Introduction
Ascension Island is a small, isolated volcanic island, part of the Mid-Atlantic Ridge region, which lies at 07°57′S 14°22′W in the tropical south Atlantic. It is one of the most remote islands in the world; the nearest landmass is St Helena Island, 1200 km to the south. It is 2300 km away from Brazil and 1500 km from the west coast of Africa. This isolation, combined with Ascension's comparative geological youth (~1.5 million years old) (Evangelidis et al., Reference Evangelidis, Minshull and Henstock2004), is thought to have in part contributed to a relatively depauperate shallow marine fauna (Brickle et al., Reference Brickle, Brown, Küpper and Brewin2017).
There has been limited research on the sponge fauna of Ascension Island (Table 1). The first specimen collected from Ascension Island was a shore-collected dry specimen in 1902 collected by the German South Polar expedition; however, the first specimens known to have originated on Ascension Island were collected by the R.R.S. Discovery between 1925 and 1929 (Burton, Reference Burton1932). Sponges were collected by scuba in 1985 during a British Joint Services' Survey expedition (Taylor & Irving, Reference Taylor and Irving1985; Irving, Reference Irving1989); 67 specimens were collected, which were estimated to represent 47 different species. These were deposited at the Zoological Museum Amsterdam, now part of the Naturalis Biodiversity Center (Naturalis BioPortal, 2021). Identifications for the majority of these specimens have not yet been finalised or formally published. However, those that have, represent several species new to science (Van Soest, Reference Van Soest1990; Van Soest et al., Reference Van Soest, Beglinger and de Voogd2013, Reference Van Soest, Beglinger and de Voogd2014).
Table 1. Prior species-level sponge records from Ascension Island

Ascension Island shallow-water fauna appears to have a broadly Mid-Atlantic Ridge faunal composition, with affinities to the tropical and sub-tropical western and eastern Atlantic regions (Floeter et al., Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008). These affinities could be due to the proximal ancient Gondwana origins of the west African and northern South American continents (Rosen, Reference Rosen1975). However, these dispersal patterns are likely now maintained by large-scale oceanic gyre currents in the Atlantic Ocean, which enable the dispersal of shallow-water organisms to the remote shelf of Ascension Island (Den Hartog, Reference Den Hartog1989; Zibrowius et al., Reference Zibrowius, Wirtz, Nunes, Hoeksema and Benzoni2014; Reimer et al., Reference Reimer, Lorion, Irei, Hoeksema and Wirtz2017). For fish, Ascension Island has a stronger biogeographic connection with the Brazilian province than with the Tropical Eastern Atlantic Province, whereas nearby, but far older, St Helena has near equal affiliations with the Brazilian and Tropical Eastern Atlantic provinces (Briggs & Bowen, Reference Briggs and Bowen2012). Ascension Island zoantharians are found to have phylogeographic affinities with the western Atlantic and Caribbean regions (Reimer et al., Reference Reimer, Lorion, Irei, Hoeksema and Wirtz2017). In contrast, shallow-water Ascension Island scleractinian corals appear to have broader amphi-Atlantic distributions (Zibrowius et al., Reference Zibrowius, Wirtz, Nunes, Hoeksema and Benzoni2014).
Ascension Island has been found to have a high level of endemism, likely due to its remote position in the Atlantic Ocean, which has enabled vicariance in many types of marine organisms (Floeter et al., Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008). Both Ascension and St Helena Islands, separately, have the two highest levels of endemism in reef fishes, for all oceanic islands in the Atlantic Ocean, despite limited shelf habitat diversity and area and relatively young geological ages (Floeter et al., Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008). Briggs (Reference Briggs1974, Reference Briggs1995) originally proposed that Ascension and St Helena should be combined as an independent biogeographic province based on fish, which has been reiterated by more recent studies of the biogeography of shelf and coastal ecoregions (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007). However, later studies indicate that both Ascension and St Helena have sufficient endemism to be regarded as separate biogeographic provinces of the Eastern Atlantic Region (Floeter et al., Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008; Briggs & Bowen, Reference Briggs and Bowen2012).
Materials and methods
Specimen collection
Sponges were collected during August–September 2012 and July 2015 as part of a larger expedition to generate baseline data on Ascension Island's marine habitats (Brickle et al., Reference Brickle, Brown, Küpper and Brewin2017). In total, 58 sponge specimens were collected by scuba diving from 17 locations (Table 2, Figure 1) and at depths of 0.5–30 m. Due to the lack of a recompression chamber, diving depth was usually limited to <20 m. However, during the 2012 survey, several deeper dives were possible as a portable recompression chamber was present on the island for a commercial diving project.

Fig. 1. Location of sponge records from Ascension Island. This includes species level records from this survey and Table 1. In addition, records for Crambe sp., Demosponges 1–3, and Halicona sp. Barnes (Reference Barnes2017), Porifera × 2 (Irving, Reference Irving1989), and Porifera × 3 (Manning & Chace, Reference Manning and Chace1990). Bathymetric data supplied by Ascension Island Government.
Table 2. Sampling sites, see also Figure 1

Sponges were selected by eye: the divers attempted to sample species that looked different from those previously sampled during the dive. The aim was to sample as many different species as possible rather than gaining any quantitative information. Once selected, three photographs of each specimen were taken in situ using housed digital SLR cameras (Nikon D70 and Nikon D90 in Ikelite housings with Ikelite DS125 substrobe both with 60 mm macro lenses). A small piece (~1 cm2 of tissue) was then removed.
Laboratory methodology
After collection, notes were made on colour, sponge texture, and any presence of any smell or slime. The samples were transferred to 95% ethanol for storage. Tissue slides were prepared by sectioning a very thin portion of tissue at a 90° angle through the sample. The tissue was then dehydrated in absolute ethanol for 4 min and placed in clove oil for a further 4 min to clarify the tissue before being mounted on a microscope slide in Canada balsam. A coverslip was then placed on the slide. To isolate spicules for examination, pieces of sponge were placed in undiluted household bleach overnight to remove tissue, then rinsed four times in distilled water and cleaned in two washes of 95% ethanol. Spicules were allowed to settle for at least 15 min between rinses, and then the upper layer of liquid was pipetted off, leaving the spicules undisturbed. Cleaned spicules were dried on glass slides, mounted in Canada balsam and imaged on a compound microscope (Olympus BX43). For scanning electron microscopy (SEM), cleaned spicules were placed on metal stubs, coated with gold and viewed with a JEOL 6400 SEM (UNB, Fredericton). Spicule measurements (N = 20, unless otherwise noted) were made using an Olympus SC50 camera and Olympus cellSens standard 1.16 software. Measurements are reported as minimum (mean) maximum.
Histology
Histology was undertaken at the Microscopy and Microanalysis Facility at the University of New Brunswick. Histological processing of samples was based on a previously incompletely documented protocol (as acknowledged in Eerkes-Medrano et al., Reference Eerkes-Medrano, Feehan and Leys2014). The full modified protocol is as follows. Sponge samples preserved in 70% ethanol were dissected into embeddable pieces no bigger than 1.5 cm cubed. These pieces were rehydrated to water before being fixed in 4% paraformaldehyde overnight and then rinsed in phosphate-buffered saline (PBS) and dehydrated again through an ethanol series back to 70% ethanol. Specimens were separately desilicified overnight in 4% hydrofluoric acid (in 70% ethanol) to remove spicules and decalcified overnight in undiluted cal-ex decalcifier to remove coral using the same rehydration to water, treatment, PBS rinse, and dehydration through an ethanol series as the fixation step. Specimens were then processed by hand using the following steps: dehydrated to 100% ethanol with two 30 min washes of 95% ethanol and three 10 min washes of 100% ethanol, all at room temperature, cleared in toluene with one 30 min wash of 1:1 ethanol: toluene at room temperature and two 30 min washes of toluene at 60°C, and infiltrated with paraffin wax overnight after two 4 h washes of paraffin all at 60°C before embedding the next day. Embedded samples were cut in 7 μm sections for choanocyte chambers, and 12 μm sections for skeletal structures using a Leica rotary microtome and sections were dried on slides using Haupt's adhesive. Slides were stained with Masson's trichrome with the following times for the stains: 1 min and 20 s with Gill III Hematoxylin, 3 min Ponceau Acid Fuchsin, and 4 min Acetic Aniline Blue with 5 min differentiation step with 1% phosphomolybdic acid after the Ponceau Acid Fuchsin and Acetic Aniline Blue staining steps.
Sequencing
Sequencing was done by the Canadian Rivers Institute Genomics Laboratory, University of New Brunswick, Saint John. DNA was extracted from sponge samples using E.N.Z.A. Tissue DNA Kit by Omega BioTek according to the manufacturer's directions. A 20–30 μg piece of tissue was ground in the lysis buffer in a MixerMill at 25.0 freq 1/s for 3 min and then was left to incubate overnight at 55°C. The C2-D2 region of 28S (Chombard et al., Reference Chombard, Boury-Esnault and Tillier1998), and CO1 (Meyer et al., Reference Meyer, Geller and Paulay2005) were amplified by polymerase chain reaction following the thermal regime used by Erpenbeck et al. (Reference Erpenbeck, Voigt, Al-Aidaroos, Berumen, Büttner, Cantania, Guirguis, Paulay, Schätzle and Wörheide2016). Clean up and sequencing of PCR products were performed by Génome Québec, Montréal, QC. Sequences were submitted to GenBank (Clark et al., Reference Clark, Karsch-Mizrachi, Liman, Ostell and Sayers2016); accession numbers are provided for the four specimens from three different species where sequencing was successful (Monanchora downesae sp. nov.; Chondrosia browningorum sp. nov.; and two specimens of Ircinia simae sp. nov.).
Type specimens
The majority of the type material is in the Atlantic Reference Centre Museum, New Brunswick, Canada (ARC). One paratype is in Ulster Museum Belfast (BELUM). Information on extant species was obtained from the World Porifera Database (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Kelly, Vacelet, Dohrmann, Diaz, Cárdenas, Carballo, Ríos, Downey and Morrow2021). The publication and species names were registered in ZooBank: publication urn:lsid:zoobank.org:pub:FD98928F-BEF3-45D5-A6D3-F612A7D5C9B9.
Results
Systematics
Phylum PORIFERA Grant, 1836
Class DEMOSPONGIAE Sollas, 1885
Subclass HETEROSCLEROMORPHA Cárdenas, Pérez & Boury-Esnault, 2012
Order HAPLOSCLERIDA Topsent, 1928
Family NIPHATIDAE Van Soest, Reference Van Soest, Hummelinck and Van der Steen1980
Genus Niphates Duchassaing & Michelotti, 1864
Niphates verityae Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:9D46D0C2–77B7-4EBD-AE43-4BE7DA54CB9F
(Figure 2A–E)
Type Material
Holotype: ARC 81586, Pan Am, Ascension Island, −7.96962° −14.4124°, depth 6.5–10.5 m, 14 July 2015, collected by Rob Mrowicki.
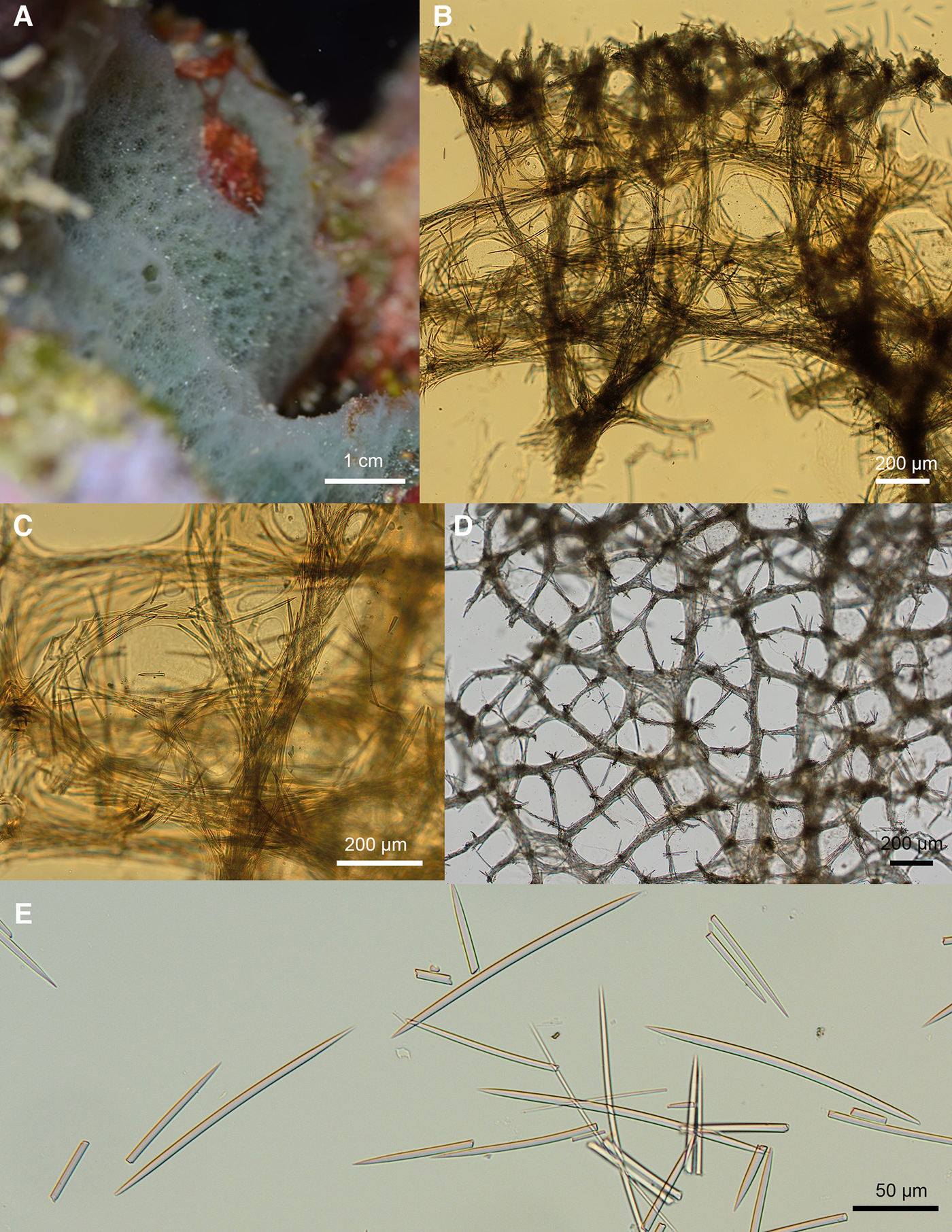
Fig. 2. Niphates verityae sp. nov. ARC 81586. (A) In vivo appearance. (B) Choanosomal skeleton. (C) Choanosomal fibres. (D) Ectosomal skeleton. (E) Oxea.
Paratype: ARC 81549, Wigan Pier, Ascension Island, −7.89397° −14.3835°, depth 6.2 m, 25 August 2012, collected by Judith Brown.
Diagnosis
Niphates with an encrusting form and pale green colour in vivo. Relatively short oxea (168(176)185 × 5(6)9 μm) compared with other species in the region.
Etymology
Named after Verity Goodwin, daughter of author Claire Goodwin, who was born shortly after the initial fieldwork expedition.
External Appearance
In vivo (Figure 2A): Pale green, thickly encrusting sponge forming a low mound on bedrock. Holotype around 5 cm in diameter and up to 1 cm thick. Skeletal mesh and apertures between it are visible through the surface, giving the sponge a delicate, lacy appearance. The sponge surface is slightly conulose due to the protruding ends of the primary choanosomal fibres. Some small oscules (up to 5 mm in diameter) are visible on the sponge surface.
Preserved: Buff in ethanol with firm but compressible texture.
Skeleton
Choanosomal skeleton (Figure 2B, C): Formed of a meshwork of fibres, most of which have thick spongin sheaths. The primary ascending fibres are 97–190 μm in diameter and cored by 6–12 spicules. The ascending fibres subdivide and diverge as they get towards the ectosome. They are joined by secondary fibres 23–66 μm in diameter cored by 2–6 spicules. The meshes formed between the fibres are irregular in shape and size. The larger meshes are 239–450 μm in diameter and the smaller 84–133 μm in diameter.
Ectosomal skeleton (Figure 2D): Paratangential (three-dimensional) ectosomal skeleton formed of two sizes of irregular mesh. The larger meshes are 426–660 μm in diameter and formed from primary fibres 46–96 μm in diameter and cored by 8–12 spicules. These large meshes are subdivided into smaller meshes 169–382 μm in diameter by secondary fibres 27–47 μm in diameter and cored by 4–8 spicules. The ends of the primary skeletal fibres of the choanosome protrude through the ectosome, creating a conulose surface.
Spicules (Figure 2E)
Oxea which abruptly taper to fine points 168(176)185 × 5(6)9 μm.
Remarks
We assign this specimen to Niphates rather than Callyspongia due to the three-dimensional ectosomal skeleton and the irregular nature of the meshes in its choanosomal skeleton (Van Soest & Hooper, Reference Van Soest, Hooper, Hooper and Van Soest2002). There are 22 valid species in the genus Niphates. Eight species are known from the tropical Atlantic and Caribbean (reviewed in Van Soest, Reference Van Soest, Hummelinck and Van der Steen1980 and Santos et al., Reference Santos, Docio and Pinheiro2014). These can all be distinguished from Niphates verityae sp. nov. by their form, colour or spiculation. Niphates alba Van Soest, Reference Van Soest, Hummelinck and Van der Steen1980, Niphates arenata Rützler et al., Reference Rützler, Piantoni, Van Soest and Díaz2014, and Niphates lutea Lehnert & Van Soest, 1999 possess strongyle rather than oxeote megascleres. Niphates luizae Santos, Docio & Pinheiro, Reference Santos, Docio and Pinheiro2014 differs from Niphates verityae sp. nov, and all other regional Niphates, in possessing very robust oxea (270 × 18 μm). Niphates amorpha Van Soest, Reference Van Soest, Hummelinck and Van der Steen1980 has a similar encrusting form but possesses larger oxea (183–252 × 4–7 μm), may have sigmata (12–18 μm) and is purplish-grey when alive. Niphates caycedoi (Zea & Van Soest, 1986) has larger oxea (199–285 × 5–19 μm), possesses toxa, and is a vivid blue to violet colour. Niphates digitalis (de Lamarck, Reference de Lamarck1814) differs in having a cup-shaped rather than encrusting form and may possess sigmata. Niphates erecta Duchassaing & Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864 has larger oxea (154–232 × 2.5–9 μm), may have sigmata, and has a ramose form with solid branches.
This species is found in Ascension Island's north and south-western sectors, encrusting on volcanic bedrock and boulders.
Family PETROSIIDAE Van Soest, Reference Van Soest, Hummelinck and Van der Steen1980
Genus Petrosia Vosmaer, 1885
Subgenus Petrosia (Petrosia) Vosmaer, 1885
Petrosia (Petrosia) ernesti Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:C29AD1FF-6496-46EA-A87A-9C5DBBCB8E88
(Figure 3A–E)
Type Material
Holotype: ARC 81579, Red Rock Archway, −7.89423° −14.3946°, depth 30 m, 6 September 2012, collected by Judith Brown.
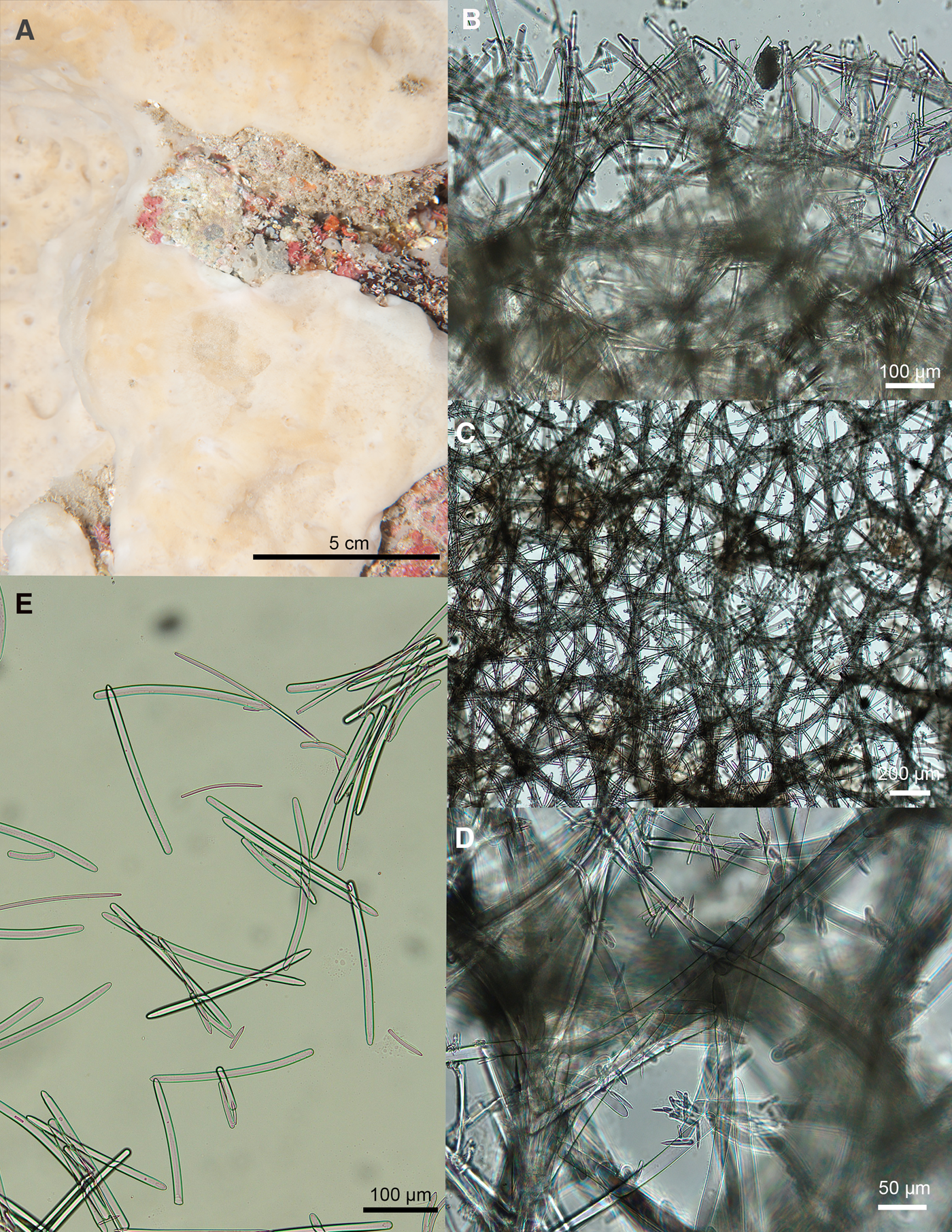
Fig. 3. Petrosia (Petrosia) ernesti sp. nov. ARC 81579. (A) In vivo appearance. (B) Choanosomal skeleton. (C) Ectosomal skeleton. (D) Close up of ectosomal fibres showing clusters of microscleres at nodes. (E) Spicules.
Diagnosis
Petrosia with four spicule categories comprising of strongyle megascleres (187–262 and 65–134 μm) and strongyle (18–32 μm) and oxea (25–38 μm) microscleres.
Etymology
Named after Ernest Goodwin, son of author Claire Goodwin, who was born shortly after the initial fieldwork expedition.
External Appearance
In vivo (Figure 3A): Thickly encrusting beige sponge with a smooth surface. Holotype formed a very large patch – over 1 m in diameter. The sponge was inside a small cave under the ‘Archway’. Preserved: Thin crust with a glassy smooth ectosome. The ectosome does detach but not in flakes.
Skeleton
Choanosomal skeleton (Figure 3B): A reticulation of fibres 50–90 μm in diameter (around 5–10 spicules thick). There is no distinction between primary and secondary fibres. Fibres are echinated by the smallest styles and strongyles.
Ectosomal skeleton (Figure 3C, D): Meshwork of fibres 30–60 μm (around 2–5 spicules) thick, echinated by the smallest styles and strongyles.
Spicules (Figure 3E)
Large strongyles: 187(237)262 × 13(15)18 μm. Ends are variable. Some are both rounded. In others one other is slightly pointed, giving an almost style or oxea-like appearance (although the points are not sharp enough to be true styles or oxeas). The majority are curved.
Medium strongyles: 65(97)134 × 7(14)21 μm. Most with rounded ends, although a few variants as above. The majority are curved.
Small strongyles: 18(25)32 × 3(5)6 μm.
Small stylote oxea: 25(32)38 × 3(5)8 μm. One rounded end, similar to the strongyles. The other end comes to an abrupt point.
Remarks
We assign this species to Petrosia (Petrosia) rather than Petrosia (Strongylophora) as its ectosomal skeleton is reticulate network rather than a dense irregular tangential reticulation of free microxeas and strongyles (Desqueyroux-Faúndez & Valentine, Reference Desqueyroux-Faúndez, Valentine, Hooper and Van Soest2002). Ten species are known from the Atlantic and Caribbean (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Kelly, Vacelet, Dohrmann, Diaz, Cárdenas, Carballo, Ríos, Downey and Morrow2021; Table 3). Only Petrosia (Petrosia) ficiformis (Poiret, 1789) and Petrosia (Petrosia) weinbergi Van Soest, Reference Van Soest, Hummelinck and Van der Steen1980 have more than two categories of spicules. Petrosia (Petrosia) ficiformis has 2–3 categories of spicules, but these are oxea, which in some cases may be modified to strongyles. Its smallest category of spicules is 45–65 μm, considerably larger than the small styles and strongyles found in our specimen (information on spicule dimensions from de Weerdt & Van Soest, Reference De Weerdt and Van Soest1986). Petrosia (Petrosia) weinbergi is a green sponge. It also has oxea tending to strongyles rather than mainly strongyles, and its smallest category of spicules has a broader size range than those found in our specimen (29–58 × 1.5–4 μm). None of the known species possesses the style-like microscleres (presumably modified oxea) found in our specimen.
Table 3. Petrosia (Petrosia) species from the Atlantic and Caribbean

This species was found in a rocky environment in the northern sector of Ascension Island.
Order POECILOSCLERIDA Topsent, 1928
Family CRAMBEIDAE Lévi, 1963
Genus Monanchora Carter, 1883
Monanchora downesae Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:61054234-9CD3-4032-9CF6-FA9277BED72B
(Figure 4A–H)
Type Material
Holotype: ARC 81596, Red Rock, Ascension Island, −7.89435° −14.39475°, depth 14.7 m, 19 July 2015, collected by Paul Brickle and Stevie Cartwright.

Fig. 4. Monanchora downesae sp. nov. (A) In vivo appearance ARC81596. (B) In vivo appearance Mc6222, (C) Spicules. (D) Choanosomal skeleton. (E) Ectosomal tylostyles. (F) Basal styles. (G) Basal style ends. (H) Ectosomal tylostyle ends.
Paratype: BELUM Mc6222, English Bay, Ascension Island, −7.89292°, −14.3868°, depth 5–11.5 m, 3 December 2009, collected by Claire Goodwin.
Other specimens: BELUM Mc6234, BELUM Mc6235, BELUM Mc6236, One Hook, English Bay, Ascension Island, −7.98292°, −14.3868°, depth 8–20 m, 8 December 2009, collected by Claire Goodwin.
GenBank accession number for ARC 81596: MW488272.
Diagnosis
Monanchora lacking microscleres. Spicules ectosomal tylostyles 217(271)294 × 4(5)6 μm and basal styles 288(311)343 × 6(7)9 μm. Bright orange-red colour in vivo, prominent stellate channels surround oscules but not of a contrasting colour.
Etymology
Named for Kate Downes, a young, bright, talented scientist whose precious life sadly ended in 2020. Kate's passion for Ascension drove her work on the island and continued after she left to pursue a PhD on yellowfin tuna that utilize this unique tropical ecosystem. Kate's sad passing has left large voids in her family, friends and colleagues' lives. We are honoured to name this common beautiful sponge species in her cherished memory.
External Appearance
In vivo (Figure 4A, B): Red thinly encrusting (up to 1 cm thick) sponge forming patches up to 10 cm in diameter. Large pore sieves, often slightly elevated. Stellate subsurface channels radiate from the pore sieves. The area between the channels is punctate.
Preserved: Thin red crust.
Skeleton (Figure 4D)
A basal layer of large styles echinates the substrate. Ascending columns of up to 15 of the smaller ectosomal tylostyles ascend to the surface.
Spicules (Figure 4C)
Ectosomal tylostyles (Figure 4E, H): 217(271)294 × 4(5)6 μm. Head can be slightly tylote, forming an oval swelling. Taper to a fine point.
Basal styles (Figure 4F, G): 288(311)343 × 6(7)9 μm. Head not tylote. Fairly straight-sided then terminating in an abrupt point.
Remarks
Seventeen species of Monanchora are currently regarded as valid (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Kelly, Vacelet, Dohrmann, Diaz, Cárdenas, Carballo, Ríos, Downey and Morrow2021). Of these, six are known from the Atlantic: Monanchora arbuscula (Duchassaing & Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864), Monanchora bahamensis Esteves et al., 2018, Monanchora brasiliensis Esteves et al., Reference Esteves, Lerner, Lôbo-Hajdu and Hajdu2012, Monanchora coccinea Esteves et al., 2018, Monanchora megasigma Esteves et al., 2018, and Monanchora stocki Van Soest, Reference Van Soest1990.
Monanchora stocki Van Soest 1990 was described from the Cape Verde Islands and Ascension Island. It has styles of only a slightly shorter size range (ectosomal 175–263 by 2–3.5 μm and basal 161–362 by 4–7 μm) and is also a red crust. However, it also has isochelae 16–24 μm, and although we did search carefully, we could not find any in any of our specimens. Although both Monanchora (Esteves et al., Reference Esteves, Paula, Lerner, Lôbo-Hajdu and Hajdu2018) and the related genus Crambe (Van Soest, 2002) are known to lose microscleres in some specimens, we feel it is unlikely that this would occur in all the specimens collected. Additionally, the large styles of M. stocki have a pronounced tylote head, and both categories are thinner and can be much shorter than those in our specimen.
Esteves et al. (Reference Esteves, Paula, Lerner, Lôbo-Hajdu and Hajdu2018), in their review of tropical western Atlantic Monanchora, note that Monanchora arbuscula can have very rare or absent microscleres, unlike the other species present in the region. M. arbuscula also can take a red encrusting form. However, as the name suggests, other specimens can be erect and ramified. They measured a large number of specimens they categorized as M. arbuscula. Some of these, particularly those from Fernando de Noronha had very similar-sized styles to our specimens (223–339 μm) and lacked microscleres, as did specimens from Rio Grande do Norte in Brazil. Their specimens from Abrolhos, Brazil were very similar in appearance to ours, being a red crust without veins. However, they had sigmoid chelae, which our specimens lack. Additionally, specimens of M. arbuscula that lack chelae also lack two categories of megascleres, having just the thinner category (Esteves pers. comm.). This indicates that our specimens are probably a distinct species. There was also only 93% alignment with 28S sequences from our specimen (ARC 81596, GenBank MW488272) and a specimen of M. arbuscula (GenBank KC869447.1, Thacker et al., Reference Thacker, Hill, Hill, Remond, Collins, Morrow, Spicer, Carmack, Zappe, Pohlmann, Hall, Diaz and Bangalore2013).
The genus Crambe has species with a similar appearance and also often has a reduced spicule complement, lacking microscleres. However, sequences of Crambe crambe (GenBank KX688742.1, Idan et al., Reference Idan, Shefer, Feldstein, Yahel, Huchon and Ilan2018) had only 83% similarity with our sequenced specimen so we feel our species is unlikely to belong in this genus.
This species was very abundant on volcanic bedrock in the shallow water around northern and western Ascension Island.
Order SCOPALINIDA Morrow & Cárdenas, 2015
Family SCOPALINIDAE Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs & Allcock, 2012
Genus Svenzea Alvarez, Van Soest & Rützler, 2002
Svenzea weberorum Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:15D06575-04EB-4880-BE3D-4B4CBAA03FE6
(Figure 5A–H)
Type Material
Holotype: ARC 81544, Wigan Pier, Ascension Island, −7.89397° −14.3835°, depth 6 m, 25 August 2012, collected by Judith Brown.

Fig. 5. Svenzea weberorum sp. nov. ARC 81544. (A) In vivo appearance. (B) Close up of surface. (C) Choanosomal skeleton. (D) Granular cells. (E) Close up of choanosomal skeleton showing granular cells. (F) Styles. (G) Oxea. (H) Close up of ends of spicules.
Paratype: ARC 81600, Pyramid Point, Ascension Island, −7.90617° −14.40522°, depth 18 m, 22 July 2015, collected by Emma Nolan and Jerry Pierce.
Other specimens: ARC 81591, Red Rock, Ascension Island, −7.89435°, −14.39475°, depth 14.7 m, 19 July 2015, collected by Paul Brickle and Stevie Cartwright.
ARC 81588, Red Rock, Ascension Island, −7.89423° −14.3946°, depth 16 m, 18 July 2015, collected by Paul Brewin and Jerry Pierce.
ARC 81555, Porpoise Point, Ascension Island, −7.8981° −14.35125°, depth 13 m, 29 August 2012, collected by Judith Brown.
Diagnosis
Svenzea with massively encrusting form and yellow-orange colour in vivo. Spicules are styles 260–318 × 6–12 μm and oxeas 236–348 × 7–11 μm.
Etymology
Named for Drs Nicola and Sam Weber; two scientists who have spent many years on Ascension Island. They were instrumental in generating a biodiversity baseline and environmental management, which ultimately led to the creation of the island's Marine Protected Area. Sam and Nicola continue to work closely with Ascension Island Government's Conservation Department and were critical to this project's success.
External Appearance
In vivo (Figure 5A, B): Thickly encrusting yellow-orange sponge. The holotype is around 30 cm in diameter and up to 5 cm thick, but this species often forms larger patches. Oscules up to 5 mm across are irregularly scattered over its surface; they are slightly raised and surrounded by a ring of solid, non–punctate tissue. The rest of the ectosome is punctate, with the ectosomal mesh visible through the surface.
Preserved: Cream in ethanol. Holds its form but fairly soft and compressible. Oscules have a smooth area around them, distinct from the rest of the sponge's punctate surface.
Skeleton
Choanosomal skeleton (Figure 5C): Reticulate. Ascending columns of 3–6 spicules are irregularly joined by either single spicules or short bundles of up to 3 spicules. Columns are surrounded by a spongin sheath which is obvious on some tissue sections. Granular cells (9(12)17 μm in diameter) are very abundant in the tissue and sometimes obscure the skeleton when viewed on the light microscope (Figure 5D, E). They form a particularly dense layer in the ectosome. There is no distinct ectosomal skeleton, but there is a translucent layer of tissue at the surface which can be peeled off. This has very abundant granular cells in it.
Spicules
The majority of the spicules are styles (Figure 5F). These are usually curved, sometimes smoothly, but sometimes with one or more angular bends. The point is fairly abrupt and can be smooth but is often telescoped (Figure 5H). Styles measure 267(289)318 × 9(10)12 μm in the holotype (ARC 81544) and 266(284)295 × 6(8)10 μm in the paratype (ARC 81600). Oxea, presumably modifications of the styles, are also present but much less abundant than the styles (Figure 5G). These are of a similar form to the styles but usually slightly longer. Oxea measured 278(321)348 × 7(9)11 μm in the holotype (ARC 81544) and 236(297)343 × 7(9)11 μm in the paratype (ARC 81600). There are occasional very thin styles (1–2 μm thick present), presumably modifications of the other styles.
Remarks
This sponge was very common in the shallow waters around Ascension. It is eaten by turtles.
The other specimens listed here, but not as holotypes or paratypes, do vary in some characters, and therefore we have not assigned them as type specimens. However, all of these had similar skeletal forms and granules, so we think they are the same species. Additional sampling will help determine the level of variation in spiculation that occurs in Svenzea weberorum sp. nov.
ARC 81555 is very similar in appearance to the type specimens but is brown rather than orange in colour and has slightly shorter and narrower styles (228(264)284 × 7(8)9 μm). While oxea were present, they were scarce. ARC 81591 is yellow (a good photograph of external appearance was not available). It has a similar size range of styles (276(298)328 × 11(14)17 μm), but oxeas do not seem to be present. ARC 81588 also has styles of a similar, but slightly longer, length (290(316)348 × 9(11)14 μm), but while oxea were present, they were very rare. This specimen was not photographed.
There are six valid species of Svenzea (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Kelly, Vacelet, Dohrmann, Diaz, Cárdenas, Carballo, Ríos, Downey and Morrow2021; Table 4). Of these Svenzea zeai (Alvarez et al., Reference Alvarez, Van Soest and Rützler1998), Svenzea cristinae Alvarez et al., Reference Alvarez, Van Soest and Rützler2002 (Lehnert and Van Soest, Reference Lehnert and Van Soest1999), Svenzea germanyanezi Gómez and Calderón-Gutiérrez, Reference Gómez and Calderón-Gutiérrez2020 and Svenzea tubulosa (Alcolado & Gotera, Reference Alcolado and Gotera1986) have been recorded from the Atlantic or Caribbean. S. tubulosa can be distinguished by its tubular form and much larger styles. S. flava has ‘styloid’ spicules, mostly with two blunt ends, rather than true styles, and these are larger than found in our specimens (310–395 × 12–15 μm). S. cristinae has much larger styles (320–460 × 4–11 μm). S. germanyanezi is a small, cone-shaped, cave-dwelling sponge, with two categories of oxea, both of which are considerably larger than those of S. weberorum sp. nov. (390–490 × 9–10.6 μm and 325–410 × 1.8–5.5 μm). S. zeai has shorter styles and oxea and is a purple-brown sponge with volcano-shaped, oscular mounds up to 6 cm high.
Table 4. Comparison of currently valid Svenzea species. Information from original descriptions and Zea et al. (Reference Zea, Henkel and Pawlik2014)

This species is found in the north-eastern and western sectors of the island, on volcanic bedrock, boulders, and on the under hangs of vertical rocks.
Order TETRACTINELLIDA Marshall, 1876
Suborder ASTROPHORINA Sollas, 1887
Family GEODIIDAE Gray, 1867
Subfamily ERYLINAE Sollas, 1888
Genus Erylus Gray, 1867
Erylus williamsae Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:22A91166-24FB-4960-BB99-84806CDE1717
(Figure 6A–H)
Type Material
Holotype: ARC 81577, Red Rock Archway, Ascension Island, −7.89423° −14.3946°, depth 30 m, 6 September 2012, collected by Judith Brown.

Fig. 6. Erylus williamsae sp. nov. ARC 81577. (A) In vivo appearance. (B) Skeleton. (C) Oxea. (D) Microrhabd. (E) Orthotriaenes/Dichotriaenes. (F) Aspidaster. (G) Aspidaster surface. (H) Strongylasters.
Diagnosis
Erylus with oxeas 483(578)662 μm long. Microscleres include strongylasters 9(13)16 μm and microrhabds/microxea 38(47)54 × 3(4)6 μm.
Etymology
Named for Natasha Williams, Conservation Assistant for Ascension Island Government's Conservation Department who has been with the department since she left school on Ascension Island. Natasha played an important role in facilitating this study.
External Appearance
In vivo (Figure 6A): Small white lobe around 4 cm in length, 2.5 cm in width and 2 cm in height. Smooth surface with a single oscule on its apex.
Preserved: White in ethanol. Very firm texture.
Skeleton (Figure 6B)
Columns of oxeas in cortex 300–500 μm thick. Composed of orthotriaenes which join the ends of the columns of oxeas, interspersed with a dense layer of aspidasters.
Spicules
Large oxeas (Figure 6C): 483(578)662 × 12(17)25 μm. Some are true oxea, some strongylote (rounded ends), and occasionally there are stylote forms.
Orthotriaenes/Dichotriaenes (Figure 6E): Clades 200(242)284 μm, rhabdome 145(191)251 μm. In some one or more clades are bifurcate.
Microrhabds/microxea (Figure 6D): 38(47)54 × 3(4)6 μm. Rounded ends. Often centrotylote.
Aspidasters (Figure 6F, G): 80(111)146 × 4(62)69 μm. Uneven ovals. Some have slightly pointed ends, tending towards diamond shaped.
Strongylasters (Figure 6H): 9(13)16 μm. Seem to take two forms, both with 5–6 uneven rays. In one form rays are usually not branched, and one ray is significantly longer than the others. In the other form, the rays are shorter in comparison to the centrum, and each ray is divided into 2–5 segments at its end. There are also intermediate forms.
Remarks
This species can be distinguished from most other Erylus species, of which there are 68, based on its smaller megascleres and differing combinations of microscleres (see Vieira et al., Reference Vieira, Cosme and Hajdu2010) for a review, and Lehnert & Stone (Reference Lehnert and Stone2019) and Van Soest (Reference Van Soest2017) for subsequent descriptions of E. imperator Lehnert & Stone, Reference Lehnert and Stone2019, E. rhabdocoronatus, Van Soest, Reference Van Soest2017 and E. surinamensis Van Soest, Reference Van Soest2017. Those with similar spicule combinations are E. bahamensis Pulitzer-Finali, Reference Pulitzer-Finali1986, and E. corneus Boury-Esnault, Reference Boury-Esnault1973. Erylus bahamensis has larger oxeas (530–850 × 6–15 μm) and its asterose microscleres are tylasters which can be twice the size of those found in our specimen (15–28 μm). Erylus corneus can be distinguished as it has oxyasters (with pointed tips) rather than strongylasters. This species was found in a rocky environment in the northern sector of the island.
Subclass KERATOSA Grant, 1861
Order DICTYOCERATIDA Minchin, 1900
Family IRCINIDAE Gray, 1867
Genus Ircinia Nardo, 1833
Ircinia nolanae Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:47C13975-9EC1-4D8E-9B25-377033DA2E73
(Figure 7A–E)
Type Material
Holotype: ARC 81543, The Arches, Ascension Island, −14.41951667° −7.918116667°, depth 5 m, 25 August 2012, collected by Judith Brown.
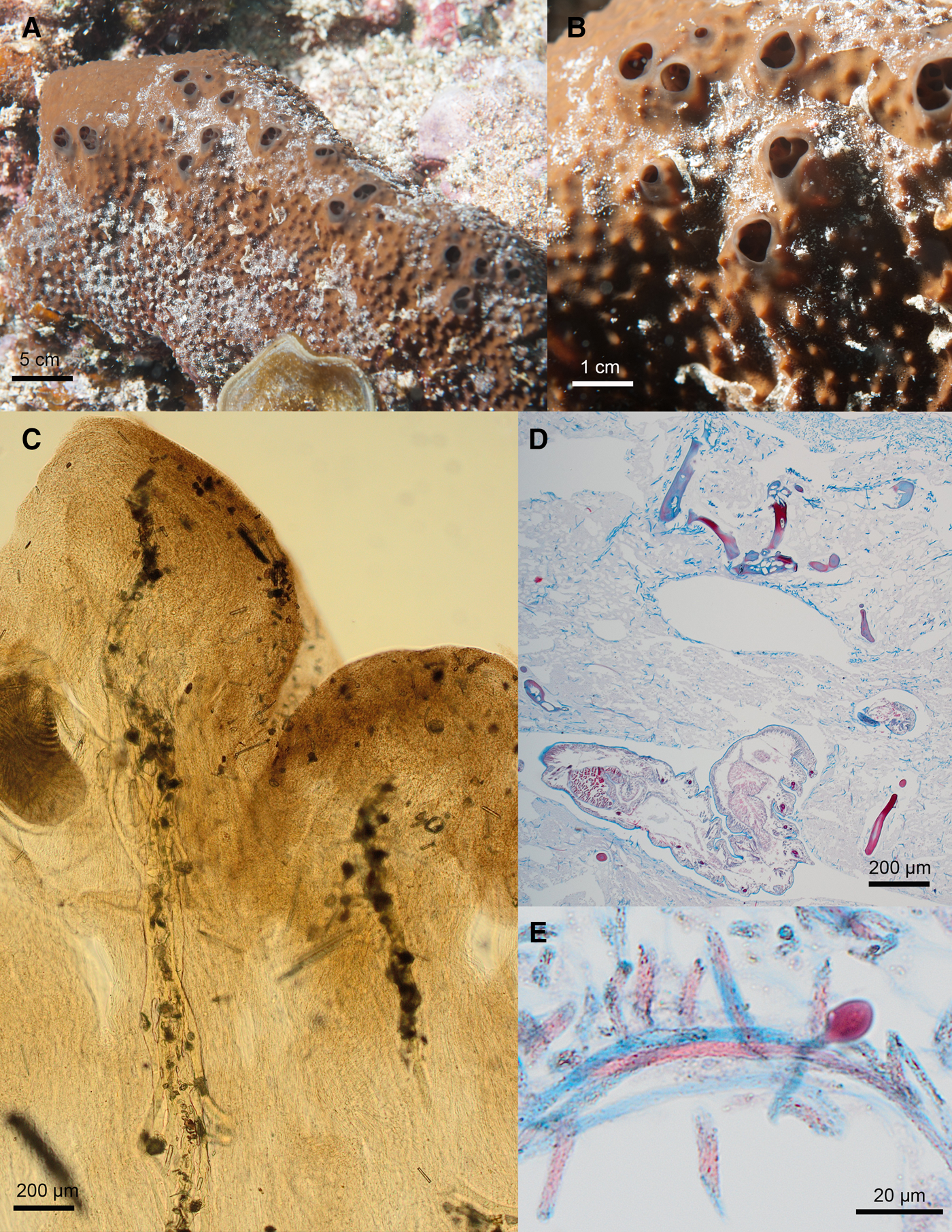
Fig. 7. Ircinia nolanae sp. nov. ARC 81543. (A) In vivo appearance. (B) Close up of surface. (C) Choanosomal skeleton. (D) Stained section of choanosome. (E) Ircinid filaments.
Paratype: ARC 81595, Red Rock, Ascension Island, −7.89435° −14.39475°, depth 14.7 m, 19 July 2015, collected by Paul Brickle and Stevie Cartwright.
Diagnosis
Massively encrusting Ircinia which is reddish-brown in vivo and takes the form of a massive (up to 1 metre in length) encrusting lobe. Large, fasciculate, primary fibres (100–160 μm width) cored with debris. Sparse secondary fibres (around 30–40 μm in width) with no or little debris.
Etymology
Named for Dr Emma Nolan, a marine scientist on the Ascension Island Marine Sustainability Project. Emma took part in the SMSG/SAERI Surveys and helped with the logistics.
External Appearance
In vivo (Figure 7A, B): Very large sponge forming massive lobes around 50 −100 cm in length and 5 −15 cm high. The form is typically an elongate, thickly encrusting lobe with a central ridge along which large oscules (up to 75 mm in diameter) are arranged. The colour is reddish-brown on the outside. The interior of the sponge, when cut, is beige. The surface of the sponge is covered with closely spaced, rounded conules up to 10 mm high.
Preserved: Firm but compressible lobe. Wrinkled, slightly shiny, exterior layer with obvious conules. The exterior is dark brown on the top and cream on the bottom of the sponge. Cream interior.
Skeleton (Figure 7C–E)
Large, fasciculate, primary fibres (100–160 μm width) cored with debris. Sparse secondary fibres (around 30–40 μm in width) with no or little debris join them at intervals. Spongin filaments are very abundant, especially in the ectosome. They are 3(4)5 μm in width on the shaft and have oval heads 6(7)10 μm wide. There is a light crust of debris in the ectosome.
Remarks
Many of the other species of Ircinia present in the region can be distinguished based on form, being globular: Ircinia strobilina (de Lamarck, Reference de Lamarck1816), cup-shaped Ircinia campana (de Lamarck, Reference de Lamarck1814); ramose: Ircinia dickinsoni (de Laubenfels, Reference De Laubenfels1936), Ircinia repens Sandes & Pinheiro, Reference Sandes and Pinheiro2014, Ircinia reteplana (Topsent, Reference Topsent1923); or thinly encrusting: Ircinia hummelincki Van Soest, 1978 (Table 5). Ircinia ectofibrosa (George & Wilson, Reference George and Wilson1919) is white in vivo. Those with similar thickly encrusting/lobular forms and, where known, a brown colour, are Ircinia felix (Duchassaing & Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864), Ircinia pauciarenaria Boury-Esnault, Reference Boury-Esnault1973; and Ircinia sergipana Sandes & Pinheiro, Reference Sandes and Pinheiro2014 (Table 5). Ircinia pauciarenaria Boury-Esnault, Reference Boury-Esnault1973 has much larger primary fascicular fibres (570–760 μm) and secondary fibres (lower width of 96 μm). In Ircinia sergipana Sandes & Pinheiro, Reference Sandes and Pinheiro2014 both the primary and secondary fibres are cored, and it has thinner filaments (2.5–5 μm with heads 5–7.5 μm). Ircinia felix (Duchassaing & Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864) is a common species in the Caribbean, known colloquially as the stinker sponge. It is typically globular or cake shaped rather than an elongate lobe, and has pronounced white conules linked with pale webbing, whereas the surface of our specimens is uniform in colour. Ircinia richardsoni sp. nov. is similar in form to this species but differs in having strongly cored secondary fibres and is black rather than brown.
Table 5. Comparison of currently valid Ircinia species from the Atlantic and Caribbean. Information from type descriptions except where stated otherwise

Species in ‘Incertae sedis’ have not been included: Ircinia marginalis (Duchassaing and Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864); Ircinia procumbens (Poléjaeff, Reference Poléjaeff1884), Ircinia tintinnabula (Duchassaing and Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864), Ircinia tristis (Duchassaing and Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864).
This species is common on bedrock in the shallow waters around the north and north-western side of Ascension.
Ircinia richardsoni Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:A99F0D6A-1B3E-49D7-A063-B51EEA7F16D8
(Figure 8A–E)
Type Material
Holotype: ARC 81552, Soudan Wreck, Ascension Island; −7.8876° −14.37621667°; depth 8 m, 27 August 2012; collected by Judith Brown.
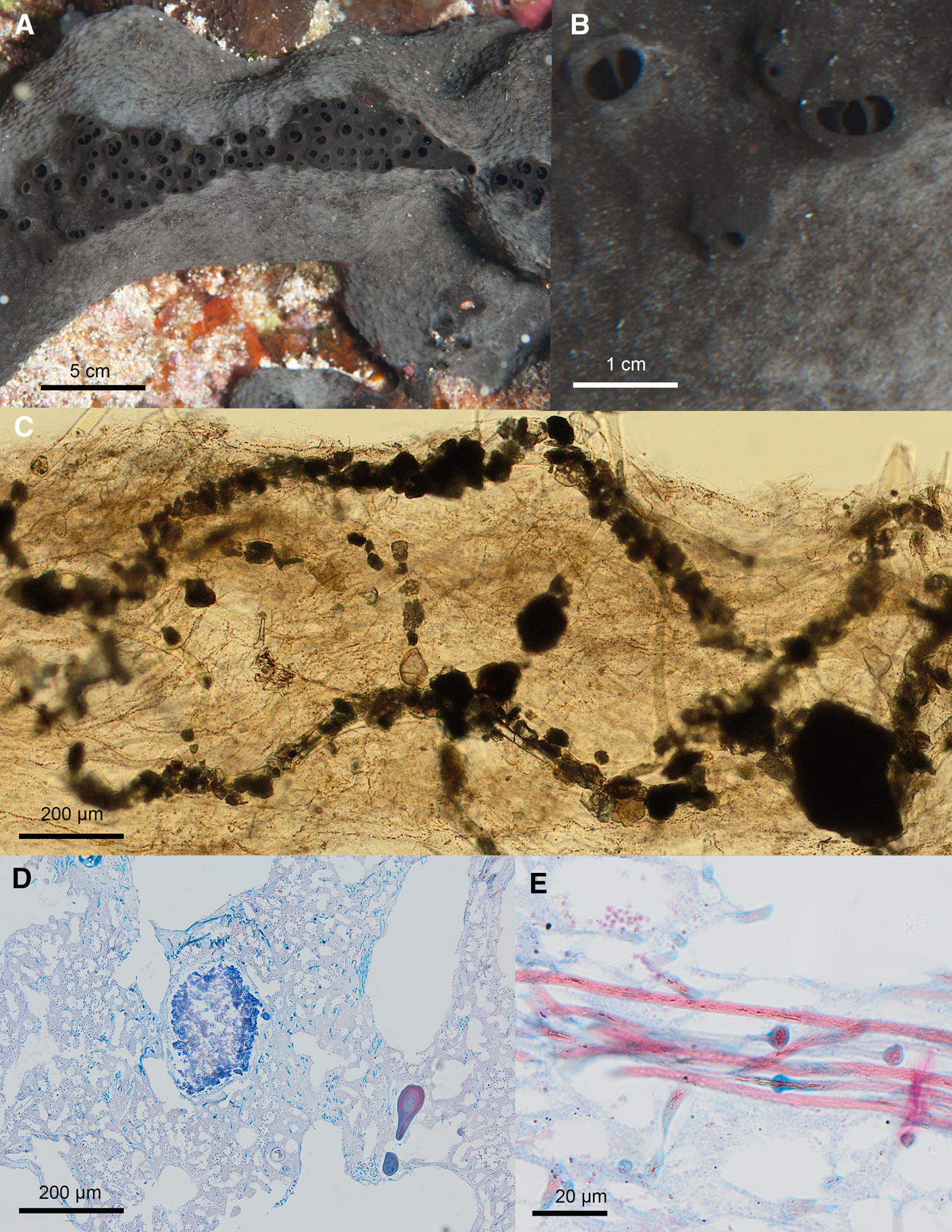
Fig. 8. Ircinia richardsoni sp. nov. ARC 81543. (A) In vivo appearance. (B) Close up of surface. (C) Choanosomal skeleton. (D) Stained section of choanosome. (E) Ircinid filaments.
Paratypes: ARC 81599 and ARC 81590 Red Rock, Ascension Island; −7.894233° −14.3946°; depth 8 m, 20 July 2015, collected by Stevie Cartwright.
Diagnosis
Ircinia with a massively encrusting form, black external colour in vivo, and small surface conules. Primary fibres (100–170 μm width) fasciculate and heavily cored with debris. Secondary fibres (40–60 μm wide) are also debris cored.
Etymology
Named for Dr Andrew (Andy) Richardson who was the Senior Marine and Fisheries Scientist on Ascension Island until 2019. Andy helped with surveys and sample collections and was significant in driving the AIMS (Ascension Island Marine Sustainability) Project.
External Appearance
In vivo (Figure 8A, B): Large sponge forming massive lobes up to 50 cm in length and 20 cm high. The form is typically an elongate lobe with a central ridge along which oscules (up to 50 mm diameter) are arranged. The colour is black on the outside. The interior of the sponge, when cut, is beige. The sponge's surface is covered with very small conules that appear only as slight bumps on the surface (unlike the more pronounced conules found in other species of the genus).
Preserved: In ethanol, dark brown exterior with conulose surface. Cream interior. Texture fairly soft and compressible.
Skeleton (Figure 8C, D)
Large, fasciculate, primary fibres (100–170 μm width) heavily cored with debris. Secondary fibres (40–60 μm wide) join them; these are also fairly heavily cored and, due to this, the distinction between primary and secondary fibres is not always clear. Spongin filaments (Figure 8E) are very abundant, especially in the ectosome. They are 3(3)4 μm in width on the shaft and have oval heads 5(6)7 μm wide. There is a light crust of debris in the ectosome.
Remarks
Many of the other species of Ircinia present in the region can be distinguished based on form, being globular, cup-shaped, or ramose (Table 5), see summary for Ircinia nolanae sp. nov. above. Those with similar thickly encrusting/lobular forms and, where known, a black or dark brown colour are Ircinia felix (Duchassaing & Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864), Ircinia pauciarenaria Boury-Esnault, Reference Boury-Esnault1973, and Ircinia sergipana Sandes & Pinheiro, Reference Sandes and Pinheiro2014. Ircinia pauciarenaria Boury-Esnault, Reference Boury-Esnault1973 has much larger primary fascicular fibres (570–760 μm) and secondary fibres (lower width of 96 μm). Ircinia felix (Duchassaing & Michelotti, Reference Duchassaing de Fonbressin and Michelotti1864) is typically globular or cake shaped rather than an elongate lobe. It has pronounced white conules linked with pale webbing, whereas the surface of our specimens is uniform in colour. Ircinia sergipana Sandes & Pinheiro, Reference Sandes and Pinheiro2014 is similar in that both its primary and secondary fibres are cored. However, it is much more strongly conulose than our specimens with conules up to 5 mm high. It is also a much smaller sponge; the type specimen is only 8 cm in width. Ircinia nolanae sp. nov. is similar in form to this species but differs in having uncored secondary fibres, being brown rather than black, and having much larger conules.
This species has been found on rocky environments in the northern sector of Ascension Island.
Ircinia simae Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:7B551BC9-C91C-43DC-8F9C-B9B484C4C8F7
(Figure 9A–G)
Type Material
Holotype: ARC 81559, Boatswain Bird Island, Ascension Island, −14.3081° −7.936766667°; depth 6 m, 1 September 2012, collected by Steve Brown.

Fig. 9. Ircinia simae sp. nov. (A) In vivo holotype ARC 81559. (B) Close up of ectosome ARC 81559 (note surface reticulation seen in upper half). (C) Choanosomal skeleton ARC 81559. (D) Ectosomal skeleton Paratype ARC 81561. (E) Ircinid filaments ARC 81559. (F) Paratype ARC 81561 In vivo. (G) Stained choanosomal section ARC 81559.
Paratype: ARC 81561 Boatswain Bird Island, Ascension Island, −7.936766667° −14.3081°; depth 6 m, 1 September 2012, collected by Steve Brown.
Other specimens: ARC 81560; ARC 81564 and ARC 81562, Boatswain Bird Island, Ascension Island, −7.936766667° −14.3081°; depth 6 m, 1 September 2012, collected by Steve Brown.
ARC 81580, Red Rock Archway, Ascension Island, −7.894233333° −14.3946°; depth 30 m, 6 September 2012, collected by Judith Brown.
ARC 81594, Red Rock, Ascension Island, −7.89435° −14.39475°; depth 14.7 m, 19 July 2015, collected by Paul Brickle and Stevie Cartwright.
ARC 81589, Red Rock, Ascension Island, −7.894233° −14.3946°; depth 16 m, 18 July 2015, collected by Paul Brewin and Jerry Pierce.
GenBank accession number for ARC 81559 MW488269; for ARC 81561 MW488270.
Diagnosis
Ircinia with white colour in vivo, strongly conulose surface, and massively encrusting form. Primary fibres are cored and strongly fasciculate with a width of 120–240 μm. Secondary fibres 40–80 μm and always uncored.
Etymology
Named for Jolene Sim who is the Conservation Officer at Ascension Island Government's Conservation Department. Jolene was instrumental in helping with the logistics of the SMSG/SAERI surveys that enabled the collection of these samples. Jolene has also done a phenomenal amount of work for conservation on Ascension Island.
External Appearance
In vivo (Figure 9A, B, F): Thickly encrusting white sponge forming patches up to 15 cm in diameter and 10 cm thick. In some specimens, the form is that of a low lobe. The surface is covered with dense, spiky conules, which often come to a fine point, giving it a hispid appearance. Occasional large oscules are scattered over the surface, sometimes on the apex of low mounds. In some specimens, the surface is tinged bright purple-pink, presumably due to symbiotic algae; this may be patchy or cover the entirety. In some specimens, the surface reticulation is visible, giving the sponge a webbed appearance when viewed closely.
Preserved: Cream with conulose surface. Texture soft and compressible.
Skeleton
Choanosome (Figure 9C, G): Primary fibres are strongly fasciculate with a width of 120–240 μm. The central core takes up around a third of the column. The secondary fibres join the primary columns at regular intervals; the fibres branch dendritically where they join the columns. They are also often fasciculate along their length, although less strongly than the primaries. They are much narrower than the primary fibres (40–80 μm) and always uncored.
Ectosome (Figure 9D): The ectosome has a thick crust of debris. In some patches, this forms meshes with uneven apertures. Spongin filaments (Figure 9E) are very dense, especially in the ectosome. They measure 4(4)5 μm on the shaft and 5(7)8 μm on the head.
Remarks
Very few other species of Atlantic and Caribbean Ircinia are pale coloured; the majority, where in vivo colour is known, are dark brown or black (Table 5). Ircinia ectofibrosa (George & Wilson, Reference George and Wilson1919) is light in vivo but is plate-shaped with cylindrical projections. The in vivo colours of Ircinia hummelincki Van Soest, 1978, Ircinia procumbens (Poléjaeff, Reference Poléjaeff1884), Ircinia reteplana (Topsent, Reference Topsent1923), Ircinia sergipana Sandes & Pinheiro, Reference Sandes and Pinheiro2014, and Ircinia repens Sandes & Pinheiro, Reference Sandes and Pinheiro2014 are not known, but they all differ in form.
The closest species seems to be Ircinia variabilis (Schmidt, Reference Schmidt1862) which is also thickly encrusting and often a white or pinkish colour, although it has also been recorded as yellow, brown, purple or greenish (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Kelly, Vacelet, Dohrmann, Diaz, Cárdenas, Carballo, Ríos, Downey and Morrow2021). The type locality of this species is in the Adriatic, and it is widespread in the Mediterranean and on southern European coasts. It has also been recorded from several oceanic Atlantic locations, including the Canaries, Cape Verde (Topsent, Reference Topsent1928) Gulf of Guinea (Burton, Reference Burton1956). It has been recorded from the Bahamas (de Laubenfels, Reference De Laubenfels1950), but these specimens differ in being an underlying blue-grey colour rather than white, and further investigation is needed to determine if they are conspecific. Images of Mediterranean specimens indicate that it appears to have a more strongly mounded form than our specimens with strong lobes with prominent terminal oscules that are often white ringed (Parco Nazionale Cinque Terre, 2019). Comparison with the lectotype figured in Pronzato et al. (Reference Pronzato, Malva, Manconi, Pansini, Pronzato, Bavestrello, Manconi and Sarà2004) shows that the primary fibres are less fasciculate and narrower than those found in our specimens with the core taking up a greater part of the fibre. The filaments are also wider than those found in our specimens (2–8 μm on shaft and 5–11 μm on their head). Comparison of our specimen (ARC 8155, GenBank MW488269) with a sequence from Ircinia variabilis from the Mediterranean (GenBank KX688725.1 (Idan et al., Reference Idan, Shefer, Feldstein, Yahel, Huchon and Ilan2018)) showed only 92.56% similarity; our specimens were more similar (98.72%) to Ircina campana (GenBank KC869531 (Thacker et al., Reference Thacker, Hill, Hill, Remond, Collins, Morrow, Spicer, Carmack, Zappe, Pohlmann, Hall, Diaz and Bangalore2013)).
This species was common in both the east and northern sectors of Ascension Island, under boulders and in a sea cave.
Subclass VERONGIMORPHA Erpenbeck, Sutcliffe, De Cook, Dietzel, Maldonado, Van Soest, Hooper & Wörheide, 2012
Order CHONDROSIA Boury-Esnault & Lopes, 1985
Family CHONDROSIIDAE Schulze, 1877
Genus Chondrosia Nardo, 1847
Chondrosia browningorum Goodwin & Downey, 2021 sp. nov.
urn:lsid:zoobank.org:act:EA4CF59E-1919-4632-8F75-ECACD8B4472C
(Figure 10A–E)
Type Material
Holotype: ARC 81575, Red Rock Archway, Ascension Island, −7.89423° −14.3946°; depth 30 m, 6 September 2012, collected by Judith Brown.

Fig. 10. Chondrosia browningorum sp. nov. (A) ARC 81575 In vivo. (B) ARC 81545 In vivo. (C) ARC 81575 Stained section showing cortex and choanosome. (D) ARC 81575 Sphaerulous cells. (E) ARC 81575 Choanocyte chambers.
Paratypes: ARC 81545, ARC 81550 and ARC 81547 Wigan Pier, Ascension Island, 7.89397° −14.3835°; depth 6.5–12 m, 25 August 2012, collected by Judith Brown.
GenBank accession number for ARC 81575: MW488271.
Diagnosis
An Atlantic species of Chondrosia with a cortex around 600–700 μm thick without inclusions, sphaerulous cells 1.2(1.6)1.7 μm in diameter and oval choanocyte chambers 30–40 μm in length and 17–23 μm in diameter.
Etymology
Named for Sarah Browning and Lt Col. Simon Browning (retd). Simon and Sarah are long-term members of SMSG and participated in all the SMSG/SAERI surveys to Ascension Island and collected material for this study. Simon was also instrumental with regards to the logistics for the surveys through the facilitation of shipping of dive gear, compressors and sample equipment on the MOD Atlantic airbridge.
External Appearance
In vivo: The holotype (Figure 10A) is light grey with patches of darker grey. It has prominent, slightly raised, oscules irregularly scattered over its surface. It forms a large patch up to 20 cm in diameter and 5 cm thick. The paratypes (Figure 10B) are smaller patches (around 5 cm maximum diameter and 2 cm thick) that are dark black. Presumably, as with other species of Chondrosia this difference can be attributed to light exposure, with specimens exposed to light becoming darker (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002). The texture of this sponge was very firm and rubbery, very tough to cut. The holotype was taken from an archway without much light exposure.
Preserved: Crust with a very smooth surface. In specimens that were black in vivo this colour is retained in the surface layer, the interior is grey. Texture very hard.
Skeleton (Figure 10C–E)
The cortex is around 600–700 μm thick with upper and basal layers of around 40–50 μm (Figure 10C). The sphaerulous cells (Figure 10D) are slightly more abundant in the cortex's upper layer but are also fairly numerous through the other cortex layers and in the choanosome. They occur in clusters of up to 11 but usually around 5–9. They are 1.2(1.6)1.7 μm in diameter. The choanocyte chambers (Figure 10E) are oval, 30–40 μm in length and 17–23 μm in diameter.
Remarks
Three species of Chondrosia have been recorded from the Atlantic or Caribbean: Chondrosia plebeja Schmidt, 1868; Chondrosia collectrix (Schmidt, Reference Schmidt1870) and Chondrosia reniformis Nardo, 1847 (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Kelly, Vacelet, Dohrmann, Diaz, Cárdenas, Carballo, Ríos, Downey and Morrow2021). C. reniformis is a massively encrusting species that can reach 30 cm in diameter. It is black when exposed to light and white when not exposed. It has a cortex 1–3 mm thick without inclusions (Lazoski et al., Reference Lazoski, Solé-Cava, Boury-Esnault, Klautau and Russo2001). Its sphaerulous cells contain about 20 spherules of about 3 μm in diameter, and it has choanocyte chambers 40 μm in diameter (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002). Boury-Esnault (Reference Boury-Esnault, Hooper and Van Soest2002) notes that only Chondrosia reniformis from the Mediterranean Sea, and the nearest Atlantic coasts (Spain, Portugal and Morocco) belong to this species. Specimens from localities outside the Mediterranean vary principally in the localization and abundance of foreign materials and sphaerulous cells and are certainly different species (Lazoski et al., Reference Lazoski, Solé-Cava, Boury-Esnault, Klautau and Russo2001). Our specimens differ in having a much thinner cortex and smaller sphaerulous cells.
C. plebeja was initially described from the coast of Algiers. It was re-described by Kirkpatrick from specimens from St Helena. Schmidt distinguished C. plebeja from C. reniformis on account of C. plebeja's irregular surface and the presence of foreign bodies on the surface and in the interior (Kirkpatrick, Reference Kirkpatrick and Cunningham1910). Kirkpatrick's specimen was mug shaped (14.5 × 13.5 × 3.5 cm), and in life, specimens were buff coloured with reddish-brown patches. The surface is irregular and deeply pitted due to the incorporation of foreign material. It has a very thin, delicate cortical layer. It has pear-shaped choanocyte chambers (30 × 24 μm) and a dendritic canal system in which the terminal axes end in numerous ‘bristle’ canals. Stephens (Reference Stephens1915) also noted this species from St Helena, also with a cup-shaped form and with abundant foreign material. Sphaerulous cells are not abundant. Wiedenmayer (Reference Wiedenmayer1977) believed C. plebeja was a synonym of C. reniformis, but Kirkpatrick's, and Stephens (Reference Stephens1915) specimens appeared to be distinct. Our specimens lack foreign material in their choanosome and lack the distinctive choanocyte chambers described by Kirkpatrick.
Chondrosia collectrix (Schmidt, Reference Schmidt1870) was described from Florida and is known from Bermuda, the Caribbean and Brazil. It has an irregular cortex 250 μm thick. Inclusions are sometimes present in its cortex and choanosome (Lazoski et al., Reference Lazoski, Solé-Cava, Boury-Esnault, Klautau and Russo2001). Wiedenmayer (Reference Wiedenmayer1977) describes it as cushion-shaped and up to 15 cm wide and 1.5 cm thick with a purplish brown to black colour. The colour can be obscured by large quantities of sand in the cortex. Our specimens differ from C. collectrix in having a much thicker cortex, without inclusions. A sequence of 28S from our specimen ARC 81575 only shows 93% similarity with Chondrosia collectrix from Panama GenBank specimen KC869640.1 (Thacker et al., Reference Thacker, Hill, Hill, Remond, Collins, Morrow, Spicer, Carmack, Zappe, Pohlmann, Hall, Diaz and Bangalore2013).
A further undescribed species is noted by Lazoski et al. (Reference Lazoski, Solé-Cava, Boury-Esnault, Klautau and Russo2001) with a smooth cortex 1–2 mm thick, with no inclusions. Specimens were taken from Brazil and Bermuda.
This species was found in rocky areas in the northern sector of Ascension Island.
Discussion
Before this investigation, taxonomic knowledge about shallow-water Ascension Island sponge fauna was limited and patchy (Hentschel, Reference Hentschel1914; Burton, Reference Burton1932; Van Soest, Reference Van Soest1990; Van Soest et al., Reference Van Soest, Beglinger and de Voogd2013, Reference Van Soest, Beglinger and de Voogd2014). Before 2012, a total of 17 species from 10 genera, and eight families, had been fully described for Ascension Island (previously identified species in the genera: Clathria, Dysidea, Monanchora, Ascandra, Arturia, Clathrina, Geodia, Pione, Mycale and Spongia). Hentschel's (Reference Hentschel1914) record of Ircinia variabilis (Schmidt, Reference Schmidt1862) is not included in this total as it was a drift specimen and therefore, this record is believed to be inaccurate (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Kelly, Vacelet, Dohrmann, Diaz, Cárdenas, Carballo, Ríos, Downey and Morrow2021). Some of the species-level identifications, specifically for Dysidea fragilis, and Spongia (Spongia) officinalis, are not yet confirmed as definitive Ascension Island sponge fauna. Therefore, these broadly Atlantic-distributed species, will need future work to determine their exact identity.
Many previously collected specimens have been preliminarily identified to genera, which include Crambe, Eurete, Aplysilla, Batzella, Callyspongia, Chalinula, Chelonaplysilla, Chondrosia, Cinachyrella, Clathrina, Cliona, Darwinella, Dendrilla, Halichondria, Hippospongia, Holopsamma, Hymeniacidon, Ircinia, Leucandra, Leucosolenia, Niphates, Penares, Plakina, Siphonodictyon, Spirastrella, Stronglyacidon, Svenzea, Sycon, Tedania, Terpios, Tethya and Haliclona, which adds 29 genera and 16 families to this pre-2012 list (Barnes, Reference Barnes2017; OBIS, 2021). In addition to this, there are many remaining specimen records that are only identified to Class or Phylum (Taylor & Irving, Reference Taylor and Irving1985; Irving, Reference Irving1989; Manning & Chace, Reference Manning and Chace1990; Barnes, Reference Barnes2017; OBIS, 2021). Operation Origin (Taylor & Irving, Reference Taylor and Irving1985; Irving, Reference Irving1989) collected 67 specimens which they hypothesized represented 47 different species, but only a limited number of these identifications for this material have been finalized and published (Van Soest, Reference Van Soest1990; Van Soest et al., Reference Van Soest, Beglinger and de Voogd2013, Reference Van Soest, Beglinger and de Voogd2014; BioPortal Naturalis, 2021; R. Van Soest, pers. comm. 2021).
This new investigation has identified nine additional new species, added two new genera, and one new family to what we previously knew about Ascension Island sponge fauna. Twenty-six species, plus additional higher taxonomic level records, from the shallow waters have now been described, and these occur in 45 genera and 29 families. Most records are from the Demospongiae Class; however, nine records are from the Calcarea Class, which represents four described species, and in totality, with genera level records, seven genera, and five families. One record represents the genus Eurete, which is in the Hexactinellida class, and one genus level record, Plakina, represents the Homoscleromorpha Class. This new investigation has more than doubled the number of previously known species for Ascension Island. Currently, at least 10 species are classed as endemics, including the nine described herein, and the species Mycale (Microciona) ascensionis. This means that close to 40% of the sponge species currently known from Ascension Island, are potentially found nowhere else in the world. Previous studies of Ascension Island's shallow marine fauna have generally found very low levels of endemic species (5–20%), such as in shrimps (Chace & Manning, Reference Chace and Manning1972), molluscs (Rosewater, Reference Rosewater1975), echinoderms (Pawson, Reference Pawson1978) and fish (Lubbock, Reference Lubbock1980). The limited number of endemics is believed to be caused by the island's young age, isolation, and the impact of competition and predation in sub-littoral environments (Irving, Reference Irving and Sheppard2013). However, the diversity and endemism of sponges could be due to their relative unpalatability and the large number of crevices and overhangs in shallow-water environments (Irving, Reference Irving and Sheppard2013).
Few Ascension Island sponge species have broad distributions that have been substantiated, with only five species in the World Porifera Database (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Kelly, Vacelet, Dohrmann, Diaz, Cárdenas, Carballo, Ríos, Downey and Morrow2021) verified by taxonomic experts. These broad ranging species from Ascension Island, have been found, although rarely, at the islands of St Helena, Cape Verde Islands, Azores, and the Senegalese coast. However, records of identified species from Bioportal Naturalis, which add an additional eight described species, indicate that many of these sponges have type localities at the islands of Cape Verde, Tenerife, the Azores, the Senegalese and Gulf of Guinean coast, and Mediterranean Sea (R. Van Soest pers. comm.; WoRMS, 2021). Two species (Mycale (Arenochalina) laxissima and M. (Carmia) microsigmatosa) have type localities around the islands, seas, and coasts of Brazil, the Caribbean, the island of Bermuda, and the remote islands of Trindade and Martim Vaz Islands, and two species (Geodia gibberosa and Ascandra atlantica) are distributed on both sides of the mid-Atlantic. These broader-ranging species generally place shallow-water sponges of Ascension Island into a stronger biogeographic affinity with the eastern tropical/sub-tropical side of the Atlantic Ocean. Ascension Island's oceanographic position indicates that it should be more greatly influenced by the westward-flowing Southern Equatorial Current from the western side of the African continent. However, only one sponge species, Clathria (Microciona) ascensionis Van Soest et al., 2013, is found at both the islands of St Helena and Ascension, that could have been enabled by this current. The limited connectivity between these two islands has also been found in the distributions of shallow-water Scleractinia corals (Zibrowius et al., Reference Zibrowius, Wirtz, Nunes, Hoeksema and Benzoni2014). Our results indicate a stronger affinity of Ascension Island to fauna found in the North Atlantic gyre, which could be due to the mixing of the cooler Canary Island current, and the Southern Equatorial Counter-Current in the mid-Atlantic Ocean (Den Hartog, Reference Den Hartog1989). Currently, Ascension Island sponge distributions appear to be at the southerly limit of this eastern subtropical North Atlantic distribution. Four species indicate, however, an affinity with the western Atlantic and Caribbean, which was found to be important for the biogeography of Ascension Island shallow-water zoantharians and scleractinians (Zibrowius et al., Reference Zibrowius, Wirtz, Nunes, Hoeksema and Benzoni2014; Reimer et al., Reference Reimer, Lorion, Irei, Hoeksema and Wirtz2017). This has been posited to be caused by either the presence of more species in the Brazilian coastal regions, compared to the west African, or the Atlantic Equatorial undercurrent, which flows from west to east to depths of less than 100 m, enabling population connectivity (Zibrowius et al., Reference Zibrowius, Wirtz, Nunes, Hoeksema and Benzoni2014; Reimer et al., Reference Reimer, Lorion, Irei, Hoeksema and Wirtz2017). The sponge biodiversity in many of these remote islands in the Atlantic Ocean is poorly known. Further research is needed on sponges from surrounding mid-Atlantic islands and South American and West African coastlines to determine biogeographic affinities with Ascension Island (Floeter et al., Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008).
Burton (Reference Burton1932) hypothesized that the presence of Spongia officinalis at Ascension Island demonstrated how oceanic islands might act as stepping stones in the colonization of new areas by sponges, as it explained the presence of species found in both Australia and the West Indies. He accepted wide variation in spicule sizes and forms within species resulting in much synonymization and belief that many sponge species were cosmopolitan (Burton, Reference Burton1932). With the advantages of in situ imagery and molecular analysis now available to us, it is becoming apparent that there is much undiscovered sponge biodiversity including many cryptic species (Klautau et al., Reference Klautau, Russo, Lazoski, Boury-Esnault, Thorpe and Solé-Cava1999; Xavier et al., Reference Xavier, Rachello-Dolmen, Parra-Velandia, Schönberg, Breeuwer and Van Soest2010). Populations of species separated by a wide geographic range are unlikely due to (when known), the often short-lived existence of lecithotrophic larvae in sponge species, which limits their dispersal capabilities (Maldonado, Reference Maldonado2006). Burton's specimens of Spongia officinalis should be re-examined, and their identification evaluated.
Limited knowledge of sponge fauna from the closest oceanic island, St Helena, has restricted our biogeographic understanding of how these islands’ fauna is potentially connected. Preliminary surveys do indicate that the sponge faunas differ. Several of the St Helena species are new to science, indicating this area may also have endemic species (Authors’ unpublished data; Brown 2014). The St Helena sponge fauna could be more influenced by the cooler Benguela Current from the South Atlantic gyre; this could explain limited faunal connectivity between these two islands. Currently, most sampling of Ascension Island has been in the northern sector. Therefore, future sampling in the south of the island could also improve our understanding of biogeographic connections with St Helena, other mid-Atlantic oceanic islands, and coastal regions in the South Atlantic's eastern sector.
Our findings indicate that more research is needed on Ascension Island sponge fauna due to the very high incidence of new species from the relatively small number of samples collected in this study. This should be undertaken at St. Helena too, to improve our knowledge base of Mid-Atlantic shallow-water sponge fauna. A more detailed comparison between sponge fauna from these two isolated oceanic islands would determine the biogeographic affinities and extent of endemism of the phylum. The entire Exclusive Economic Zone (EEZ) of Ascension Island was designated as a Marine Protected Area in 2019 (Ascension Island Government, 2020). Continued work will aid better management of risks to marine biodiversity in Ascension Island and the wider Mid-Atlantic region.
Acknowledgements
We are incredibly grateful to the following people who participated in the coastal fieldwork for the Darwin Challenge and Darwin Plus Awards: Sam Weber (Ascension Island Government), Nicola Weber (Ascension Island Government), Martin Collins (SMSG), Stephen Cartwright (SMSG), Wetjens Dimmlich (SMSG), Steve Brown (SMSG), Dion Poncet (SMSG), Juliet Hennequin (SMSG), Rob Mrowicki (SMSG), Vladimir Laptikhovsky (SMSG), Lt Col. Simon Browning (British Forces South Atlantic Islands/SMSG), Sarah Browning (SMSG), Jerry Pierce (SMSG), Simon Morley (British Antarctic Survey), Alexander Arkhikpin (Falkland Islands Government Fisheries Department), Zhanna Shcherbich (Falkland Islands Government Fisheries Department), Peter Wirtz (Universidade do Algarve), Konstantinos Tsiamis (Hellenic Centre for Marine Research), Frithjof Kuepper (University of Aberdeen), Pieter Van West (University of Aberdeen), Caz Young (Ascension Island Dive Club) and Jimmy Young (George Town, Ascension Island).
Thank you to the organization team of the 4th international workshop on the taxonomy of Atlanto-Mediterranean deep-sea and cave sponges (10–15 September 2018) which provided a useful forum to discuss taxonomic issues. Thanks particularly to participants Paco Cárdenas and Cristina Diaz for their helpful suggestions on these specimens during the workshop.
Financial support
Funding for this work came from a grant to the Shallow Marine Surveys Group (SMSG) and to Ascension Island Government and SAERI from the Darwin Initiative (EIDCF012; DPLUS021). The three expeditions were organized by SMSG and SAERI. We thank the Ascension Island Government, the staff at the Conservation Centre and the Ascension Island Dive Club for their logistical support, cooperation, accommodation and hospitality. We are very grateful to British Forces South Atlantic Islands for their logistical support.

















