Introduction
For several reasons over the past decade, the interest in (bovine) preantral ovarian follicle physiology has substantially increased. Firstly, there is a growing awareness that cohorts of preantral follicles constitute an untapped source of immature oocytes that might be available for reproductive purposes (Aerts & Bols, Reference Aerts and Bols2010). Mammalian ovaries contain a large stock of quiescent, immature follicles that is still believed to be limited at birth in most species, despite several reports on oogenesis after birth in mice and prosimian primates (Abir et al., Reference Abir, Fisch, Nitke, Okon, Raz and Ben Rafael2001; Johnson et al., Reference Johnson, Canning, Kaneko, Pru and Tilly2004; Bukovsky et al., Reference Bukovsky, Caudle, Svetlikova, Wimalasena, Ayala and Dominguez2005; Kerr et al., Reference Kerr, Duckett, Myers, Britt, Mladenovska and Findlay2006). While early follicular stages can develop and sustain oocyte growth, about 99.9% finally disintegrate following atresia (Oktem & Urman, Reference Oktem and Urman2010). Unfortunately, while in vitro culture of early immature follicles still depends on the development and future availability of culture techniques, primordial follicle culture has thus far only been routinely achieved in mice (Eppig & O’Brien, Reference Eppig and O’Brien1996). Although no recent substantial progress has been made on long-term in vitro culture techniques, a few reports have been published on successful short-term in vitro culture using bovine, caprine and ovine tissue (Cecconi et al., Reference Cecconi, Barboni, Coccia and Mattioli1999; Figueiredo et al., Reference Figueiredo, Rodrigues, Silva and Santos2011).
Secondly, preantral ovarian follicles can play a crucial role in restoring and preserving human female fertility. Fortunately, an increasing number of women survive ootoxic radiotherapy and chemotherapy treatments. As a result, there is an increasing demand for fertility preservation strategies based on cryopreservation of ovarian tissue combined with in vitro culture of isolated or in situ preantral follicles (Abir et al., Reference Abir, Nitke, Ben-Haroush and Fisch2006; Aerts et al., Reference Aerts, De Clercq, Andries, Leroy, Van Aelst and Bols2008a; Fabbri et al., Reference Fabbri, Pasquinelli, Keane, Mozzanega, Magnani, Tamburini and Venturoli2009). These follicles can be reintroduced subsequently to these patients to restore fertility. Although the risk of reintroducing cancer cells cannot be completely excluded, several live births have been reported in humans following autologous ovarian tissue transplantation (Donnez et al., Reference Donnez, Dolmans, Demylle, Jadoul, Pirard, Squifflet, Martinez-Madrid and van Langendonckt2004; Lee et al., Reference Lee, Yeoman, Battaglia, Stouffer, Zelinski-Wooten, Fanton and Wolf2004). Culture and transplantation of previously isolated follicles on the other hand can largely minimize the reintroduction of cancer cells. However, the availability of human ovarian tissue for research is extremely limited, hampering research progress in this field. Therefore, animal and ruminant models in particular have recently gained considerable importance as a possible alternative for human reproductive research (Campbell et al., Reference Campbell, Souza, Gong, Webb, Kendall, Marsters, Robinson, Mitchell, Telfer and Baird2003; Malhi et al., Reference Malhi, Adams and Singh2005; Petro et al., Reference Petro, Covaci, Leroy, Dirtu, De Coen and Bols2010).
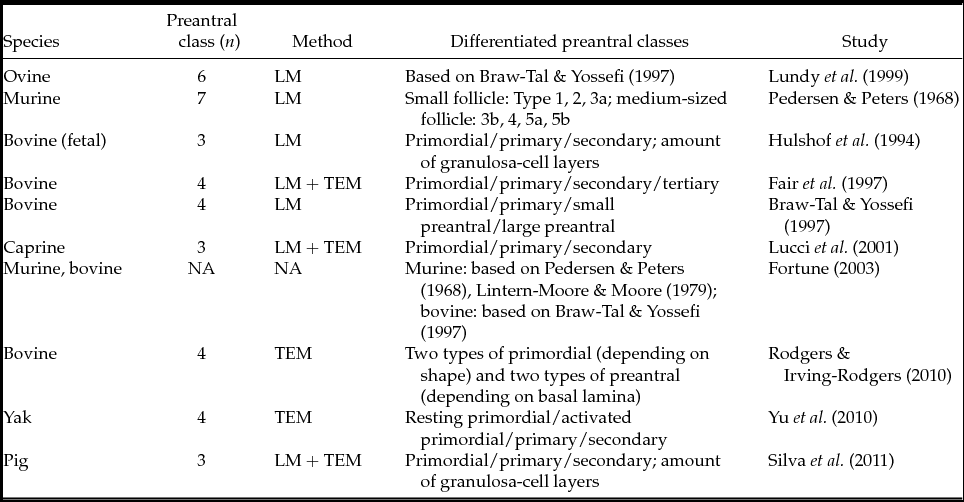
Isolation, processing and culture of early preantral follicles requires the development of non-invasive morphological identification and quality assessment protocols for follicles upon retrieval. Surprisingly, there is a lack of papers that specifically describes morphological classifications performed on freshly retrieved follicles – a prerequisite to study preantral follicular populations and determine their viability (Roy & Greenwald, Reference Roy and Greenwald1985; Greenwald & Moor, Reference Greenwald and Moor1989; Chambers et al., Reference Chambers, Gosden, Yap and Picton2010) for the purpose of immediate in vitro follicular culture. Current classification systems (Fair, Reference Fair2003) are nearly all based on light microscopy assessment of follicles in (HE)-stained sections (LM) (van Wezel & Rodgers, Reference van Wezel and Rodgers1996) or on invasive transmission electron microscopy (TEM) micrographs, as summarized in Table 1. While these techniques have defined generally accepted morphological characteristics, the use of invasive morphology and/or quality assessment methods renders the studied follicles useless for subsequent in vitro experiments, thereby troubling the link between morphology and developmental capacity. When it comes to follicle isolation, both mechanical and enzymatic methods (Figueiredo et al., Reference Figueiredo, Hulshof, Van den Hurk, Ectors, Fontes, Nusgens, Bevers and Beckers1993; Dolmans et al., Reference Dolmans, Michaux, Camboni, Martinez-Madrid, Van Langendonckt, Nottola and Donnez2006) are used randomly to free follicles from the surrounding stroma cells, the ultimate goal being to develop a fast and simple way to maximize viable preantral follicle yield (Hulshof et al., Reference Hulshof, Figueiredo, Beckers, Bevers and van den Hurk1994; Carambula et al., Reference Carambula, Goncalves, Costa, Figueiredo, Wheeler, Neves and Mondadori1999; Lucci et al., Reference Lucci, Amorim, Rodrigues, Figueiredo, Bao, Silva and Goncalves1999b, Reference Lucci, Rumpf, Figueiredo and Bao2002; Itoh & Hoshi, Reference Itoh and Hoshi2000; Gupta et al., Reference Gupta, Nandi, Ravindranatha and Sarma2001; Nayudu et al., Reference Nayudu, Fehrenbach, Kiesel, Vitt, Pancharatna and Osborn2001). Enzymatic digestion of small ovarian tissue fragments is reasonably fast, but it easily damages the basal membrane, impairing the follicle's viability (Figueiredo et al., Reference Figueiredo, Hulshof, Van den Hurk, Ectors, Fontes, Nusgens, Bevers and Beckers1993). Mechanical follicle isolation, on the other hand, is generally considered to be a safe but labour-intensive and time-consuming method (Figueiredo et al., Reference Figueiredo, Hulshof, Van den Hurk, Ectors, Fontes, Nusgens, Bevers and Beckers1993; Park et al., Reference Park, Lee, Park, Song and Chun2005).
Table 1 Classification of different stages in the development of preantral follicles according to different authors

LM: sectioned for light microscopy evaluation; TEM: ultrasectioned for transmission electron microscopy evaluation.
With the overall objective to describe features of freshly retrieved preantral follicles in order to develop relevant models and thus contribute to the knowledge of early preantral ovarian follicular physiology, we focused on three different ruminant species – ovine, caprine and bovine – aiming to: (1) define a standardized follicle isolation protocol for early preantral follicle retrieval, applicable to all three species, resulting in minimal damage to the retrieved follicles; (2) morphologically classify viable early preantral ruminant ovarian follicles immediately upon retrieval; and (3) briefly describe morphological features of freshly retrieved follicles in comparison with follicle characteristics reported using existing invasive assessment and classification systems.
Materials and methods
Collection of ovaries and processing of ovarian cortex fragments
All chemicals were purchased from Sigma® (Bornem, Belgium), unless stated otherwise. Ovaries were collected at a local slaughterhouse in batches of 10–40, depending on the availability of animals for slaughter. They were transported to the laboratory in physiologic saline solution (0.9% NaCl, 25°C) within 2 h after slaughter. Upon arrival, three to four early follicular stage ovaries were selected per species. Following removal of the meso-ovaric structures, the ovaries were washed three times in warm (37°C) physiologic solution supplemented with kanamycin (0.25%). After puncturing the visible antral follicles for other in vitro purposes, the ovarian cortex was peeled away, preferably in an area without antral follicular activity, using a scalpel and tweezers over a surface area of approximately 2 cm3. The ovarian cortex tissue was subsequently cut into pieces of approximately 1 mm3 (sample size) with a scalpel.
Ovarian cortex samples of all three species were treated in the same way. The samples of ovarian cortex were transferred to an isolation medium: minimum essential medium (MEM; Life Technologies®), supplemented with 10 mM HEPES (Life Technologies®) and 3 mg/ml bovine serum albumin (BSA), filtered through a 0.2 μm filter. Ovarian cortex tissue was not processed by a tissue chopper but subsequently mixed and dispersed with an Ultra Turrax T18 basic homogenizer (see Fig. 1; S18D-14G-KS dispersing element, IKA®, Germany) for 3 min at speed 1–1.5. The resulting follicle suspension was filtered through a 100 μm filter (BD Falcon®, USA) and the filtrate transferred into a conical tube to enable selecting of follicles in their early preantral follicular stage. To isolate the follicles from the stroma cell debris, the suspension was centrifuged for 10 min at 3.5 g after which the supernatant was discarded and the pellet was re-suspended in 5 ml of isolation medium and transferred into a large Petri dish. An inverted microscope (Olympus CKX 41; magnification: ×100–400) was used to find follicles.

Figure 1 Disperser to grind ovarian tissue samples into pieces. (a) Disperser. (b) The inner rotor rotates with a maximum circumferential speed of 12 m/s.
Viability testing of isolated early preantral follicles by neutral red staining
In five replicates per species, the follicle suspension (2.5 ml) was poured into a Petri dish with a grid scratched on the bottom to which 2.5 ml neutral red (NR; 0.05 mg/ml) containing medium was added. NR is a non-toxic, non-fluorescent dye, soluble in aqueous solvents. The staining is based on the ability of living cells to incorporate the dye in their lysosomes (Elliott & Auersperg, Reference Elliott and Auersperg1993; Chambers et al., Reference Chambers, Gosden, Yap and Picton2010) as a measure for cell viability. Metabolically active, living follicles stain positive for NR. At the onset of use of the dye, the concentration of the residual quantity of NR in the medium (Fotakis & Timbrell, Reference Fotakis and Timbrell2006) was determined by spectrophotometry (absorbance 540 nm).
The NR medium consisted of MEM + glutamine (Life Technologies®) supplemented with 2 mM hypoxanthine, 100 ng/ml penicillin/streptomycin (PenStrep; Life Technologies®), a mixture of insulin, transferrin and selenium (ITS; Life Technologies®, 8 μg/ml, 8 μg/ml and 8 ng/ml respectively), 50 ng/ml follicle stimulating hormone (FSH; Merck-Serono®) and 1.25 mg/ml BSA. The medium was filtered through a 0.2 μm filter and equilibrated for at least 2 h before use. The final solution thus had a concentration of 0.025 mg/ml. The cell suspension was incubated in NR medium for 2 h at 38.5°C.
Morphological characterization and viability assessment of early preantral follicles: methodology
Following 2 h of incubation in the presence of NR, another search was made for preantral follicles at ×100 and they were morphologically classified by visual assessment using an inverted phase-contrast microscope (Olympus CKX 41; magnification: ×400) and the method described by Hulshof et al. (Reference Hulshof, Figueiredo, Beckers, Bevers and van den Hurk1994), albeit the latter used histological slides, because no routinely available classification system exists for fresh follicles. Granulosa-cell gross morphology (flattened-cuboidal) and the number of granulosa-cell layers were evaluated as follows. Pictures were taken (magnification ×400) of each individual follicle and these were subsequently classified as ‘primordial’ (one layer of flattened granulosa cells), ‘primary’ (one layer of cuboidal granulosa cells) or ‘secondary’ (at least two layers of cuboidal granulosa cells; see Fig. 2). Hereafter, two perpendicular diameters were measured on each follicle and the calculated mean was used as outcome parameter ‘follicle diameter’ (see Table 3 and Fig. 2). Finally, 100 early preantral follicles (minimal five replicates per species, see above), were selected randomly, irrespective of follicle class, to determine the percentage of viable follicles based on NR positive staining. It is important to note that first, the follicles were classified on the basis of the morphological appearance of the follicular cells and only then the follicle diameters were measured.

Figure 2 Representative images of different classes of preantral follicles of different ruminant species following a mechanical isolation procedure. Perpendicular diameters are measured as indicated on the secondary ovine follicle. (a) Primordial follicles; (b) primary follicles; (c) secondary follices. Scale bar = 20 μm.
Statistics
Differences in follicle diameter among species and developmental stages were analyzed statistically using a two-way analysis of variance (ANOVA), with the logarithm of the diameter as outcome, and follicular stage and species as independent (categorical) variables. To check whether the differences in diameter among developmental stages were uniform across species, a model with an interaction term (species*stage) was fitted. Significance of the interaction term was tested via an F-test, comparing the fit of this latter model to a model containing only the main effect terms. Significance of the terms in the main effect model was also tested using an F-test. Calculations were performed using the statistical software package R, version 2.13.1 (www.r-project.org). Data are expressed as means ± standard deviation (SD). P-values < 0.05 were considered as indicative of significant difference among groups.
Results
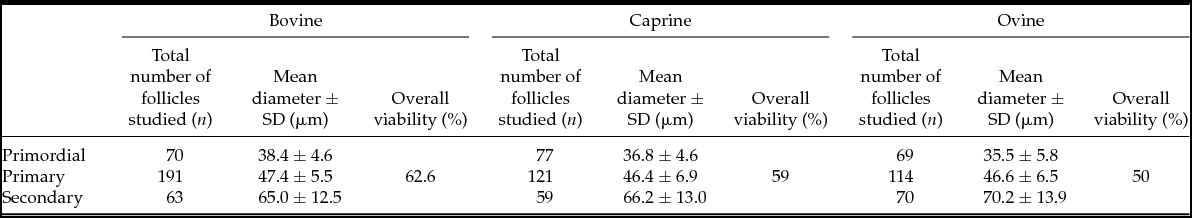
Follicles could be routinely isolated from ovarian tissue in the three ruminant species studied using the same mechanical isolation protocol through dispersion of ovarian tissue samples. Between 59 and 191 early preantral follicles were studied in a single specific morphological class, over the three species (see Table 2). Representative pictures of different morphological classes of early preantral follicles retrieved from bovine, caprine and ovine ovarian cortical tissue following mechanical isolation are presented in Fig. 2. Positive and negative NR stained follicles for the bovine species are demonstrated in Fig. 3. Descriptive characteristics such as the mean follicle diameter per morphological class for all three species as well as the percentages of viable follicles per class are summarized in Table 2.
Table 2 Total number of follicles studied, mean diameters ± SD and percentage viability (assessed by neutral red staining) of different classes of follicles in three different species

SD, standard deviation.
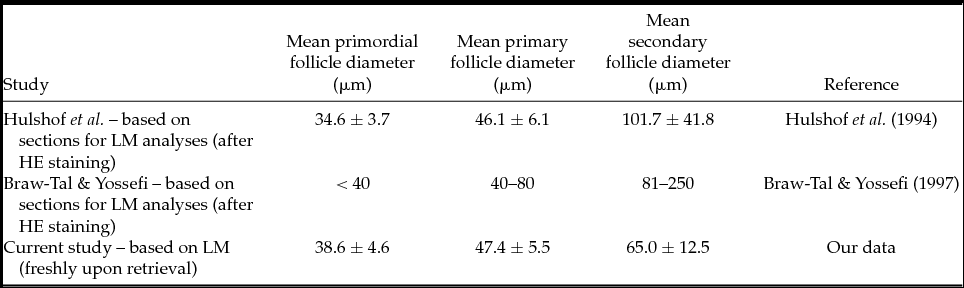
Table 3 Comparison of the mean bovine primordial, primary and secondary follicle diameters among different authors

HE: haematoxylin–eosin; LM: sectioned for light microscopy evaluation.

Figure 3 Representative images of positive and negative NR-stained bovine preantral follicles following a mechanical isolation procedure as described in Materials and methods. (a) Primordial follicles; (b) primary follicles; (c) secondary follices. Positive staining (NR+: left-hand panels) and negative staining (NR−: right-hand panels). Scale bar = 20 μm.
Across all species, there was a significant increase in follicular diameter with developmental stage (Fig. 4). A two-way ANOVA analysis indicated that these differences are not entirely uniform across all three species (P = 1.5 × 10−4 for interaction between class and species). In secondary follicles, the largest diameters were observed in sheep and the smallest in cows, whereas this order was inverted in the case of primordial follicles. Limiting the two-way ANOVA to main effects, a highly significant difference in diameter was observed among the three developmental stages (P < 10−16) across the three species. However, no disparity in diameter could be detected among the three species across the developmental stages (P = 0.22, Fig. 4). On average, 57% of all follicles retrieved stained positive, indicating they probably were alive and viable based on the uptake of the NR stain (Table 2).

Figure 4 Mean diameter by stage and species. The lines show the mean diameter for the three developmental classes, separately for bovine (bo: black), caprine (cap: red) and ovine (ov: green) species. Error bars denote standard errors of the mean.
Discussion
As described above, we successfully applied the same mechanical follicle isolation protocol on the ovarian cortex tissue of three ruminant species allowing us to isolate viable early preantral follicles through tissue dispersion. In addition, the follicles were classified immediately upon retrieval, first using morphological characteristics of the granulosa cells and only then determining the follicular diameter and viability. In the discussion below, we will address the necessity of non invasive follicle quality assessment including the differences, similarities, advantages and disadvantages between, from the follicle point-of-view, invasive and non-invasive – fresh upon retrieval – follicle classification systems with subsequent in vitro follicle culture in mind.
The need for morphological characterization of biological entities in (assisted) reproduction, following isolation, retrieval and/or culture, has been recognized for decades, whether it be follicles, cumulus–oocyte complexes (Hazeleger et al., Reference Hazeleger, Hill, Stubbing and Walton1995; Mayes & Sirard, Reference Mayes and Sirard2001) or in vitro-produced embryos (Lindner & Wright, Reference Lindner and Wright1983). As it was not our intention to compare different follicle isolation protocols, we used mechanical isolation, which is assumed to cause the least damage to the follicles depending on the studied species (Roy & Greenwald, Reference Roy and Greenwald1996), including equine (Haag et al., Reference Haag, Magalhaes-Padilha, Fonseca, Wischral, Gastal, King, Jones, Figueiredo and Gastal2013), pig (Greenwald & Moor, Reference Greenwald and Moor1989), hamster (Roy & Greenwald, Reference Roy and Greenwald1985), mouse (Demeestere et al., Reference Demeestere, Delbaere, Gervy, Van Den Bergh, Devreker and Englert2002), bovine (Itoh & Hoshi, Reference Itoh and Hoshi2000), ovine and caprine (Lucci et al., Reference Lucci, Amorim, Rodrigues, Figueiredo, Bao, Silva and Goncalves1999b). We did investigate whether this technique would be applicable to the ruminants studied, as the most important factor for choosing mechanical or enzymatic digestion is the density of the ovarian collagen matrix (Aerts et al., Reference Aerts, Martinez-Madrid, Leroy, Van Aelst and Bols2010; Park et al., Reference Park, Lee, Park, Song and Chun2005). While in goat and sheep (Lucci et al., Reference Lucci, Amorim, Bao, Figueiredo, Rodrigues, Silva and Goncalves1999a; Amorim et al., Reference Amorim, Rodrigues, Lucci, Figueiredo and Goncalves2000) the mechanical approach often is used, this is not the case in species with a dense collagenous matrix, such as humans and cattle (Figueiredo et al., Reference Figueiredo, Hulshof, Van den Hurk, Ectors, Fontes, Nusgens, Bevers and Beckers1993; Lucci et al., Reference Lucci, Rumpf, Figueiredo and Bao2002), where it is considered a difficult and time-consuming method (Dolmans et al., Reference Dolmans, Michaux, Camboni, Martinez-Madrid, Van Langendonckt, Nottola and Donnez2006; Aerts et al., Reference Aerts, Martinez-Madrid, Flothmann, De Clercq, Van Aelst and Bols2008b). Although enzymatic digestion has proven to be effective in the latter (Dolmans et al., Reference Dolmans, Michaux, Camboni, Martinez-Madrid, Van Langendonckt, Nottola and Donnez2006; Xu et al., Reference Xu, West-Farrell, Stouffer, Shea, Woodruff and Zelinski2009; Abir et al., Reference Abir, Roizman, Fisch, Nitke, Okon, Orvieto and Ben Rafael1999), it requires an elaborate search for customized digestion protocols, taking into account species differences and the various enzymes that could be used (collagenase, DNase, liberase, etc.). In addition, enzymatically isolated human primordial and primary follicles are known to deteriorate and exhibit oocyte release, which has been attributed to compromised follicle integrity (Abir et al., Reference Abir, Roizman, Fisch, Nitke, Okon, Orvieto and Ben Rafael1999; Hovatta et al., Reference Hovatta, Wright, Krausz, Hardy and Winston1999). Bovine follicles are reported to be especially vulnerable when the tissue is digested by collagenase Ia (Figueiredo et al., Reference Figueiredo, Hulshof, Van den Hurk, Ectors, Fontes, Nusgens, Bevers and Beckers1993), which is apparently not the case for human and murine tissue (Eppig & O’Brien, Reference Eppig and O’Brien1996). Conclusively, the mechanical isolation protocol, based on tissue dispersion and suspension filtration, was shown to be applicable for all three species, regardless of cortical tissue composition.
While it was beyond the aim of the current study to compare viability assessment methods, we have used NR as a non toxic viability indicator to generate descriptive preliminary data on follicle survival. This allowed us to successfully select viable follicles in a non invasive way for subsequent in vitro culture (Nottola et al., Reference Nottola, Cecconi, Bianchi, Motta, Rossi, Continenza and Macchiarelli2011). As the latter is not available yet for ruminant preantral follicles, we could not use it as a viability test as such, because it was impossible to distinguish culture and follicle quality effects. On average, about 60% of the isolated follicles incorporated NR in their lysosomes, indicating they were alive and viable. However, this might be an underestimation, because it is known that primordial follicles are less metabolically active, which can hamper the uptake of NR. Chambers et al. (Reference Chambers, Gosden, Yap and Picton2010) reported, on average, 50% positively stained follicles in situ in fresh ovine ovarian tissue fragments, which they validated by preparing HE coloured slides. The current experiment used suspensions of isolated preantral follicles, technically hampering the subsequent validation through HE slides. Isolated follicles indeed are difficult to process and prepare for HE staining without changing morphological characteristics. While previous research confirmed that NR stains viable follicles in a reversible way, it can be assumed that this dye is not detrimental to tissue and apparently does not compromise in vitro culture (Chambers et al., Reference Chambers, Gosden, Yap and Picton2010), which is a crucial advantage for the use of NR. Red coloured follicles are considered viable, as they also stain positive for CFDA-SE (Chambers et al., Reference Chambers, Gosden, Yap and Picton2010). Nevertheless additional studies are needed to monitor the developmental competence of NR-positive follicles under short-term as well as under long-term in vitro culture conditions.
Uniform follicle classification systems permit comparisons among species and/or laboratories, subsequently allowing the development of quality assessment methods. Surprisingly, there is a scarcity of recent papers (Figueiredo et al., Reference Figueiredo, Hulshof, Van den Hurk, Nusgens, Bevers, Ectors and Beckers1994; Hulshof et al., Reference Hulshof, Figueiredo, Beckers, Bevers and van den Hurk1994) describing morphological characteristics of freshly retrieved follicles. While, for obvious reasons, invasive quality assessment methods cannot be used to select follicles for subsequent in vitro culture or cryopreservation, the currently available classification systems do use stained sections (HE) from fixed ovaries, as illustrated in cattle (Braw-Tal, Reference Braw-Tal2002; Rodgers & Irving-Rodgers, Reference Rodgers and Irving-Rodgers2010), rat (Mandl & Zuckerman, Reference Mandl and Zuckerman1952) and mouse (Pedersen & Peters, Reference Pedersen and Peters1968; Torrance et al., Reference Torrance, Telfer and Gosden1989), goat (Lucci et al., Reference Lucci, Silva, Carvalho, Figueiredo and Bao2001), dog (Durrant et al., Reference Durrant, Pratt, Russ and Bolamba1998) and guinea pig (Roy & Greenwald, Reference Roy and Greenwald1985) (for an overview, see Table 1). We used the system proposed by Braw-Tal & Yossefi (Reference Braw-Tal and Yossefi1997) and Pedersen & Peters (Reference Pedersen and Peters1968), as a basis for follicle grading upon retrieval. In addition, Fair (Reference Fair2003) defined a ‘primordial’ follicle as an oocyte surrounded by a single layer of flattened (pre-)granulosa cells, while primary oocytes initiated growth and showed a single layer of cuboidal granulosa cells (Hulshof et al., Reference Hulshof, Figueiredo, Beckers, Bevers and van den Hurk1994). When granulosa cell layers start to multiply, and the formation of the zona pellucida has started, the follicle is denominated ‘secondary’, i.e. the final preantral stage. Apart from the major disadvantage that invasive classification renders the follicles useless for in vitro culture, it has been reported that fixation and subsequent processing can cause shrinkage of tissue and imbedded follicles (Chambers et al., Reference Chambers, Gosden, Yap and Picton2010), which challenges morphology assessment. However, as summarized in Table 3, our data do not support this concern when invasive (Hulshof et al., Reference Hulshof, Figueiredo, Beckers, Bevers and van den Hurk1994) and low-invasive morphology assessment strategies (our data) are compared. In line with previous studies (Rajakoski, Reference Rajakoski1960; Turnbull et al., Reference Turnbull, Braden and Mattner1977), we report a significant difference among the three early preantral follicle stages across the three species, but not a significant difference among the three species across the developmental stages. In other words, freshly retrieved early preantral ruminant follicles differ enough to be fairly easily allocated to a developmental stage, based on their morphological characteristics upon retrieval. As expected, our results show that irrespective of the species, the follicle diameter increased with the developmental stage. It should be stressed that, to obtain these results, granulosa-cell morphology was used as the first characteristic to allocate a certain follicle to a specific category and only secondarily, the follicle diameter was measured. Interestingly, as shown by the significance of the interaction term (Fig. 4) and, in the extent of our knowledge, hitherto never demonstrated on histological sections, the difference in follicular diameter between primordial and secondary follicles in sheep is larger as compared with cattle and goats, probably indicating a slightly different timing of early preantral follicle growth across species.
In conclusion, we have defined and applied a mechanically based, low-invasive, early preantral follicle isolation method that is applicable to three ruminant species and routinely results in the retrieval of viable follicles. By doing so, we were able to describe and classify the freshly isolated follicles based on generally accepted morphological characteristics deducted from invasive assessment protocols. In addition, the proposed isolation and classification protocol is compatible with fertility preservation strategies, as follicles turned out to be viable, based on NR staining.
Acknowledgements
The authors thank Silke Andries and Els Merckx for their excellent technical assistance and the local slaughterhouses for their cooperation in sample collection. We also thank the staff of the LAMOFOPA laboratory (Brazil) and Prof. Dr. R. Figueiredo for their cooperation and training possibilities offered.
Financial support
This research was funded by the University of Antwerp, but received no specific grant from any funding agency, commercial, or not-for-profit sectors. E.P.A. Jorssen acknowledges support from a Research Grant from the Belgian Government (Federale Overheidsdienst Volksgezondheid, Veiligheid van de Voedselketen en Leefmilieu, Cel Contractueel Onderzoek) ‘Embryoscreen RF6222’.
Conflict of interest declaration
None.