research nurses skilled at follow-up studies in young children and data were comparable between the two sites. We had good data ascertainment and quality with 92.5% of subjects completing vascular measurements suitable for analyses. We included questionnaire data on behaviour, diet and physical activity allowing us to adjust for other potential confounders of body composition.
The main limitation is the overall small sample size. Although women had given permission to be contacted for future follow-up studies, two-thirds of these women were uncontactable. This highlights the challenges of attempting a follow-up study in a mobile population, when this was not part of the original trial design. In contrast, within the reachable participants, we achieved a recruitment rate of 69%, suggesting that attrition could have been limited if we had continued regular contact with women after completion of the original trial. Future pregnancy trials should consider steps to maintain up-to-date contact details of participants if there is any consideration for longer-term child follow-up. Reference Catherine, Lever and Marcellus17 We considered opening recruitment to the additional 13 study sites that participated in EMPOWaR, but were concerned that the potential small numbers at each site might introduce variability to the precision of the follow-up measurements. In addition, our post hoc sample size calculation suggests we would have detected large clinically significant differences between the groups but would need an unfeasible large number (three to five times larger than the overall number of participants in EMPOWaR) to detect possible subtle differences.
In conclusion, our findings suggest children born to obese women treated with metformin or placebo during pregnancy have no differences in body composition or vascular measures at age 4–7 years. In the EMPOWaR trial, metformin had no effects on the offspring birthweight and there were also no differences in gestational weight gain between the metformin and placebo groups. Indeed, a Cochrane review suggests metformin has no short-term benefits for use in pregnant women who are obese. Reference Dodd, Grivell, Deussen and Hague18 Our sample is too small to draw definitive conclusions about the long-term safety profile of metformin. Further follow-up studies of children exposed to metformin in utero are needed to increase knowledge on long-term safety.
Supplementary material
For supplementary material for this article, please visit https://doi.org/10.1017/S2040174421000301
Acknowledgements
We acknowledge the support of the Edinburgh Children’s Clinical Research Facility.
Financial support
We acknowledge the support of Tommy’s and the British Heart Foundation (RE/18/5/34216).
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the UK national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees (References 17/SS/0065 and LL447019).







