Introduction
Aluminium (Al) toxicity is one of the main factors limiting plant growth in acid soils, which account for about 0.40 of the world's arable lands (Delhaize et al., Reference Delhaize, Gruber and Ryan2007; Kovácik et al., Reference Kovácik, Štork, Klejdus, Grúz and Hedbavny2012; Rezaee et al., Reference Rezaee, Ghanati and Behmanesh2013). The growing acidification of soils has become a major environmental concern over recent years. Soil acidification can be accelerated by intensive farming, among others, and prevented by sustainable management practices (Singh and Agrawal, Reference Singh and Agrawal2008; Kunhikrishnan et al., Reference Kunhikrishnan, Thangarajan, Bolan, Xu, Mandal, Gleeson, Seshadri, Zaman, Barton, Tang, Luo, Dalal, Ding, Kirkham and Naidu2016).
Lithuania is located in the cold and humid zone, where mean annual precipitation exceeds mean evapotranspiration and soil acidification is an ongoing natural process. Soil acidification is caused by precipitation, which dissolves and removes (due to excess precipitation) carbonates in the soil parent material. In this region, primary carbonates are leached from more than 2 m deep (Eidukeviciene et al., Reference Eidukeviciene, Volungevicius, Marcinkonis, Tripolskaja, Karcauskiene, Fullen and Booth2010) and soil acidification is encouraged by anthropogenic activities. Soils in Lithuania tend to acidify, especially in the eastern and western regions of the country (in the hilly uplands and plateau areas). Here, more intense acidification is influenced by the heterogeneous, wedge-shaped moraine loam structure and greater age of the soil parent material compared with the surrounding moraine lowlands. Acid soils account for 0.25 in the eastern part and 0.40 of soils in the western part of Lithuania (Marcinkonis and Tripolskaja, Reference Marcinkonis and Tripolskaja2008; Staugaitis and Vaišvila, Reference Staugaitis and Vaišvila2015).
Increasing soil acidity (pH < 4.5–5) favours an increase in Al in the soil solution and potential toxicity, which has a negative effect on plant productivity (Yamamoto et al., Reference Yamamoto, Okuda, Nozawa, Furukawa and Miura2015). It is relevant that acid soils have an exchange complex saturated in Al. The occurrence of Al in the soil solution depends on pH as well as on the existence of components capable of releasing this element in the soil solid fraction. Aluminium toxicity is affected by many factors, such as pH, concentration of Al, temperature and concentrations of cations and anions in the soil solution. The critical soil pH at which sufficient Al is soluble to be toxic is difficult to predict because it depends on many other factors, including clay mineralogy, organic matter (OM), other cations and anions and total salts (Đalović et al., Reference Đalović, Jocković, Dugalić, Bekavac, Purar, Šeremešić and Jocković2012). Changes in organo–Al complexes play an important role in soil pH regulation as well as Al activity in soil solution (Eimil-Fraga et al., Reference Eimil-Fraga, Álvarez-Rodríguez, Rodríguez-Soalleiro and Fernández-Sanjurjo2015). Aluminium organic compounds of high stability, such as aluminium humates, citrates, etc., have the lowest phytotoxic effect (Álvarez et al., Reference Álvarez, Fernández-Sanjurjo, Seco and Núñez2012). The adverse effects of phytotoxic forms of aluminium could be alleviated by liming (Prado et al., Reference Prado, Natale and Rozane2007; Álvarez et al., Reference Álvarez, Viadé and Fernández-Marcos2009). The role of lime application is to raise soil pH and reduce exchangeable Al, favour the development of soil microbiota and stimulate biological activity (Fuentes et al., Reference Fuentes, Bezdicek, Flury, Albrecht and Smith2006). Eidukeviciene et al. (Reference Eidukeviciene, Volungevicius, Marcinkonis, Tripolskaja, Karcauskiene, Fullen and Booth2010) and Repsiene and Karcauskiene (Reference Repsiene and Karcauskiene2016) reported that long-term periodic soil liming with limestone and its combination with farmyard manure decreased soil acidity up to the 40–50 cm depth and mitigated this process in deeper horizons up to a depth of 1 m.
In highly acidic soils, lime incorporation is recommended to ensure a faster return on the lime application investment (Conyers et al., Reference Conyers, Heenan, McGhie and Poile2003). However, long-term liming can provide a greater economic return (Caires et al., Reference Caires, Churka, Garbuio, Ferrari and Morgano2006) and reduce the risk of soil losses, which is even more important in highly degraded soils.
The objective of the current work was to study the effect of long-term liming on pH, the forms of Al in the solid phase and total Al in soil solution by comparing a limed soil profile with an unlimed soil profile, both originating from the same parent material under the same climatic conditions.
Materials and methods
Description of the study site
Research was carried out at the site of a cultivated long-term experiment, conducted at Vėžaičiai Branch of the Lithuanian Research Centre for Agriculture and Forestry (55°41′39.9″N 21°29′56.9″E). The experiment is located in West Lithuania, in the cold and humid zone, where the mean annual precipitation (748 mm) exceeds the mean evapotranspiration (512 mm) and the average annual air temperature is 6.7 °C. This region is strongly affected by the maritime climate.
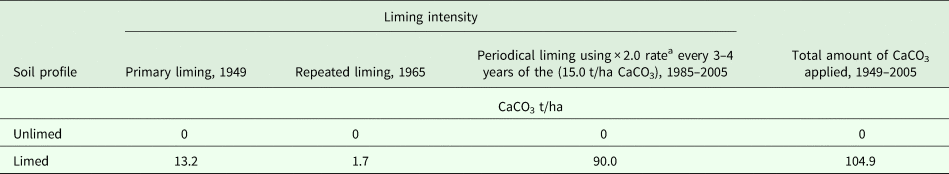
This long-term field experiment was started in 1949 on a Dystric Glossic Retisol (Loamic) (WRB IUSS Working Group, 2014), developed on marginal moraine (till), with a texture of sandy loam. The carbonate horizon is found deeper than 2 m below the surface. Two contrasting profiles were studied. One soil profile was not limed, with the following horizon sequence according to the WRB IUSS Working Group (2014): Ahp, the ploughing horizon, which has humus accumulation (0–30 cm); E, the eluvial horizon from which clay particles are leached (30–38 cm); E/B, a horizon with retic properties and predominantly coarser-textured albic material (38–75 cm); B/E, a horizon with retic properties and predominantly finer-textured argic material (75–108 cm); BCl, an intermediate horizon which has gleyic properties and from which primary calcium carbonates are leached (108–153 cm). The other soil profile was limed (Table 1), with the following horizon sequence: Ahp (0–32 cm); E (32–50 cm); E/B (50–80 cm); B/E (80–125 cm) and BCl (125–155 cm).
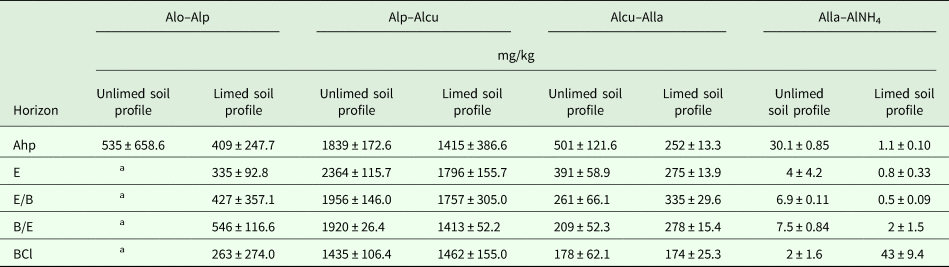
Table 1. Liming intensity of soil profiles
CaCO3, calcium carbonate.
a Liming rate calculated based on the soil hydrolytic acidity.
A four-course crop rotation was used: spring barley under-sown with grasses; perennial grasses of the first harvest year; winter wheat and spring oilseed rape. The crop rotation was fertilized annually with nitrogen (N), phosphorus (P) and potassium (K) N60P60K60 and conventional soil tillage was used. Organic fertilization was minimal, i.e. 40 t/ha of farmyard manure per crop rotation until 2005.
In 2016, soil samples were collected at different horizons from both soil profiles and placed into plastic bags. Each horizon had three replicates.
Soil analysis
Soil samples were dried at 40 °C, sieved through 2 mm mesh size and visible roots and plant residues were removed.
The pH was determined in 1.0 m potassium chloride (KCl) at a soil to water ratio of 1 : 5 and in distilled water at a soil to water ratio of 1 : 5. Soil organic carbon was measured by the Tyurin method modified by Nikitin (Reference Nikitin1999) using dichromate oxidation. Effective cation exchange capacity (eCEC) was determined as the sum of exchangeable calcium (Ca), magnesium (Mg), sodium (Na), K and Al (Kamprath, Reference Kamprath1970) displaced by 1 m ammonium chloride (NH4Cl; Peech et al., Reference Peech, Alexander and Dean1947).
Different forms of Al were extracted from the solid fraction by non-sequential extraction with different reagents:
• Total non-crystalline Al (Alo) – extracted with 0.2 m acid ammonium oxalate (ratio soil : extractant 1 : 100, with 4 h shaking in the dark) (Blakemore, Reference Blakemore and Smith1978).
• Total organically bound Al (Alp) – extracted with 0.1 m sodium pyrophosphate (ratio soil : extractant 1 : 100, with 16 h shaking) (Bascomb, Reference Bascomb1968).
• Organo–Al complexes of low to medium stability (Alcu) were extracted with 0.5 m copper chloride (ratio soil : extractant 1 : 10, 30 min shaking) (García-Rodeja et al., Reference García-Rodeja, Novoa, Pontevedra, Martinez-Cortizas and Buurman2004).
• The most labile organo–Al complexes (Alla) were extracted with 0.33 m lanthanum chloride (LaCl3; ratio soil : extractant 1 : 10, 2 h shaking) (Hargrove and Thomas, Reference Hargrove and Thomas1984).
• Exchangeable Al (AlNH4) was extracted with unbuffered 1 m NH4Cl (ratio soil : extractant 1 : 20, contact time, 12 h) (Peech et al., Reference Peech, Alexander and Dean1947).
The extracts with different reagents were filtered. Aluminium in the extracts was determined by atomic absorption spectroscopy (PerkinElmer AAnalyst 100, PerkinElmer, Waltham, MA, USA). The difference between Alo and Alp showed as inorganic non-crystalline Al (Alo − Alp); the difference between Alp and Alcu showed as high stability Al complexes with OM (Alp − Alcu); the difference between Alcu and Alla was estimated as medium stability Al complexes with OM (Alcu − Alla); the difference between Alla and AlNH4 was estimated low-stability Al complexes with OM (Alla − AlNH4) (Urrutia et al., Reference Urrutia, Macías and Garcíıa-Rodeja1995; Álvarez et al., Reference Álvarez, Monterroso and Fernández-Marcos2002).
The soil solution was approximated by aqueous extracts (ratio soil : extractant 1 : 10, suspension was shaken for 10 min, allowed to rest for 3 days, filtered at 0.45 µm) and total water-soluble Al (Alw) determined by visible spectrophotometry following the pyrocatechol violet method (Dougan and Wilson, Reference Dougan and Wilson1974).
Statistical analysis
A one-way analysis of variance was used to compare the soil characteristics and Al compounds of unlimed and limed soil horizons at each profile. Means were compared using Fisher's least significant difference test at P < 0.05. Moreover, Pearson correlation between parameters was performed. The statistical software package SAS (2011) was used for all analyses.
Results
General soil parameters
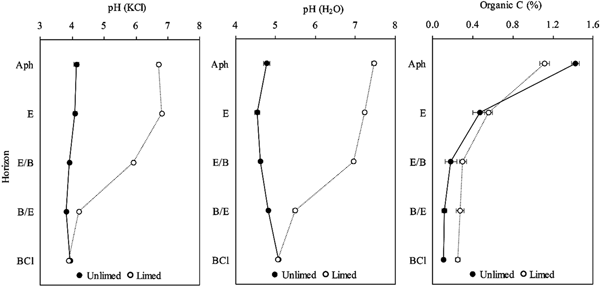
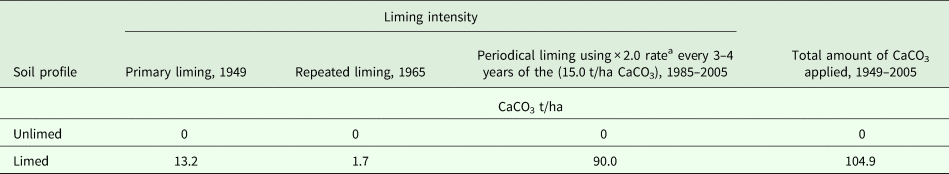
Soil pH values changed little in the completely unlimed soil profile: pH (KCl) 3.90–4.12 (Fig. 1) and pH (H2O) 4.53–5.08 (Fig. 1). Long-term liming increased the pH in most horizons of the soil profile and the effect was significant (P < 0.001) up to 125 cm depth (up to B/E horizon) of the soil. The maximum increase was observed in the Ahp and E horizons of the limed soil profile compared with the unlimed soil profile (increased pH (KCl): 2.58 and 2.72 for Ahp and E, respectively; increased pH (H2O): 2.68 and 2.70 for Ahp and E, respectively). In the two deepest horizons (B/E–BCl), the differences in pH (KCl) and pH (H2O) values between the two soil profiles decreased (Fig. 1).
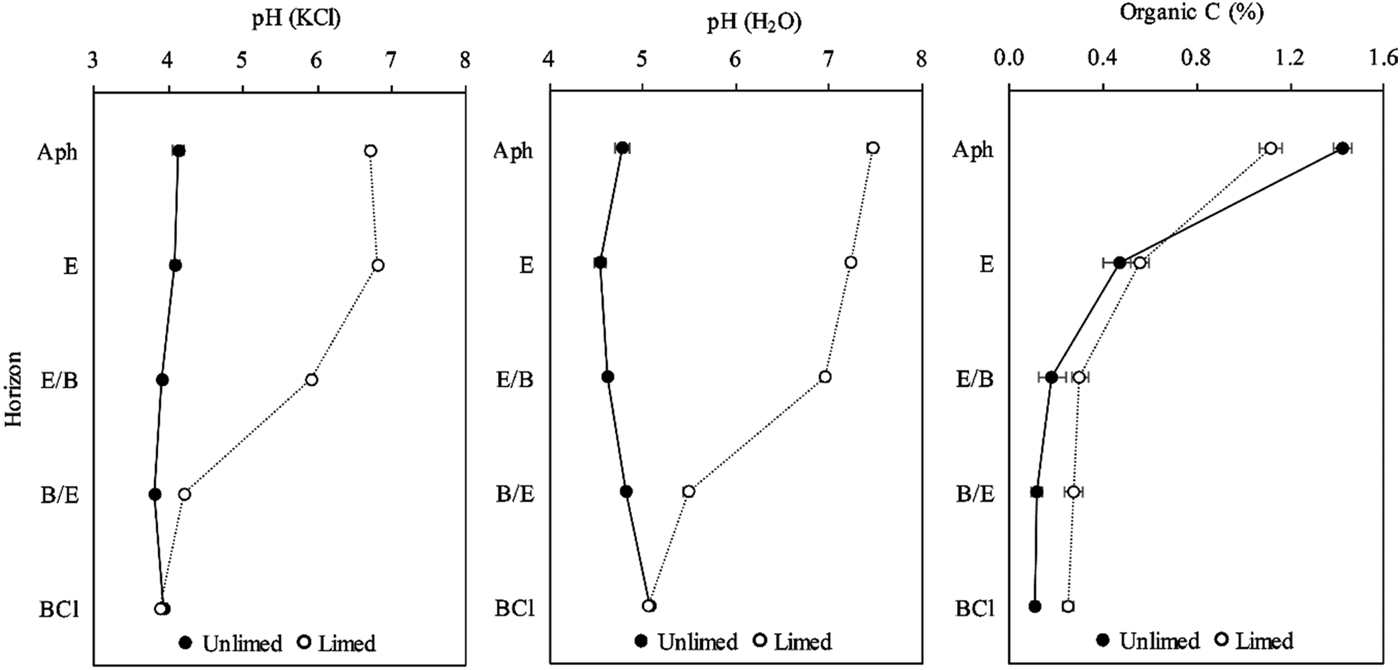
Fig. 1. Values of pH (KCl and H2O) and organic C in unlimed and limed soil profiles of a Retisol. All values are means of three replicates and standard deviations are shown as error bars. Significant differences between horizons of unlimed and limed soil were indicated when P < 0.05.
In the unlimed soil, the concentration of organic C was 1.42% in Ahp horizon (Fig. 1), 0.47% in E and decreased in deeper horizons (E/B–BCl) to 0.10–0.18% (Fig. 1). It was found that long-term liming decreased organic C concentration in the topsoil significantly (P = 0.012) and increased it slightly in the deeper horizons. Organic C was correlated significantly with pH (KCl) (r = 0.488, P = 0.006) and pH (H2O) (r = 0.412, P = 0.024).
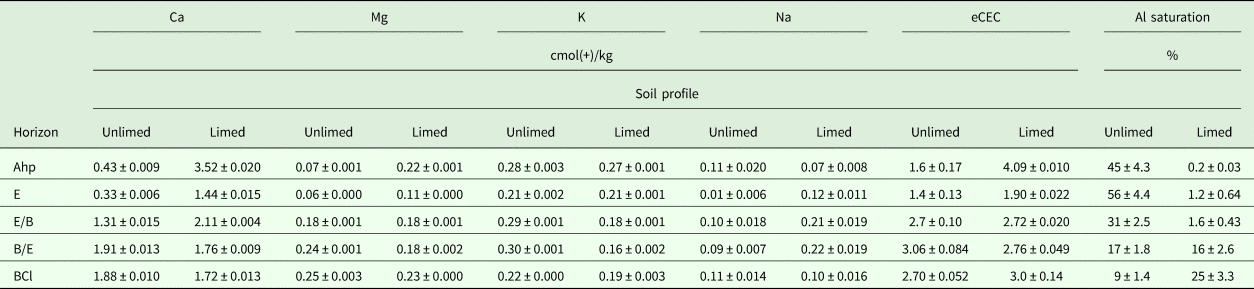
The concentration of exchangeable Ca was significantly higher (P < 0.001) in Ahp–E–E/B horizons of limed soil profile (2.11–3.52 cmol(+)/kg) than unlimed soil profile (0.33–1.31 cmol(+)/kg) (Table 2). The concentrations of Ca were significantly (P < 0.001) lower in B/E and BCl horizons of the limed soil profile (1.72–1.76 cmol(+)/kg) than the unlimed soil profile (1.88–1.91 cmol(+)/kg). Long-term liming increased significantly (P < 0.01) the concentrations of exchangeable Mg in Ahp–E–E/B horizons (0.11–0.22 cmol(+)/kg) but decreased it significantly (P < 0.001) in B/E–BCl horizons. The concentration of exchangeable K was significantly (P < 0.01) higher in all horizons of unlimed soil profile (0.21–0.30 cmol(+)/kg) than in limed soil profile (0.16–0.27 cmol(+)/kg). Exchangeable Na was significantly (P = 0.046) higher in Ahp horizon of unlimed soil profile and there was significantly (P < 0.01) higher in E–E/B–B/E horizons of limed soil profile (0.12–0.22 cmol(+)/kg).
Table 2. Average values and standard deviation of concentrations of exchangeable calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), effective cations exchange capacity (eCEC) and aluminium (Al) saturation in unlimed and limed soil profiles

Ahp, ploughing horizon, which has humus accumulation; E, eluvial horizon from which clay particles are leached; E/B, a horizon with retic properties and predominantly coarser-textured albic material; B/E, a horizon with retic properties and predominantly finer-textured argic material; BCl, an intermediate horizon which has gleyic properties and from which primary calcium carbonates are leached.
All values are means of three replicates. Statistically significant differences are among the horizons in the unlimed and limed soil profile for P < 0.05.
Long-term liming increased the eCEC in Ahp–E horizons of the limed soil profile significantly (P < 0.01) (1.90–4.09 cmol(+)/kg, Table 2). The eCEC was correlated positively with pH (H2O) (r = 0.38, P = 0.037). The Al saturation (%) was significantly (P < 0.001) lower in Ahp–E–E/B horizons of limed soil profile, and it was correlated negatively with pH (KCl) (r = −0.62, P < 0.001), pH (H2O) (r = −0.76, P < 0.001) and eCEC (r = −0.62, P < 0.001).
Aluminium in the solid fraction
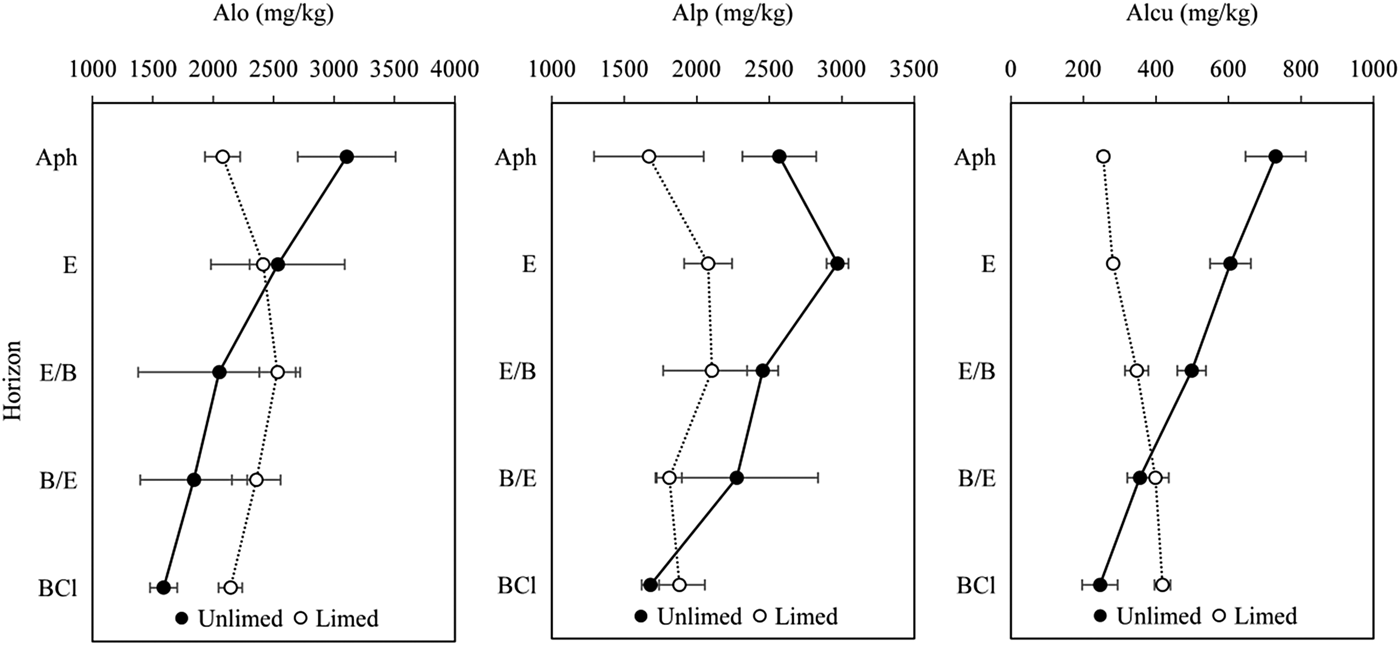
The total non-crystalline Al ranged from 1589.8 to 3104.3 mg/kg in the unlimed soil profile, while in long-term limed soil profile Alo ranged from 2078.0 to 2534.8 mg/kg (Fig. 2). The total non-crystalline Al values in both soil profiles showed that long-term liming decreased significantly (P = 0.014) its concentration in Ahp (about 30 cm depth) (Fig. 2). The total non-crystalline aluminium was significantly correlated with organic C (r = 0.55, P = 0.002) and pH (H2O) (r = −0.45, P = 0.013).
Fig. 2. Concentration of total non-crystalline Al (Alo), total organically bound Al (Alp) and organo–Al complexes of low to medium stability (Alcu) in unlimed and limed soil profiles of a Retisol. All values are means of three replicates and standard deviations are shown as error bars. Significant differences between horizons of unlimed and limed soil were indicated when P < 0.05.
The total organically bound Al ranged from 1680.7 to 2969.6 mg/kg in the unlimed soil profile (Fig. 2). In the first two horizons of the limed soil profile, Alp was significantly (P = 0.027) lower and ranged between 1669.4 and 2104.5 mg/kg (Fig. 2): it was significantly negatively correlated with pH (H2O) (r = −0.45, P = 0.013).
The concentration of Al extracted with copper chloride (CuCl2), which estimates the organo–Al complexes of low to medium stability, ranged from 730.3 (Ahp horizon) to 245.8 mg/kg (BCl horizon) in the unlimed soil profile (Fig. 2) and accounted for 15.46–28.43% Alp. The organo–Al complexes of low to medium stability concentration at 0–155 cm depth increased from 254.9 (Ahp horizon) to 418.2 mg/kg (BCl horizon) in the limed soil profile (Fig. 2) and accounted for 13.55–22.24% of Alp. Liming reduced the Alcu significantly (P < 0.01) in the first three horizons of the soil profile. The organo–Al complexes of low to medium stability was significantly positively correlated with organic C (r = 0.39, P = 0.032), Alo (r = 0.47, P = 0.008) and Alp (r = 0.73, P < 0.001), negatively correlated with pH (KCl) (r = −0.48, P = 0.007), pH (H2O) (r = −0.61, P < 0.001) and eCEC (r = −0.64, P < 0.001).
The concentration of the most labile Al complex varied from 229.1 mg/kg in Ahp to 68.0 mg/kg in the BCl horizon of unlimed soil profile (Fig. 3). The most labile Al in the limed soil profile increased from 2.8 mg/kg in the Ahp horizon to 244.0 mg/kg in the BCl horizon. The concentration of Alla was significantly (P < 0.01) lower in the Ahp, E and E/B horizons in limed soil profile (Fig. 3). The most labile Al was significantly negatively correlated with pH (KCl) (r = −0.80, P < 0.001) and pH (H2O) (r = −0.85, P < 0.001) and Alla was significantly positively correlated with Alcu (r = 0.69, P < 0.001) and Alp (r = −0.45, P = 0.014).
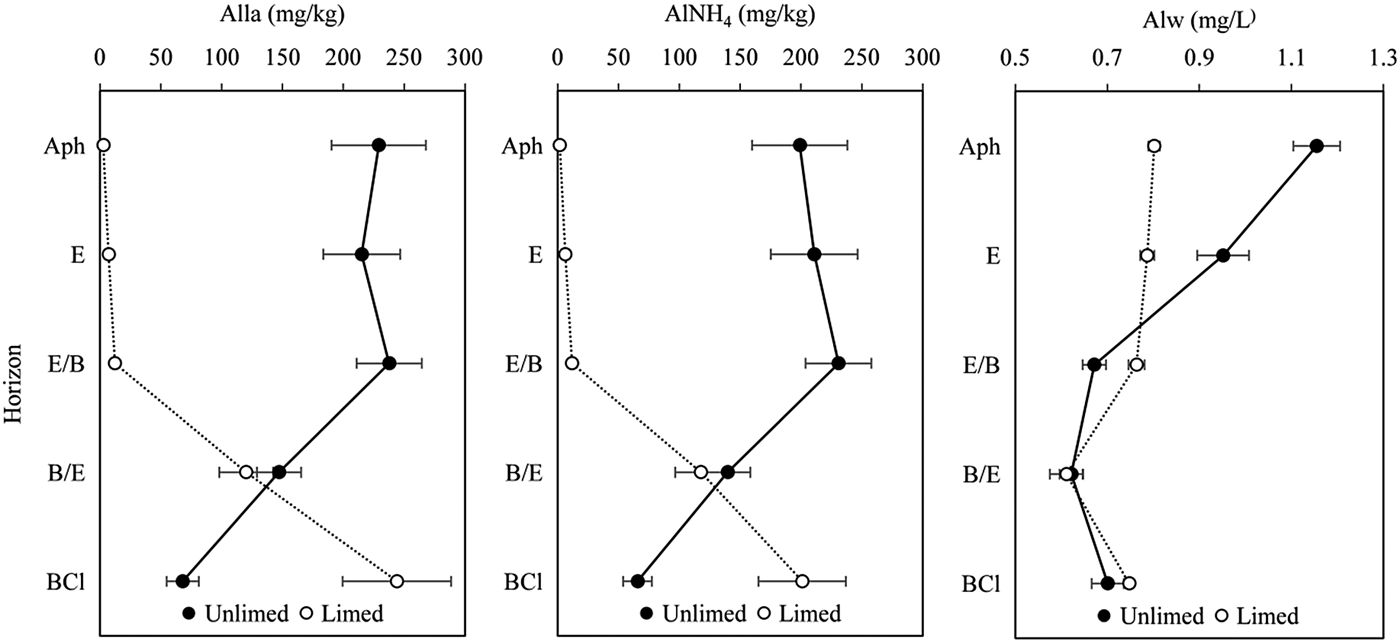
Fig. 3. Concentration of labile organo–Al complexes (Alla), exchangeable Al (AlNH4) and water-soluble Al (Alw) in unlimed and limed soil profiles of Retisol. All values are means of three replicates and standard deviations are shown as error bars. Significant differences between horizons of unlimed and limed soil were indicated when P < 0.05.
The data showed that the difference between Alo and Alp (inorganic non-crystalline Al) was 535.3 mg/kg in the Ahp horizon of unlimed soil profile and it was negative in the E–BCl horizons of unlimed soil profile (Table 3). Inorganic non-crystalline Al concentration ranged between 262.9 and 546.0 mg/kg in the limed soil profile (Table 3).
Table 3. Average values and standard deviation of concentrations of inorganic non-crystalline Al (Alo–Alp) and the organo–Al complexes of different stability: high – Alp–Alcu, medium – Alcu–Alla, low – Alla–AlNH4 in unlimed and limed soil profiles
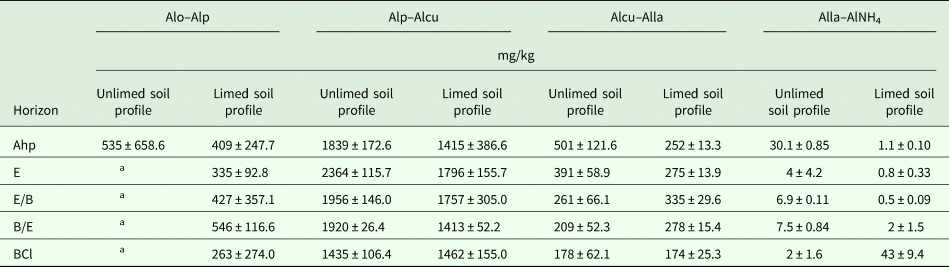
Ahp, ploughing horizon, which has humus accumulation; E, eluvial horizon from which clay particles are leached; E/B, a horizon with retic properties and predominantly coarser-textured albic material; B/E, a horizon with retic properties and predominantly finer-textured argic material; BCl, an intermediate horizon which has gleyic properties and from which primary calcium carbonates are leached.
All values are means of three replicates. Statistically significant differences are among the horizons in the unlimed and limed soil profile for P < 0.05.
a Negative subtraction Alo–Alp.
The organo–Al complexes of high stability ranged from 1434.9 to 2364.0 mg/kg in the unlimed soil profile (Table 3), while in the long-term limed soil profile Alp–Alcu ranged from 1412.6 to 1796.4 mg/kg. These results indicated that Alp–Alcu is a predominant form of organically bound Al in unlimed and limed soil profiles, representing in all cases more than 0.77 of organo–Al complexes (Table 3). Liming reduced significantly (P < 0.001) the Alp–Alcu in the E horizon of the soil. The highly stable Al complexes with OM showed a highly significant correlation with the Alp (r = 0.96, P < 0.001).
The concentration of organo–Al complexes of medium stability decreased from 501.2 mg/kg in the Ahp horizon to 177.8 mg/kg in the BCl horizon of the unlimed soil profile (Table 3). The results showed that the concentration of Alcu–Alla increased from 252.0 mg/kg in the Ahp horizon to 334.7 mg/kg in the E/B horizon (50–80 cm) in the limed soil profile (Table 3). Meanwhile, the concentration of Alcu–Alla decreased to 174.1 mg/kg in the BCl horizon (155 cm depth) of the limed soil profile. These complexes represent between 0.098 and 0.212 of organo–Al complexes in the unlimed soil profile and between 0.104 and 0.164 in the limed soil profile. Liming reduced organo–Al complexes of medium stability in the first two horizons significantly (P < 0.01). Organo–Al complexes of medium stability was significantly positively correlated with organic C (r = 0.56, P < 0.001), it was also positively correlated with Alo (r = 0.44, P = 0.014), Alp (r = 0.62, P < 0.001) and Alp–Alcu (r = 0.45, P = 0.012).
Low-stability Al complexes with OM ranged between 30.1 and 2.2 mg/kg in the unlimed soil profile and 1.1–43.0 mg/kg in the limed soil profile (Table 3), representing a very low proportion (<0.03) of Al complexed with OM. After long-term liming, the concentrations of Alla–AlNH4 were significantly (P < 0.05) decreased in the Ahp, E/B and B/E horizons. However, liming increased Alla–AlNH4 concentrations in the BCl horizon significantly (P < 0.001). Low-stability Al complexes with OM were significantly negatively correlated with pH (KCl) (r = −0.42, P = 0.020) and pH (H2O) (r = −0.41, P = 0.025) and positively significantly correlated with Alcu (r = 0.47, P = 0.009).
Unlimed soil profile presented a very high concentration of exchangeable Al (199.0–230.8 mg/kg) at 0–80 cm depth (in Ahp, E and E/B horizons) (Fig. 3). At 80–153 cm depth in the unlimed soil profile, the concentration of exchangeable Al decreased from 230.76 to 65.76 mg/kg. The high concentrations of exchangeable Al were associated with low pH values of the soil in the unlimed Retisol profile. Liming reduced the exchangeable Al concentration in the first three horizons significantly (P < 0.01, Fig. 3). Exchangeable Al was significantly negatively correlated with pH (KCl) (r = −0.81, P < 0.001) and pH (H2O) (r = −0.87, P < 0.001). Exchangeable Al were also significantly positively correlated with Alcu and Alla (r = 0.68, P < 0.001 and r = 0.99, P < 0.001).
Aluminium in the soil solution
The concentration of total water-soluble Al ranged from 0.61 to 0.80 mg/l in the limed soil profile, meanwhile in the unlimed soil profile it was 0.62–1.15 mg/l (Fig. 3). Total water-soluble Al concentration was significantly (P < 0.01) lower in the Ahp and E horizons in the limed soil profile compared with the unlimed soil. Conversely, the Alw concentration was significantly (P = 0.01) higher in the E/B horizon in the limed soil profile. In deeper horizons (B/E and BCl) the Alw concentration was similar between the unlimed and limed soil profiles. Total water-soluble Al was positively correlated with the organic C (r = 0.79, P < 0.001), Alo (r = 0.61, P < 0.001) and Alp (r = 0.46, P = 0.011). In addition, water-soluble Al was positively correlated with Alcu (r = 0.67, P < 0.001), Alcu–Alla (r = 0.74, P < 0.001) and Al saturation (r = 0.59, P < 0.001) and negatively significantly correlated with eCEC (r = −0.57, P = 0.001).
Discussion
Long-term application of liming resulted in a significant decrease in the exchangeable acidity (pH (KCl)) and the current acidity (pH (H2O)) in the first three horizons (Ahp–E–E/B) of the soil. Nazarkiewicz and Kaniuczak (Reference Nazarkiewicz and Kaniuczak2012) also documented that liming increased pH (KCl) in the Ap (0–25 cm) and Bt (26–50 cm) horizons of Haplic Luvisol. Eidukeviciene et al. (Reference Eidukeviciene, Ozheraitiene, Tripolskaja and Marcinkonis2001) observed that long-term liming at 1.0 rate (liming rate calculated based on the soil hydrolytic acidity) every 3–4 years and every 10 years over a period of two decades in a Retisol changed acid soil's pH (KCl) and exchangeable Al concentration in the whole profile up to 100 cm depth. The low pH of the unlimed soil is related to the lack of carbonates in the parent material, or the presence of carbonates but only in the deeper layers of the Retisol profile (below 200 cm), and leaching of bases due to percolation of water in a moderately humid climate (Eidukeviciene et al., Reference Eidukeviciene, Volungevicius, Marcinkonis, Tripolskaja, Karcauskiene, Fullen and Booth2010; Nikorych et al., Reference Nikorych, Szymański, Polchyna and Skiba2014). The negative correlation of Al saturation with eCEC showed that the highest effect of liming is felt in the layer most affected by the soil formation process, i.e. the Ahp–E–E/B horizons.
The content of OM decreased in the uppermost horizon of the limed profile as a result of the increase in pH values caused by liming. This increase of pH affects the processes of OM mineralization in the soil, due to increased microbial activity with consequent consumption of carbon by soil microorganisms (Fernández-Sanjurjo et al., Reference Fernández-Sanjurjo, Urrutia Mera and García-Rodeja1991; Llorente and Turrion, Reference Llorente and Turrion2010; Seco et al., Reference Seco, Fernández-Sanjurjo, Núñez-Delgado and Alvarez2014). With respect to the decrease of organic carbon during liming, it is also associated with the destruction of soil aggregates and subsequent release of carbon from them (Bronick and Lal, Reference Bronick and Lal2005). The additional carbon inputs associated with improved vegetation production from root biomass turnover cannot recover carbon losses. Humification rates of OM in the soil are slower than mineralization rates. Also, the alkaline pH would favour OM solubilization and leaching (Núñez-Delgado et al., Reference Núñez-Delgado, López-Perıago and Dıaz-Fierros-Viqueira2002; Pousada-Ferradás et al., Reference Pousada-Ferradás, Seoane-Labandeira, Mora-Gutiérrez and Núñez-Delgado2012). The slight increase in organic C observed below the Ahp horizon in the limed soil may be due to a higher contribution of roots in this soil as a consequence of higher crop production (Álvarez et al., Reference Álvarez, Viadé and Fernández-Marcos2009). A more favourable soil pH allows deeper penetration of plant roots and thereby enriches soil with OM.
Long-term liming generated a significant change in Al compounds in the whole soil profile. Regarding Al fractionation in the solid phase, in all the horizons of the two soil profiles Alp represented a high proportion (no less than 0.768) of Alo, and this indicates a predominance of organo–Al complexes over inorganic compounds of low crystallinity, which is also supported by a value >0.5 for the Alp/Alo ratio in these soil profiles (García-Rodeja et al., Reference García-Rodeja, Novoa, Pontevedra, Martinez-Cortizas and Buurman2004). In some horizons of the unlimed soil Alp was even higher than Alo, which has also been reported previously by other authors (García-Rodeja et al., Reference García-Rodeja, Novoa, Pontevedra, Martinez-Cortizas and Buurman2004; Walna et al., Reference Walna, Spychalski and Siepak2005; Álvarez et al., Reference Álvarez, Fernández-Sanjurjo, Otero and Macías2010; Lee et al., Reference Lee, Song, Moon and Moon2012; Ferro-Vazquez et al., Reference Ferro-Vazquez, Novoa-Munoz, Costa-Casais, Klaminder and Martinez-Cortizas2014; Rivas-Pérez et al., Reference Rivas-Pérez, Fernández-Sanjurjo, Núñez-Delgado, Macías, Monterroso and Álvarez-Rodríguez2016).
Long-term liming reduced the total non-crystalline Al in the uppermost horizon, and the organo–Al complexes of different stability as well as the exchangeable Al in the Ahp–E–E/B horizons. The reduction in the total non-crystalline Al in the limed soil up to a depth of 50 cm can be explained by the fact that liming reduces the organic C content (Wang et al., Reference Wang, Cammeraat, Cerli and Kalbitz2014) and consequently diminishes Al complexes with OM in the Retisol. It is noteworthy that despite the reduction of all Al OM complexes, in terms of proportions, liming favoured the formation of high stability complexes in the first two horizons, particularly in the Ahp horizon, possibly related to the greater reactivity of OM caused by a higher pH (Álvarez et al., Reference Álvarez, Viadé and Fernández-Marcos2009).
The results showed a strong relationship between soil pH and exchangeable Al for the tested soil profiles, while exchangeable Al decreased at a soil pH above 5.9. This negative correlation with pH (KCl) and pH (H2O) indicated that Al competes with base cations for exchange sites of soil under the most acidic conditions, because of the higher solubility of this element at low pH (Álvarez et al., Reference Álvarez, Viadé and Fernández-Marcos2009). Exchangeable Al also correlated negatively with eCEC, which indicates that the increase of pH caused by liming favours the appearance of negative charges in the soil components with variable charge (increase in eCEC) and also favours the precipitation of Al (decrease of exchangeable Al) and the increase of basic cations (mainly Ca) in the exchange positions. Several authors have described a decrease in the exchangeable Al following liming in acid soils, being the main objective of liming (Caires et al., Reference Caires, Garbuio, Churka, Barth and Corrêa2008; Álvarez et al., Reference Álvarez, Viadé and Fernández-Marcos2009; Moir and Moot, Reference Moir and Moot2010). In the current study, a decrease in Al in the soil solution was also observed in the first two horizons of the limed profile. This is particularly relevant because Al is most available to plants in this form. Aluminium mobilization can take place by various mechanisms, such as alteration of silicates, dissolution of hydroxides, organic decomplexation or cationic exchange (Zhu et al., Reference Zhu, Jiang and Ji2004; Guo et al., Reference Guo, Vogt, Zhang, Zhang, Seip, Xiao and Tang2006, Reference Guo, Zhang, Vogt, Xiao, Zhao, Xiang and Luo2007). However, if environmental conditions vary, solubilized Al can be reincorporated to the solid phase, forming precipitates and/or complexes, or bound to exchange sites (Fernández-Sanjurjo et al., Reference Fernández-Sanjurjo, Álvarez and García-Rodeja1998; Zhu et al., Reference Zhu, Jiang and Ji2004; Guo et al., Reference Guo, Vogt, Zhang, Zhang, Seip, Xiao and Tang2006, Reference Guo, Zhang, Vogt, Xiao, Zhao, Xiang and Luo2007; Álvarez et al., Reference Álvarez, Fernández-Sanjurjo, Otero and Macías2010). Water-soluble Al was positively correlated with Al saturation in the exchangeable complex. This indicated the existence of higher Al content in the soil solution when the saturation of this element in the exchangeable complex is high.
The reduction of all forms of Al in the solid phase and Al in solution in the same horizons of the limed soil profile can be interpreted as a tendency to the precipitation of crystalline minerals of Al. This was favoured by the significant increase of pH and mineralization of OM caused by liming. In fact, most forms of Al in the solid phase correlated negatively with pH and positively with OM content, as previously indicated in the Results section. Many authors have indicated that the addition of limestone promotes the removal of Al from the soil solution and the precipitation of inorganic non-crystalline or crystalline compounds (Fernández-Sanjurjo et al., Reference Fernández-Sanjurjo, Álvarez and García-Rodeja1995; Meriga et al., Reference Meriga, Reddy, Jogeswar, Reddy and Kishor2003; Holmström et al., Reference Holmström, Riise, Tau Strand, Geibe, van Hees, Wu and Lundström2003; Álvarez et al., Reference Álvarez, Viadé and Fernández-Marcos2009).
Author ORCIDs
Z. Kryzevicius, 0000-0002-8289-353X.
Financial support
This work was supported by the National Science Program ‘The effect of long-term, different-intensity management of resources on the soils of different genesis and on other components of the agro-ecosystems’ (grant number SIT-9/2015) funded by the Research Council of Lithuania and by the long-term research programme ‘Productivity and sustainability of agricultural and forest soils’ implemented by the Lithuanian Research Centre for Agriculture and Forestry.
Conflict of interest
The authors certify that they have no conflicts of interest with this manuscript.
Ethical standards
Not applicable.