Introduction
Japanese stiltgrass (also known as Annual Jewgrass, Mary’s grass, Nepalgrass, Nepalese browntop, and others) is an invasive C4 summer annual weed resembling a small bamboo (Bambusa vulgaris; McCarty and Hall Reference McCarty and Hall2018). It has a prostate to erect growth habit and is capable of growing up to 0.7 m tall (Judge et al. Reference Judge, Neal and Derr2005a). Furthermore, Japanese stiltgrass is one of the most troublesome invasive species in the United States, rapidly invading forests and low-light turfgrass sites in the Southeast, as well as being listed as an invasive species in Europe, South America, Oceania, and Asia. It is highly invasive in forests due to its ability to tolerate low light intensities and showing no decrease in growth at 18% full sunlight and can grow and produce seed at 2% to 8% full sunlight. Its ability to survive in low light environments is due to low respiration and low-light compensation points, which mean it can maintain positive carbon gains (Judge et al. Reference Judge, Neal and Derr2005a). Japanese stiltgrass seeds are dispersed through floating fruits during high water events, and by adhering to animals, humans, or vehicles (Frey and Schmit Reference Frey and Schmit2015). It produces cleistogamous and chasmogamous flowers (closed self-pollinating and open cross pollinating), which improves its survivability (Ward and Mervosh Reference Ward and Mervosh2012; Judge et al. Reference Judge, Neal and Derr2005a). Japanese stiltgrass can also colonize through the production of lateral tillers (Flory Reference Flory2010).
Japanese stiltgrass is currently listed as one of the 33 worst invasive species in southern forests by the USDA Forest Service (Judge et al. Reference Judge, Neal and Derr2005a). Japanese stiltgrass is native to Japan, Korea, China, Malaysia, India, and the Caucasus Mountains (Fryer Reference Fryer2011). It was originally reported in the United States in Tennessee in 1919 (Judge et al. Reference Judge, Neal and Shear2008). It is believed to have significant phenotypic plasticity between populations, which allows it to acclimatize to different environments (Ziska et al. Reference Ziska, Tomecek, Valerio and Thompson2015). Japanese stiltgrass flowers at different times and at different biomass depending on location; in northern latitudes it flowers earlier and at a lower biomass than in southern latitudes, allowing it to adapt to a wide range of environments (Ziska et al. Reference Ziska, Tomecek, Valerio and Thompson2015). The presence of Japanese stiltgrass can alter soil biota and chemistry, pollinators, wildlife, and aesthetics (Judge et al. Reference Judge, Neal and Derr2005a).
Current control methods for Japanese stiltgrass include hand removal, mowing, and application of PRE and POST herbicides. In many instances it is the only grass species found in shaded environments, which opens the possibility of using selective herbicides. Hand removal often does not provide acceptable control; Flory (Reference Flory2010) compared hand weeding with POST (fluazifop) and PRE + POST (pendimethalin + fluazifop) control treatments in a forested environment and noted that both POST and PRE + POST reduced biomass and cover over two seasons; however, hand-weeded plots were reinvaded. Hand weeding alongside POST-applied herbicide did show an improvement in native species. Judge et al. (Reference Judge, Neal and Shear2008) compared hand removal, mowing, glyphosate application in the autumn, mowing, or application of fenoxaprop-P-ethyl throughout the season. All treatments significantly reduced Japanese stiltgrass cover and seedbank over untreated plants. Native species were able to increase under selective management, hand removal, and fenoxaprop-P applications. Flory and Lewis (Reference Flory and Lewis2009) compared nonchemical methods of Japanese stiltgrass control including hand weeding, fall fire, spring fire, and mowing. Mowing and fall fire were the most effective treatments, spring fire reduced cover but not biomass, whereas hand weeding did not significantly reduce Japanese stiltgrass. From these studies it appears that chemical control of Japanese stiltgrass is required for golf course native areas. Imazapic is currently the only labeled herbicide for PRE and POST Japanese stiltgrass control (Judge et al. Reference Judge, Neal and Derr2005a). The objective of this study was to evaluate Japanese stiltgrass control with common POST herbicides in a shaded golf course natural area.
Materials and Methods
Field experiments were conducted to evaluate various herbicides for POST Japanese stiltgrass control. Experiments were conducted in summer 2018 and replicated in space. The first study was located at the Clemson University Turfgrass Research Facility in Clemson, SC, in a wooded area (34.670604°N, 82.833604°W), initial applications were made July 30, 2018, with repeat applications August 13, 2018. The second study was conducted at the Walker Golf Course in Clemson SC, in a tree-line hole (34.667675°N, 82.838148°W). Initial applications were made August 13, 2018, with repeat applications August 27, 2018. The soil at both locations was a Cecil fine loamy (kaolinitic, thermic Typic Kanhapludults). At the time of experiment commencement weed pressure at both sites was >80%.
Treatment rates and timings are presented in Table 1. All treatments were applied using a CO2-pressurized backpack sprayer calibrated to deliver 187 L ha−1 through 8003 flat-fan nozzles (TeeJet Spraying Systems Co., Wheaton, IL). Due to the nature of the plots being in low-maintenance areas, they were left unmaintained after treatments were applied. Plots were 1 × 1.5 m and set up as a randomized complete block with four replications. Plots were located along the wood line of the golf course and research facility; therefore, they were sized based on location of Japanese stiltgrass and its location to trees and other obstructions. Japanese stiltgrass density and percent control ratings were taken 2, 4, and 8 wk after treatment (WAT). Percent Japanese stiltgrass control was visually evaluated on a 0% to 100% scale (0% = no injury to Japanese stiltgrass, 100% = complete plant control).
Table 1. Japanese stiltgrass (Microstegium vimineum) control 2, 4, and 8 wk after treatment (WAT) at Clemson University Turfgrass Research Facility in 2018.
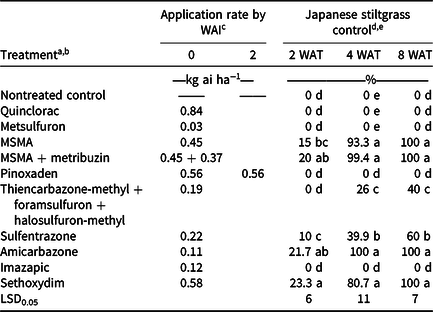
a Herbicide trade names: quinclorac, Drive XLR8; metsulfuron, Manor; MSMA, MSMA; metribuzin, Sencor; pinoxaden, Manuscript, thiencarbazone-methyl + foramsulfuron + halosulfuron-methyl, Tribute Total; sulfentrazone, Dismiss; Amicarbazone, Xonerate; Imazapic, Plateau; sethoxydim, Segment.
b All treatments included a surfactant and rate as designated by the product label.
c Initial treatments were made on July 30, 2018; WAI indicates weeks after initial treatment.
d Japanese stiltgrass control was visually evaluated on a 0% to 100% scale (0% = no injury to Japanese stiltgrass, 100% = complete plant control).
e Column values followed by different letters are significantly different according to Fisher’s protected LSD at P < 0.05.
Control data were analyzed to evaluate main effects and interaction of treatment and location. Where treatment-by-experiment interactions were not detected, data were combined for analysis and presentation. Mean comparisons between treatments were performed using Fisher’s protected LSD test. All analyses were conducted using JMP Pro version 12 (SAS Institute Inc., Cary, NC). Significant effects and differences were based on α = 0.05.
Results and Discussion
Acceptable (>80%) Japanese stiltgrass control was not achieved 2 WAT with any treatment at either site (Tables 1 and 2); however, greatest control at this time was achieved with sethoxydim at the Walker Golf Course site (~53%). At both sites, quinclorac, metsulfuron, pinoxaden, and imazapic provided minimum (<40%) control.
Table 2. Japanese stiltgrass (Microstegium vimineum) control 2, 4, and 8 wk after treatment (WAT) at the Walker Golf Course in 2018.

a Herbicide trade names: quinclorac, Drive XLR8; metsulfuron, Manor; MSMA, MSMA; metribuzin, Sencor; pinoxaden, Manuscript, thiencarbazone-methyl + foramsulfuron + halosulfuron-methyl, Tribute Total; sulfentrazone, Dismiss; Amicarbazone, Xonerate; Imazapic, Plateau; sethoxydim, Segment.
b All treatments included a surfactant and rate as designated by the product label.
c Initial treatments were made on August 13, 2018; WAI indicates weeks after initial treatment.
d Japanese stiltgrass control was visually evaluated on a 0% to 100% scale (0% = no injury to Japanese stiltgrass, 100% = complete plant control).
e Column values followed by different letters are significantly different according to Fisher’s protected LSD at P < 0.05.
Japanese stiltgrass control increased for a number of treatments 4 WAT (Table 1 and 2). MSMA, MSMA + metribuzin, amicarbazone, and sethoxydim provided (>80%) control at the Turfgrass Research Facility 4 WAT. The same four treatments provided 100% control at the Walker Golf Course site. At both sites, metsulfuron, pinoxaden, and imazapic continued to provide minimum (<40%) control; however, limited phytotoxicity (<7%) was observed from use of quinclorac at the Walker Golf Course and no control was observed at the Clemson University Turfgrass Research Facility. Limited control (<50%) was observed at both sites from thiencarbazone-methyl + foramsulfuron + halosulfuron-methyl, and sulfrentrazone.
At 8 WAT, MSMA, MSMA + metribuzin, amicarbazone, and sethoxydim provided >98% control at both locations. Quinclorac, metsulfuron, pinoxaden, and imazapic, however, provided no visible control of Japanese stiltgrass at either location. Thiencarbazone-methyl + foramsulfuron + halosulfuron-methyl, and sulfentrazone control was <60% at both sites.
Judge et al. (Reference Judge, Neal and Derr2005b) conducted greenhouse and pot studies evaluating three POST herbicides (fenoxaprop, imazapic, and sethoxydim) to control Japanese stiltgrass. All herbicides reduced biomass between 83% and 89%, reduced seedhead production between 79% and 94%, and resulted in a stand reduction of 70% to 89% vs. the untreated plots over 2 yr. These data are contrary to those of the present study in which imazapic provided 0% control. However, similar results were observed with sethoxydim in both studies. Ward and Mervosh (Reference Ward and Mervosh2012) also investigated Japanese stiltgrass control using imazapic, and several other conventional and alternative control treatments including propane torch, hand weeding, mowing, foliar application of household vinegar, pelargonic acid, pelargonic acid + pendimethalin, fenoxaprop-p-ethyl, glufosinate, and glyphosate. The authors observed that all treatments reduced Japanese stiltgrass cover and seed production and all herbicide treatments except for pelargonic acid, which completely prevented seed production in the second year of the study.
Frey and Schmit (Reference Frey and Schmit2015) investigated control of Japanese stiltgrass with three rates of sethoxydim. Treatments were applied for 2 yr followed by monitoring for 2 yr. As with the present study, sethoxydim treatments provided some control of Japanese stiltgrass (78% to 93% reduction in biomass across the first year); however, in the second year, Japanese stiltgrass had reinvaded and plots that had been treated showed no statistical difference with the control plots.
Judge et al. (Reference Judge, Neal and Derr2005a) performed greenhouse and pot studies of Japanese stiltgrass control by investigating PRE and POST herbicides. As with the present study, sethoxydim provided acceptable control by the end of the study, with two application providing better control than one application. The authors also noted quinclorac provided 0% control, as was observed in the present study. The authors also observed 0% control with MSMA; however, in the present study MSMA provided 100% control at both sites by the end of the study.
Future research should include long-term control over multiple growing seasons, repeat applications of herbicides, and evaluation of herbicides in combination for increased and longer term Japanese stiltgrass control.
Acknowledgments
No conflicts of interest have been declared. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.




