INTRODUCTION
Investigations of structure in the communities of ectoparasites infesting a host individual (hereafter known as infracommunities) in terrestrial ecosystems consistently demonstrated that these communities are non-randomly assembled (Krasnov et al. Reference Krasnov, Stanko and Morand2006a , Reference Krasnov, Stanko, Khokhlova, Mosansky, Shenbrot, Hawlena and Morand b ; Tello et al. Reference Tello, Stevens and Dick2008; Presley, Reference Presley2011). In most cases, this non-randomness is reflected in the aggregative structure of ectoparasite infracommunities. In particular, analyses of ectoparasite co-occurrences using null models demonstrated that different ectoparasites species co-occurred on the same host individual significantly more often than expected by chance (Krasnov et al. Reference Krasnov, Stanko and Morand2006a ; Presley, Reference Presley2011). In other words, ectoparasites are characterized by positive co-occurrences on the same host. Comparison of infracommunity structure of various ectoparasites harboured by the same host showed that flea infracommunities demonstrated a higher degree of interspecific aggregation than lice, mites or ticks (Krasnov et al. Reference Krasnov, Matthee, Lareschi, Korallo-Vinarskaya and Vinarski2010). Furthermore, flea communities consistently demonstrated aggregative structure not only at the scale of infracommunities, but also at the component (xenocommunity; an assemblage of parasites of all species infesting a population of a host belonging to a particular species) (Krasnov et al. Reference Krasnov, Stanko, Khokhlova, Mosansky, Shenbrot, Hawlena and Morand2006b , Reference Krasnov, Shenbrot and Khokhlova2011) and compound (an assemblage of parasites of all species infesting all co-occurring host species) community scales (Krasnov et al. Reference Krasnov, Mouillot, Shenbrot, Khokhlova and Poulin2005a ). For example, the abundance of an individual flea species in a component community or a compound community was found to correlate positively with the abundance of all other co-occurring flea species (Faulkenberry and Robbins, Reference Faulkenberry and Robbins1980; Krasnov et al. Reference Krasnov, Mouillot, Shenbrot, Khokhlova and Poulin2005a ; Brinkerhoff et al. Reference Brinkerhoff, Markeson, Knouft, Gage and Montenieri2006; see also Presley, Reference Presley2007 for streblid bat flies).
Although positive co-occurrences of different ectoparasites (in particular, fleas) has often been reported from census data (see above), the mechanisms behind this interspecific aggregation remained unclear. It has been suggested (but never tested experimentally) that the mechanisms of positive co-occurrence of ectoparasites on an individual host include not only obvious shared preferences of different parasites but also apparent facilitation (sensu Levine, Reference Levine1999) mediated via the host. Indeed, a host defends itself actively against a parasite using various behavioural and immunological tools. However, multiple challenges from a variety of parasite species may suppress host defence systems (Bush and Holmes, Reference Bush and Holmes1986; Cox, Reference Cox2001). Moreover, energetic and/or nutritional costs of the immune response are high (Demas and Nelson, Reference Demas and Nelson1998), so mounting different types of immune responses will likely be more costly than mounting one specific type of response (Taylor et al. Reference Taylor, Mackinnon and Read1998). As a result, an increase in the diversity of parasite attacks leads to a decrease in the effectiveness of energy allocation to immune defence (Jokela et al. Reference Jokela, Schmid-Hempel and Rigby2000), so that the optimal strategy for a host subjected to multiple parasite challenges would be to tolerate damage and give up its defence (Jokela et al. Reference Jokela, Schmid-Hempel and Rigby2000). From a parasite's perspective, this would be reflected in higher abundance of each parasite species that, in turn, would be caused by higher reproductive performances of parasites co-infesting a host. In other words, ectoparasites co-infestation is expected to result in an increase of their fitness in terms of either quantity or quality of the offspring or both. In this study, we tested this hypothesis using fleas Xenopsylla ramesis and Parapulex chephrenis parasitic on rodent hosts, Meriones crassus and Acomys cahirinus, in either single-species or mixed-species groups.
Both rodents are common species in the Negev desert of Israel. Meriones crassus is parasitized by several flea species among which the generalist X. ramesis is one of the most common (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova, Degen and Rogovin1996, Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). Host specialist P. chephrenis is a characteristic flea of Acomys cahirinus and a congeneric species Acomys russatus, whereas fleas of other species rarely attack it (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). Nevertheless, X. ramesis was sometimes recorded on A. cahirinus and P. chephrenis was sometimes recorded on M. crassus (G.I Shenbrot and B.R. Krasnov, unpublished data).
In our experiments, we tested reproductive performance of fleas when they exploited their characteristic host either alone or together with another flea species. We used egg production, the number of imagoes of a new generation, pre-imaginal survival and size of eggs as fitness-related variables and predicted that the values of all these variables will be higher in fleas feeding on a host in mixed-species than in single-species groups.
MATERIALS AND METHODS
Fleas and rodents
We used fleas (X. ramesis and P. chephrenis) and rodents (M. crassus and A. cahirinus) from our laboratory colonies. These colonies started from field collected specimens in 1999. For the sake of maintaining genetic diversity of the laboratory populations, we annually (starting in 2004) added 100–150 fleas of each species and 5–10 rodents of each species captured in the wild to respective colonies. Fleas were maintained on their natural host species, namely X. ramesis on M. crassus and Gerbillus dasyurus and P. chephrenis on A. cahirinus and Acomys russatus. Details of breeding and maintenance of flea and rodent colonies can be found elsewhere (e.g. Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001, Reference Krasnov, Khokhlova, Oguzoglu and Burdelova2002, Reference Krasnov, Sarfati, Arakelyan, Khokhlova, Burdelova and Degen2003; Khokhlova et al. Reference Khokhlova, Spinu, Krasnov and Degen2004, Reference Khokhlova, Ghazaryan, Krasnov and Degen2008, Reference Khokhlova, Pilosof, Fielden, Degen and Krasnov2014; Sarfati et al. Reference Sarfati, Krasnov, Ghazaryan, Khokhlova, Fielden and Degen2005). In this study we used newly emerged fleas 24–48 h old that have not fed prior to experiments and 6–8 month-old male rodents that have never been exposed to fleas prior to experiments.
Experimental design and procedures
The focus of our study was the effect of co-infestation on flea fitness. Consequently, reproductive performance of each flea species in single-species or mixed-species groups was tested when a flea exploited its characteristic host. In other words, reproductive performance of X. ramesis was evaluated when it exploited M. crassus either alone or together with P. chephrenis, whereas reproductive performance of P. chephrenis was evaluated when it exploited A. cahirinus either alone or together with X. ramesis. We did not measure reproductive variables in fleas fed on non-characteristic hosts (i.e. X. ramesis on A. cahirinus and P. chephrenis on M. crassus) intentionally because of the strong effect of host identity on reproductive performance of fleas with substantial decrease in, for example, egg production when fleas use non-characteristic host that is distantly related (but not too distantly; see Krasnov et al. Reference Krasnov, Korine, Burdelova, Khokhlova and Pinshow2007) to their principal host (e.g. Krasnov et al. Reference Krasnov, Sarfati, Arakelyan, Khokhlova, Burdelova and Degen2003; Khokhlova et al. Reference Khokhlova, Fielden, Degen and Krasnov2012), all else being equal.
Each treatment (single-species vs mixed-species infestations) for each host species was replicated 12–19 times. Experimental procedures were as follows. An individual rodent (either M. crassus or A. cahirinus) was placed in a plastic cage (60 × 50 × 40 cm3) with a floor of 3–5 mm of clean sand covered by a wire mesh (5 × 5 mm2). Then, we released a group of fleas into the cage for 3 days. To equalize ectoparasite pressure on a rodent, single-species groups were composed of 50 (30 females and 20 males) either X. ramesis or P. chephrenis, whereas mixed-species groups were composed of 25 (15 females and 10 males) X. ramesis and 25 (15 females and 10 males) P. chephrenis. This number of fleas is not higher than maximal number of fleas found simultaneously on an individual rodent (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova, Degen and Rogovin1996, Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). Under these conditions, fleas usually start to lay eggs no earlier than the 2nd day (Khokhlova et al. Reference Khokhlova, Fielden, Degen and Krasnov2012). Three days of uninterrupted access to a host guaranteed that fleas were able to copulate and produce eggs. On the 4th day of the experiment, we collected fleas from both the rodent's body (over a white plastic pan using a toothbrush until no flea was recovered) and cage substrate and counted them. We placed female fleas of the same species recovered from the same individual rodent in a Petri dish and transferred it to an incubator (FOC225E, Velp Scientifica srl, Milano, Italy) at 25 °C air temperature and 90% relative humidity (RH) for 24 h. Then, we checked the dish and counted newly-laid eggs. We measured size of at least 10 eggs from each Petri dish (maximal length and maximal width) under light microscopy on a screen using a digital microscope camera Moticam 2000 with the Motic Images Plus 2.0ML program (Motic, Speed Fair Cp., Ltd., Causeway Bay, Hong Kong) up to nearest ± 0·01 mm with 40x magnification and calibration using an object-micrometer.
After counting and measuring eggs, Petri dishes with eggs produced by the same group of females were filled with a 1 mm layer of sand and larval food medium [94% dry bovine blood, 5% millet flour and 1% grinded excrements of M. crassus (for X. ramesis) or A. cahirinus (for P. chephrenis)], and transferred into an incubator (see above) where they were maintained at 25 °C air temperature and 90% RH. Starting on the 18th day after oviposition (ca. a week less than minimal duration of pre-imaginal development; Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001), Petri dishes were checked daily until either all eggs developed into new imago or for 60 consecutive days. We recorded the number of new adults that emerged from each group of eggs.
Fitness-related variables and data analyses
For each group of fleas of each species fed on each individual rodent hosts, we calculated two variables reflecting quantitative component of fitness (i.e. the variables describing the quantity of the offspring) and two variables reflecting qualitative component of fitness (i.e. the variables describing the quality of the offspring). Rationale for using these variables as indicators of flea fitness can be found elsewhere (Krasnov, Reference Krasnov2008). The former variables were (a) egg production (mean number of eggs produced per female) and (b) new imago production (mean number of new imagoes produced per female). The latter variables were (a) mean (across eggs produced by the same group of females) number of new imagoes emerged per one egg and (b) egg volume. We considered mean number of new imagoes emerged per one egg as a proxy for pre-imaginal survival and, thus, an indicator of the offspring quality. Egg volume was calculated after Berrigan (Reference Berrigan1991) as V = 1/6π*W2*L, where V is egg volume, W is maximal egg width and L is maximal egg length.
Distribution of all four variables did not significantly deviate from normal (Shapiro–Wilk W = 0·95–0·97 for X. ramesis and Shapiro–Wilk W = 0·96–0·97 for P. chephrenis; P > 0·29 for all). We analysed the effect of treatment (single-species vs mixed-species infestations) on egg and new imago production as well as on the number of new imagoes emerged per egg using generalized linear models with normal distribution and log-link function. The effect of treatment on egg volume was analysed using linear mixed-effects models (Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009) with the individual number of a flea group (i.e. fleas that exploited the same rodent individual) as a random factor. We fitted the models using the lme function as implemented in ‘nlme’ package (version 3.1-118; Pinheiro et al. Reference Pinheiro, Bates, DebRoy and Sarkar2014) in R (version 2.14; R Development Core Team, 2013). All analyses were carried out separately for X. ramesis on M. crassus and P. chephrenis on A. cahirinus.
RESULTS
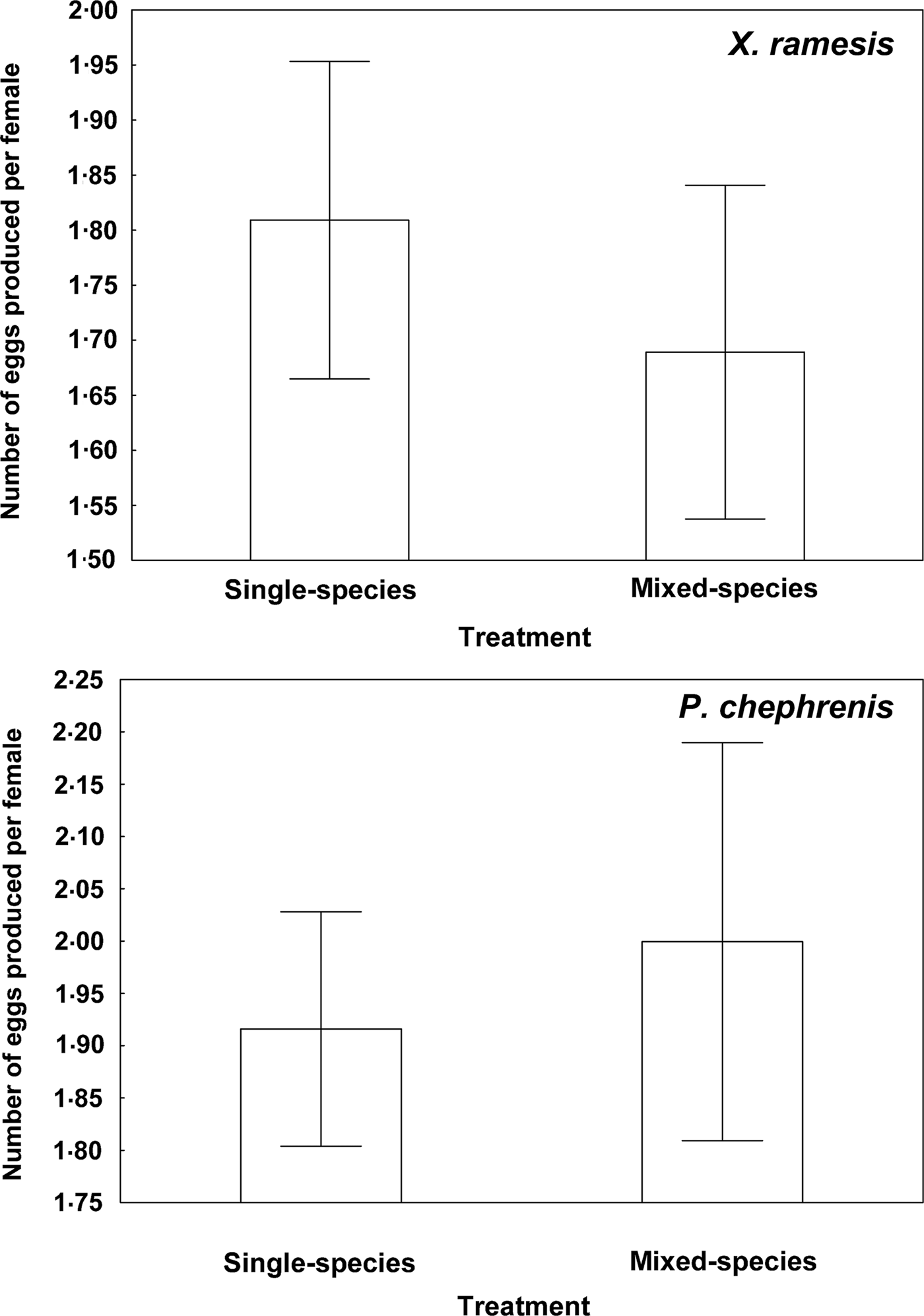
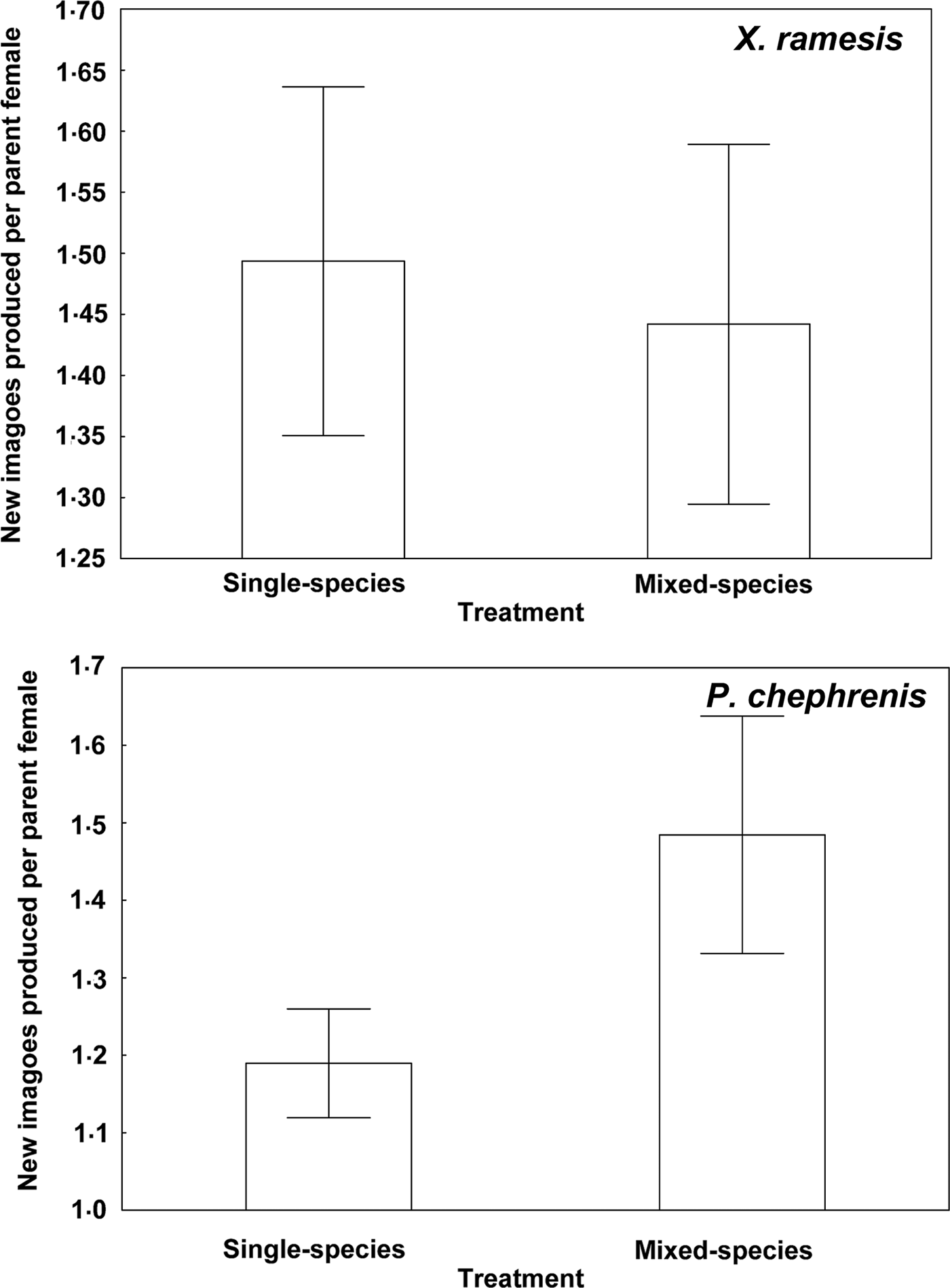
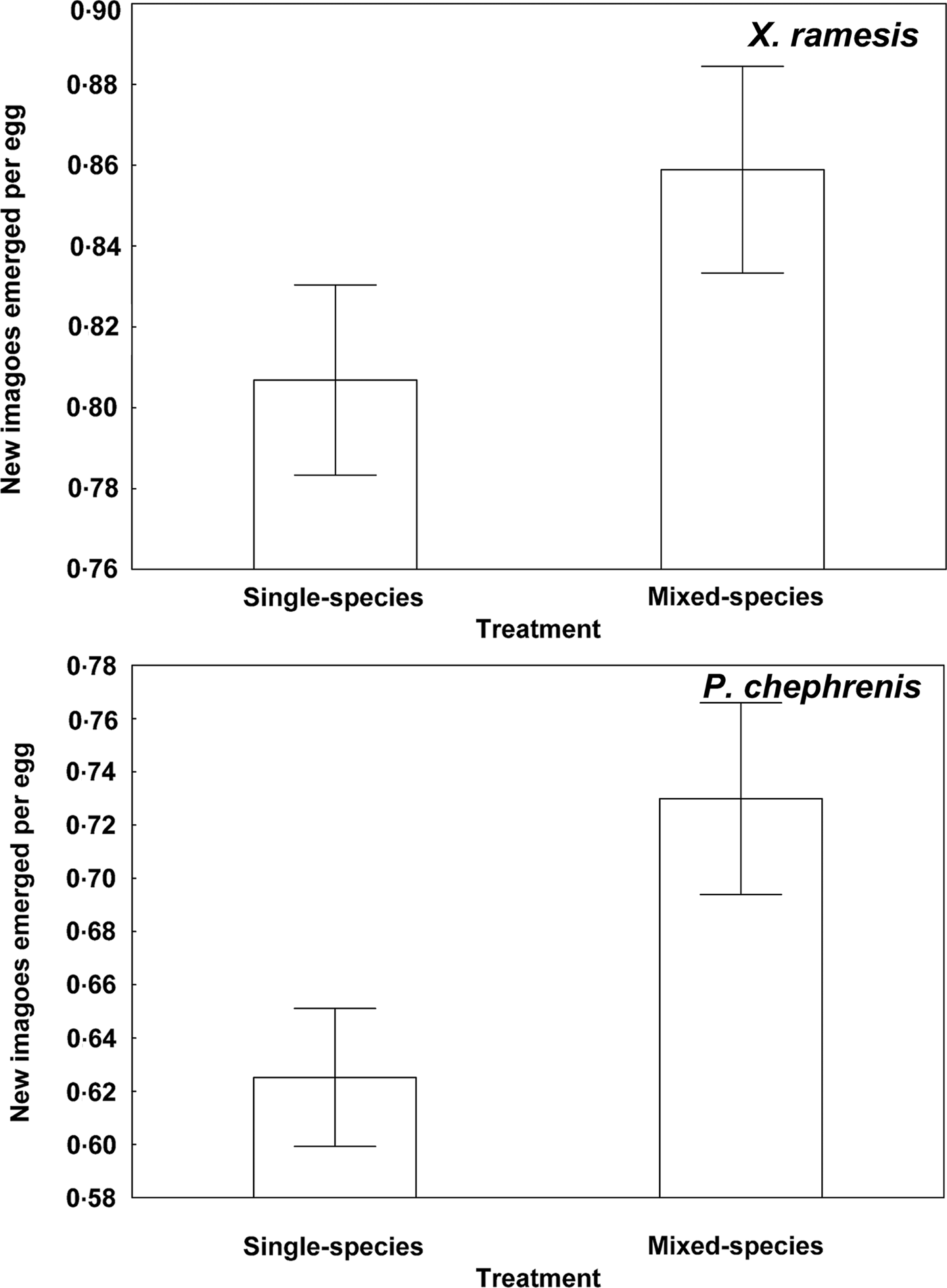
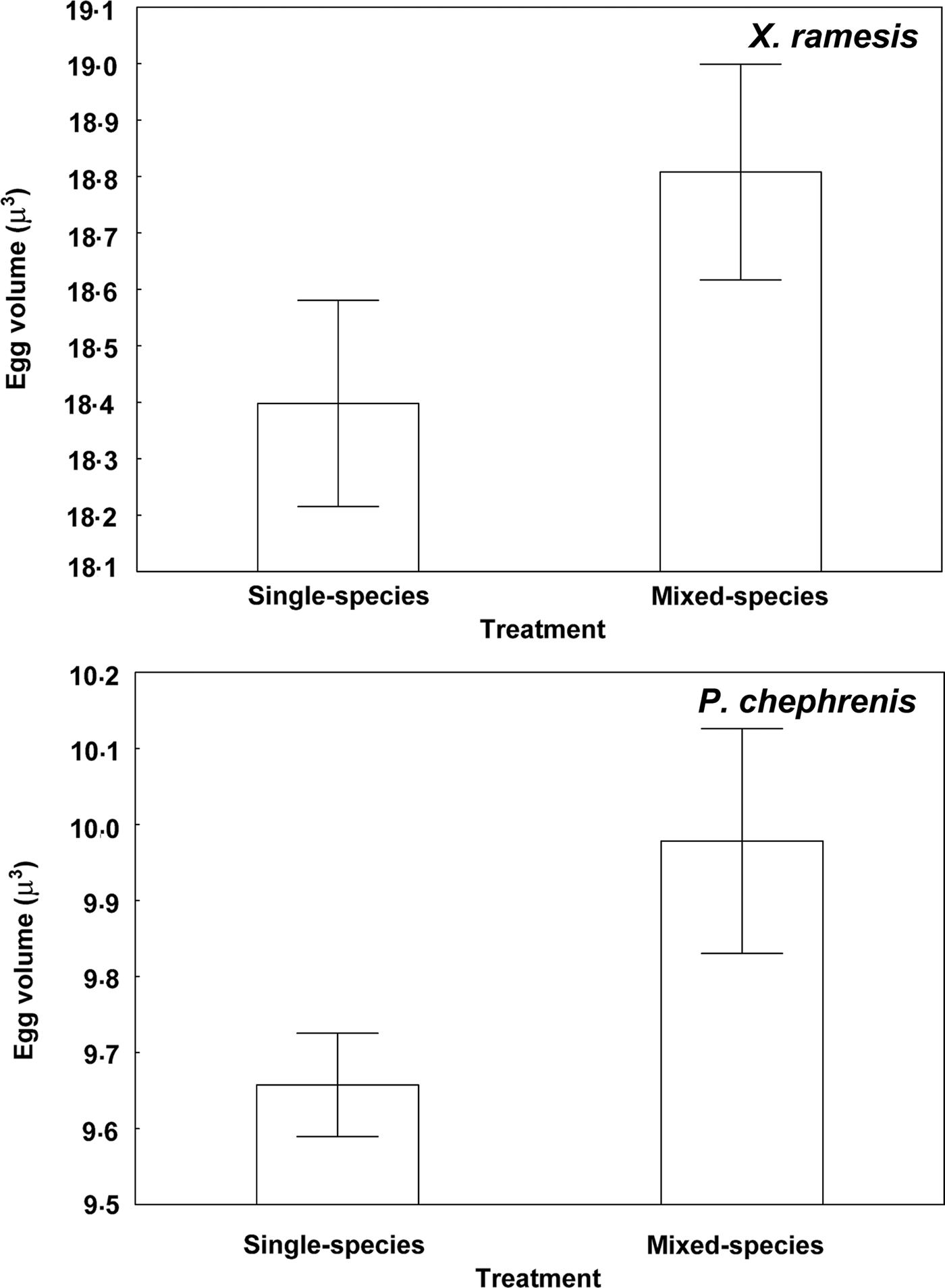
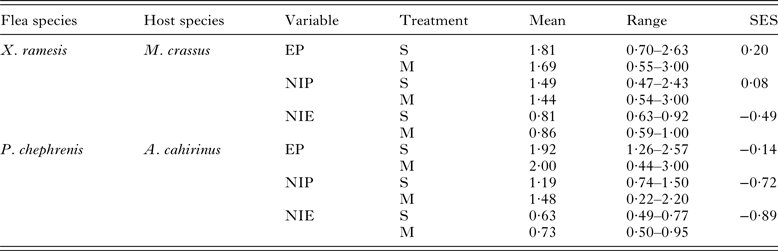
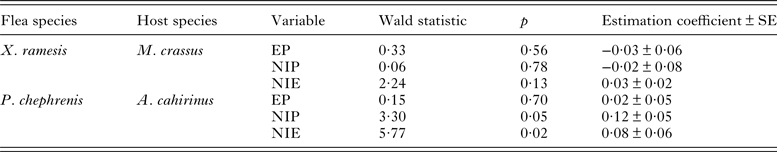
Descriptive statistics for fitness-related variables in the two fleas in single- and mixed-species infestations is presented in Table 1. A summary of generalized linear models of the effect of single- vs mixed-species infestations of a host on egg and new imago production and mean number of new imagoes emerged per egg in the two flea species is presented in Table 2. In both X. ramesis and P. chephrenis, mean number of eggs produced per female flea did not depend on whether fleas fed on a host in single- or mixed species groups (Table 2, Fig. 1). No effect of single- vs mixed-species infestation on the mean number of new imagoes produced per female flea was found for X. ramesis, whereas the number of new imagoes per parent female of P. chephrenis was significantly higher in mixed- than in single-species groups (Table 2, Fig. 2). This difference was likely a result of higher survival of eggs from females in mixed-species groups as indicated by higher number of emerged imagoes per egg in these groups as compared with single-species groups, although this was true for P. chephrenis but not X. ramesis (Table 2, Fig. 3). The results of linear mixed-effects modelling indicated that X. ramesis in single- and mixed-species groups produced eggs of similar size (Table 3, Fig. 4). In contrast, eggs produced by P. chephrenis in mixed-species groups were significantly larger than eggs produced by conspecifics fed in single-species groups (Table 3, Fig. 4). The variation in the egg volume due to differences among rodent individuals (a random term in each model) was low (Table 3).
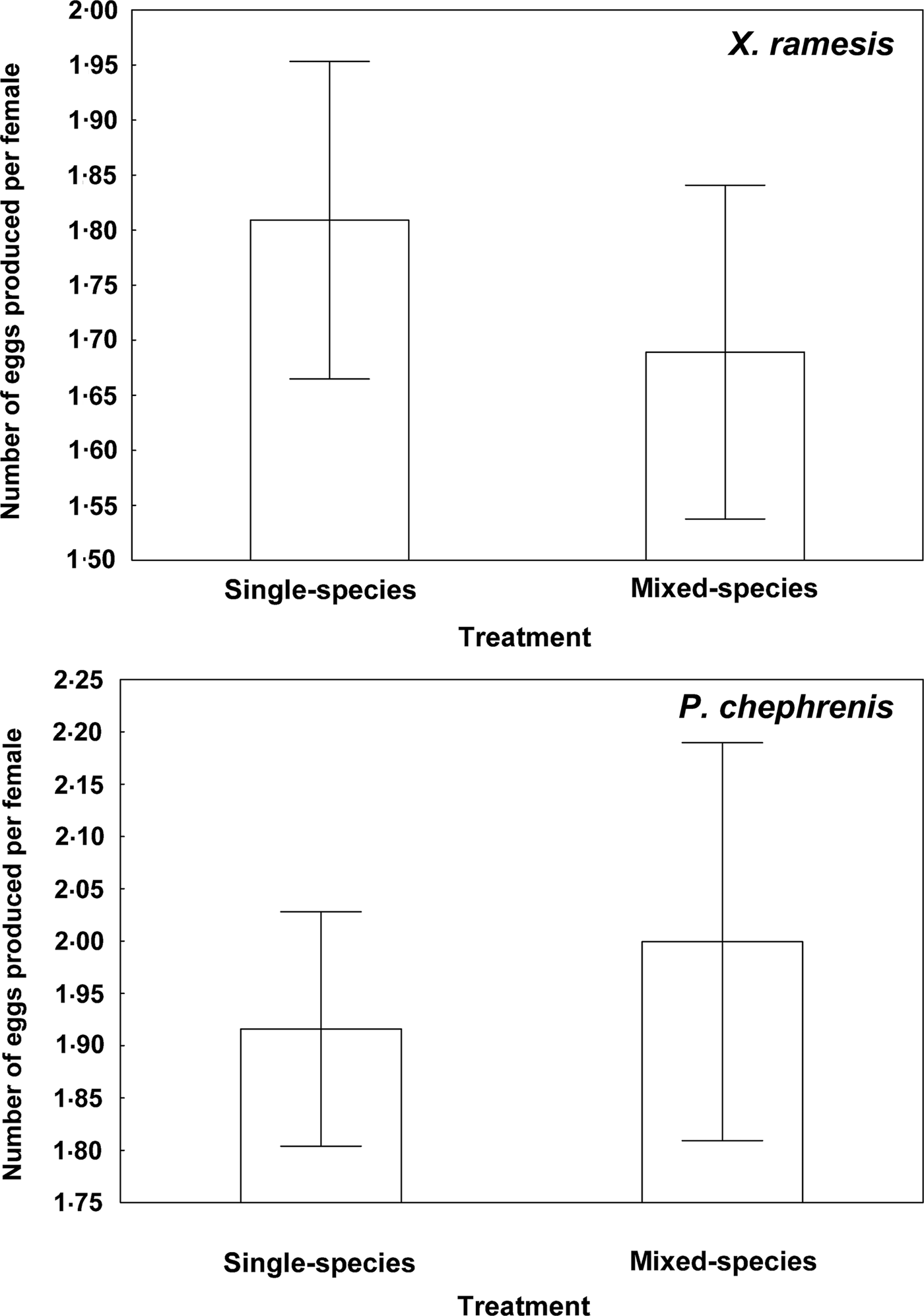
Fig. 1. Mean (±s.e.) number of eggs produced per female of Xenopsylla ramesis and Parapulex chephrenis after 3 days of uninterrupted feeding on a rodent host (Meriones crassus and Acomys cahirinus, respectively) in single-species or mixed-species infestations.
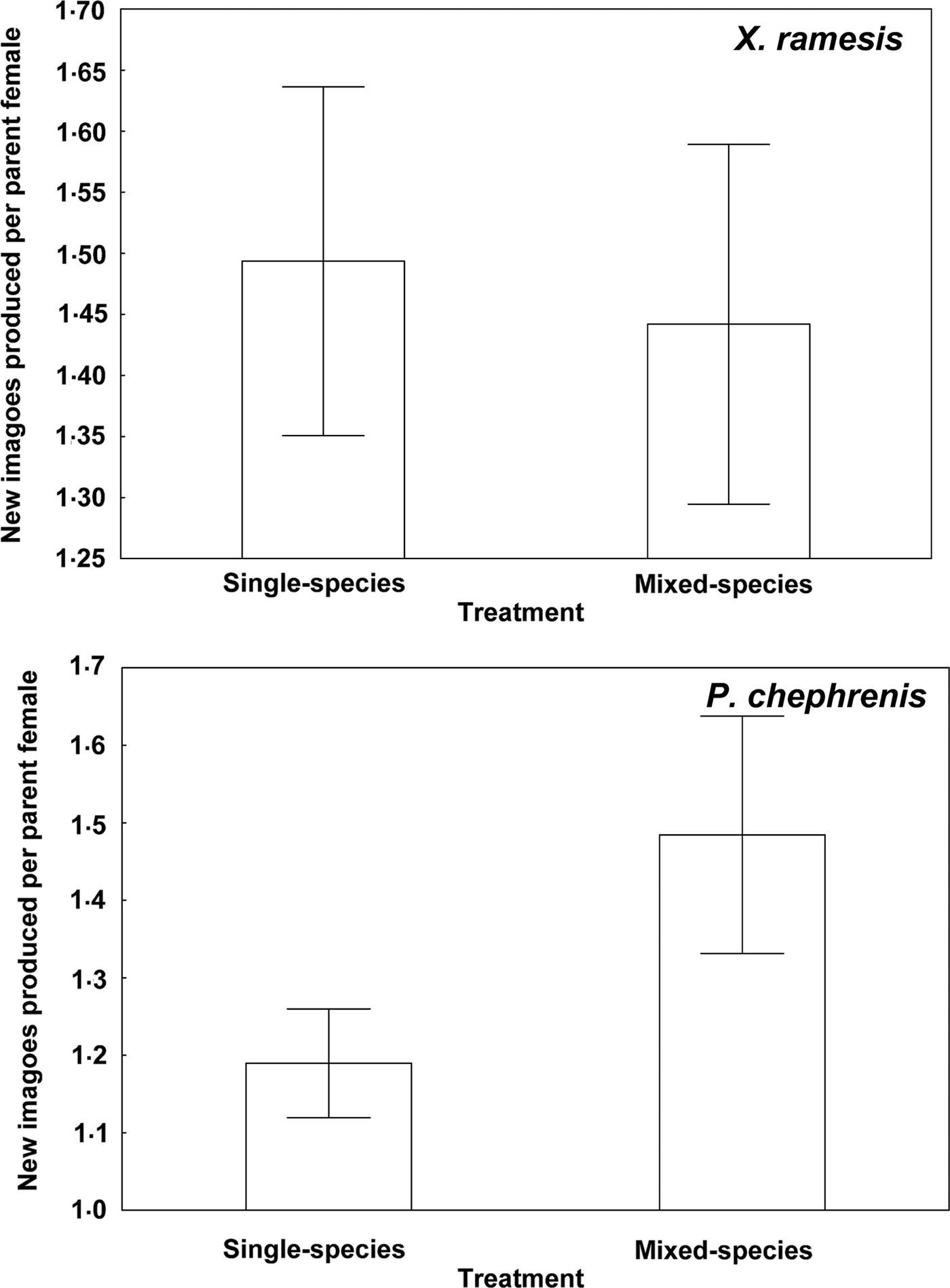
Fig. 2. Mean (±s.e.) number of new imagoes produced per female of Xenopsylla ramesis and Parapulex chephrenis after 3 days of uninterrupted feeding on a rodent host (Meriones crassus and Acomys cahirinus, respectively) in single-species or mixed-species infestations.

Fig. 3. Mean (±s.e.) number of new imagoes emerged per egg from eggs produced by Xenopsylla ramesis and Parapulex chephrenis after 3 days of uninterrupted feeding on a rodent host (Meriones crassus and Acomys cahirinus, respectively) in single-species or mixed-species infestations.

Fig. 4. Mean (±s.e.) volume of an egg produced by female of Xenopsylla ramesis and Parapulex chephrenis after 3 days of uninterrupted feeding on a rodent host (Meriones crassus and Acomys cahirinus, respectively) in single-species or mixed-species infestations.
Table 1. Descriptive statistics for fitness-related variables in Xenopsylla ramesis and Parapulex chephrenis exploiting Meriones crassus and Acomys cahirinus, respectively, in single (S)- and mixed (M)-species infestations.

Fitness-related variables were: egg production (EP; mean number of eggs produced per female), new imago production (NIP; mean number new imagoes produced per parent female) and mean number of new imagoes emerged per egg (NIE; mean number of new imagoes emerged per egg). Standardized effect size (SES) was calculated as mean differences divided by pooled standard deviation.
Table 2. Summary of general linear models of the effect of co-infestation (single-species vs mixed-species infestations) on egg production (EP; mean number of eggs produced per female), new imago production (NIP; mean number new imagoes produced per parent female) and mean number of new imagoes emerged per egg (NIE; mean number of new imagoes emerged per egg) in fleas Xenopsylla ramesis and Parapulex chephrenis exploiting their characteristic rodent host (Meriones crassus and Acomys cahirinus, respectively).
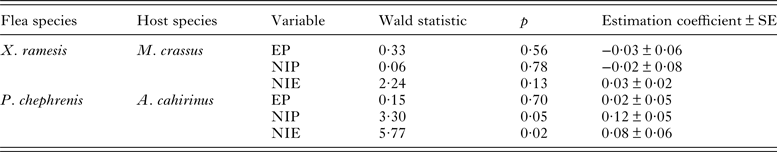
Table 3. Summary of linear mixed-effects models of egg volume in fleas Xenopsylla ramesis and Parapulex chephrenis dependent upon whether they exploited their host (Meriones crassus or Acomys cahirinus, respectively) in single-species or mixed-species groups.

The reference level for an independent variable was arbitrarily selected as the mixed-species treatment. PVRand–percentage of variation due to random term.
DISCUSSION
Our predictions appeared to be only partly true. The direct effects of single- vs mixed-species infestation were found (a) for the quality, but not quantity component of flea fitness and (b) for P. chephrenis, but not X. ramesis. Nevertheless, the quantity component of fitness (the number of imagoes of the new generation) of P. chephrenis was indirectly affected by co-infestation via its effect on survival ability of eggs.
From a mechanistic perspective, an increase in the quality of the offspring of P. chephrenis when fleas exploited their hosts simultaneously with X. ramesis, may be associated with a decrease in behavioural and/or immunological anti-parasitic defences of a host subjected to multiple parasite challenges (Jokela et al. Reference Jokela, Schmid-Hempel and Rigby2000; but see Sánchez et al. Reference Sánchez, Serrano, Gómez, Feliu and Morand2014). In fact, host resistance is often defined as host-induced loss of fitness in a parasite and, thus, represents a characteristic of a host that is measured via the parasite (Combes, Reference Combes2001). Accepting this idea, it is fair to state that the efficiency of the host's anti-parasitic defences may be assessed via its effect on parasites (e.g. Khokhlova et al. Reference Khokhlova, Ghazaryan, Krasnov and Degen2008), although traditionally it is measured in terms of host-related variables such as, for example, leukocyte concentration (Heylen and Matthysen, Reference Heylen and Matthysen2008) or the level of inflammatory cytokines (Johnston et al. Reference Johnston, Trammell, Ball-Kell, Verhulst and Toth2009). For example, multiple infestations by ticks likely led to a development of an acquired resistance in guinea pigs and resulted in decreased feeding and reproduction of ticks in subsequent infestations (Fielden et al. Reference Fielden, Rechav and Bryson1992). Similarly, increased reproductive performance of P. chephrenis in mixed-species groups likely indicates a decrease in anti-flea resistance of A. cahirinus under these conditions. A decrease of host defences under co-infestation by different parasites can also be indicated by general decrease of host's body conditions (Lochmiller and Deeremberg, Reference Lochmiller and Deeremberg2000). However, when a decrease in body condition and concomitant decrease in anti-parasitic defences under co-infestation are inferred from the field data on the records of different parasites collected from the same host individuals (e.g. Ranzani-Paiva and Silva-Souza, Reference Ranzani-Paiva and Silva-Souza2004; Risco et al. Reference Risco, Serrano, Fernández-Llario, Cuesta, Gonçalves, García-Jiménez, Martínez, Cerrato, Velarde, Gómez, Segalés and Hermoso de Mendoza2014; Serrano and Millán, Reference Serrano and Millán2014), it remains unclear whether this is because body condition (and, thus, anti-parasitic defences) deteriorates under multiple parasite attacks or because a host with originally low body condition (and, thus, low defences) represents a better source of resources for different parasites. The latter can be especially true because parasites belonging to the same taxon usually have similar trophic requirements. Nevertheless, the results of our study indicate that the former mechanism is not less likely than the latter.
Furthermore, we found P. chephrenis in mixed-species groups produced larger eggs that survived better as compared with those produced by fleas that exploited their hosts in single-species groups. Consequently, the net result of co-infestation was increased fitness of P. chephrenis under co-infestation with another flea species. Larger eggs are usually accompanied by better performance of new imagoes emerged from these eggs (Torres-Vila and Rodríguez-Molina, Reference Torres-Vila and Rodríguez-Molina2002; Pöykkö and Mänttäri, Reference Pöykkö and Mänttäri2012), although the size of new imagoes in holometabolous insects depends on other factors as well (e.g. larval food resources and density, air temperature, RH). Nevertheless, larger size and higher survival of eggs in P. chephrenis in mixed-species groups can be one of the mechanisms behind correlated abundances of co-infesting ectoparasites (Brinkerhoff et al. Reference Brinkerhoff, Markeson, Knouft, Gage and Montenieri2006; Presley, Reference Presley2007). Another, not necessarily alternative explanation of the increase in egg quality (size and survival) in P. chephrenis in mixed-species groups can be a higher investment of females into offspring quality under unfavourable conditions such as some negative interactions with a co-occurring heterospecific fleas. Although the majority of studies on fleas indicate that interspecific interactions in their communities are either positive or neutral (Krasnov et al. Reference Krasnov, Mouillot, Shenbrot, Khokhlova and Poulin2005a , Reference Krasnov, Stanko and Morand2006a , Reference Krasnov, Stanko, Khokhlova, Mosansky, Shenbrot, Hawlena and Morand b ; Brinkerhoff et al. Reference Brinkerhoff, Markeson, Knouft, Gage and Montenieri2006; Pilosof et al. Reference Pilosof, Lareschi and Krasnov2012), the possibility of the occurrence of some degree of interspecific competition between flea imagoes (but not larvae; see Krasnov et al. Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen2005b ) have also been suggested (e.g. Lindsay and Galloway, Reference Lindsay and Galloway1998). In our earlier experiments, we found that this flea responded to the unfavourable condition (feeding on a non-characteristic host) by increasing the egg size (Khokhlova et al. Reference Khokhlova, Fielden, Williams, Degen and Krasnov2013, Reference Khokhlova, Pilosof, Fielden, Degen and Krasnov2014). However, an increase in the egg size was accompanied by a decrease in egg number suggesting a well-known trade-off between quality and quantity of the offspring (Smith and Fretwell, Reference Smith and Fretwell1974). In contrast, in this study P. chephrenis invested into the egg quality without sacrificing their quantity.
From an ecological perspective, the occurrence of a fitness-related response to co-infestation in P. chephrenis and the lack of such response in X. ramesis may be associated with the characteristic species composition of flea assemblages on their main hosts. In general, interactions in a host-flea network (a system that includes all co-habitating hosts and all co-habitating fleas in a given region or locality) have been shown to be asymmetric with host-specific fleas tending to interact with hosts with high flea richness whereas host-opportunistic fleas tend to interact with hosts with low parasite richness (Vazquez et al. Reference Vazquez, Poulin, Krasnov and Shenbrot2005). However, X. ramesis and P. chephrenis do not both follow this rule. The absolute majority of M. crassus individuals harbour several flea species including host-generalist X. ramesis, whereas the absolute majority of A. cahirinus individuals harbour exclusively host-specialist P. chephrenis (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). In other words, co-infestation seems to be a usual condition for the X. ramesis, but definitely unusual for P. chephrenis. Consequently, X. ramesis may somehow be adapted to co-exploiting a host together with heterospecific fleas and thus its response to co-infestation is weak at best. In contrast, tolerance of P. chephrenis to co-occurring heterospecifics might be much lower, so it may mount a reproductive response. We recognize, however, that this explanation is highly speculative and warrants further investigation. Nevertheless, our study demonstrated that different flea species respond differently to co-infestation. In particular, this variation between flea species may explain why a positive correlation between abundance of a given flea species and abundance of co-occurring fleas of other species reported in earlier studies is, albeit general, but definitely not an universal rule (Krasnov et al. Reference Krasnov, Mouillot, Shenbrot, Khokhlova and Poulin2005a , Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen b ).
In conclusion, an increase in reproductive performance as a response to co-infestation may be one of the mechanisms behind aggregative structure of flea infracommunities. However, this response varies among flea species possibly dependent upon the level of diversity of flea assemblages on their principal hosts.
ACKNOWLEDGEMENTS
This is publication no. 875 of the Mitrani Department of Desert Ecology.
FINANCIAL SUPPORT
This study was supported by the Israel Science Foundation (Grant no. 26/12 to BRK and ISK). A post-doctoral fellowship for EMD was granted by the Blaustein Center for Scientific Cooperation, the Kreitman School of Advanced Graduate Studies and the Swiss Institute for Dryland Environmental and Energy Research (Ben-Gurion University of the Negev).