Introduction
Basic studies on reproductive aspects of a particular species of fish allow us to obtain essential data to understand the mechanisms that govern perpetuation of the species. Knowledge about first sexual maturation, reproductive cycle duration, determination of reproductive season and spawning type, allows us to understand reproductive capacity (Murua and Saborido-Rey, Reference Murua and Saborido-Rey2003), as well as develop new experiments on the viability of the species in captivity. In this regard, this information becomes especially important when it comes to understanding the life history of marine species, especially those of economic interest.
Among many marine species that are prominent in aquaria, fish from Chaetodontidae family – butterflyfish – have great ornamental importance due to their attractive colour and different morphological patterns (Nagpure et al., Reference Nagpure, Kumar, Srivastava, Kushwaha, Gopalakrishnan and Basheer2006). Currently, this family consists of 133 species belonging to 12 genera distributed in tropical and subtropical marine regions with the largest number of species belonging to the genus Chaetodon (Eschmeyer et al., Reference Eschmeyer, Fricke and Van der Laan2018).
Although Chaetodon is a popular species, being present in aquaria around the world, information on reproductive aspects in many of these species focuses on behavioural studies (Motta, Reference Motta2012) or shows superficial and rough histological aspects of the gonads, lacking essential detail. Despite some spawning successfully achieved in captivity in some Chaetodon butterflyfishes (Degidio et al., Reference Degidio, Yanong, Watson, Ohs, Cassiano and Barden2017; Nowicki et al., Reference Nowicki, O’Connell, Cowman, Walker, Coker and Pratchett2018) and particularly in Chaetodon striatus (Linnaeus, 1758) (Rising Tide Conservation, 2019), there has been no detailed morphological description of the germinal epithelium, nor the reproductive phases of the animal. Existing studies are old (Ralston, Reference Ralston1981; Colin, Reference Colin1989) and performed in paraffin, showing low resolution histology (Fowler, Reference Fowler1991). This approach has led to loss of the important information on germinal epithelium that we were able to show in this study using high resolution light microscopy. Therefore, previous studies did not provide details on basic aspects of the reproductive biology of these animals, such as ovarian and testicular development, or evolution of female and/or male gonadal tissue during reproductive stages throughout the life of the specimens.
In fish, reproductive dynamics in different Teleostei species such as changes in the germinal epithelium and gametic cell differentiation (Grier et al., Reference Grier, Uribe and Parenti2007, Reference Grier, Uribe-Aranzábal, Patino and Jamieson2009), observation and viability of the gametes, quantification of the gonadal structure or germ cells diameter (Vazzoler, Reference Vazzoler1996), sex steroid profiles (Pandian, Reference Pandian2010) and gonadosomatic index (Tyler and Sumpter, Reference Tyler and Sumpter1996) can be evaluated using macroscopic or microscopic tools. However, reproductive characteristics regardless of the evaluation methods are important aspects to consider when the objective is maintenance of a species out of its natural habitat.
Under captivity conditions, the natural reproduction process is often not complete (Mylonas et al., Reference Mylonas, Fostier and Zanuy2010) and reproductive dysfunction is frequent; this makes a basic study related to the reproductive dynamics of captive species quite relevant. Therefore, considering various mechanisms that involve reproductive events in fish, this study aimed using histological techniques to analyze gonads of C. striatus from the South Atlantic, providing further information on the reproductive biology of the species. This investigation into gonadal development of C. striatus will supply a crucial basis for developing aquaculture protocols for other marine ornamental species, including Chaetodon butterflyfish.
Materials and methods
From November 2015 to July 2017, we sampled 170 mature adults Chaetodon striatus from two sampling sites in Ubatuba, São Paulo State, Brazil: Itaguá Beach (23°27′05.17′′S, 45°02′48.49′′W) and Rapadaʼs Island (23°25′33.44′′S, 44°54′10.90′′W). Specimens (with 14–15 cm length and 80–120 g weight) were sampled using various methods that ranged from passive capture through to the use of pit-type traps implanted on the rocky shores and active capture through diving with dip nets. Captures were performed between 3 and 8 m deep, according to variation between high and low tides.
Light microscopy
Once specimens were collected, all were anaesthetized with 0.1% benzocaine and euthanized according to the institutional animal care protocols and approval (no. 805 – CEEA-IBB/UNESP). Both ovaries and testes from all 75 females and 65 males collected were quickly removed and fixed in 2% glutaraldehyde and 4% paraformaldehyde in filtered seawater (pH 8.0) for at least 24 h. For light microscopy, the gonads were dehydrated in ethanol and embedded in Historesin (Leica). Sections (3–5 µm) from all ovaries and testes were stained with Periodic acid Schiff (PAS) + iron haematoxylin + metanil yellow (MY) (Quintero-Hunter et al., Reference Quintero-Hunter, Grier and Muscato1991). These sections were used to detect the reproductive phases of C. striatus and changes in their germinal epithelium. Sections from testis were also stained using the reticulin method (RM), which enhances basement membranes (Mazzoni et al., Reference Mazzoni, Grier and Quagio-Grassiotto2014). Ovaries and testes were evaluated using a computerized image analyzer (AmScope FMA050) and a microscope (Bioptika).
Results
Ovarian structure and female germinal epithelium
The ovaries of Chaetodon striatus are even, cylindrical and elongated organs that occupy the dorsal region of the abdominal cavity, leading to urogenital papillae. They are attached to the dorsal wall of this cavity by a connective tissue membrane called the mesovary. The ovaries of C. striatus are very close to each other, being placed in a parallel longitudinal arrangement, surrounded by a thick connective tissue capsule that becomes fused in the intersection between both ovaries (Fig. 1). In addition, ovaries of C. striatus have a central lumen, internally delimited by a germinal epithelium that borders their ovigerous lamellae, which are facing the ovarian lumen (Fig. 1).

Figure 1. Cross-section of the ovaries (ov) of Chaetodon striatus showing connective tissue sharing in the region of intersection of the tunica albuginea (ta). (A–C) Different periods of gonadal development. Ovarian lumen (lu), ovigerous lamellae (ol). Staining: MY. Bars: 600 µm.
Oocyte development
In C. striatus, the female germinal epithelium (Fig. 2A) is formed by somatic and germ cells at different stages of development. In specific regions of the germinal epithelium, there are areas of intense cellular activity, called cell nests, in which proliferation of oogonia occurs. In these nests, oogonia are also recruited to enter into meiosis, originating the early prophase oocytes. At entry into diplotene, the oocyte presents with a strong basophilia near the nuclear envelope (Fig. 2A). At this stage, epithelial somatic cells, called pre-follicle cells, surround each oocyte, individualizing it and giving rise to an ovarian follicle (Fig. 2B). During this process, follicle cells initiate the formation of the basement membrane. Gradually, the basement membrane is synthesized, individualizing each ovarian follicle (data not shown).
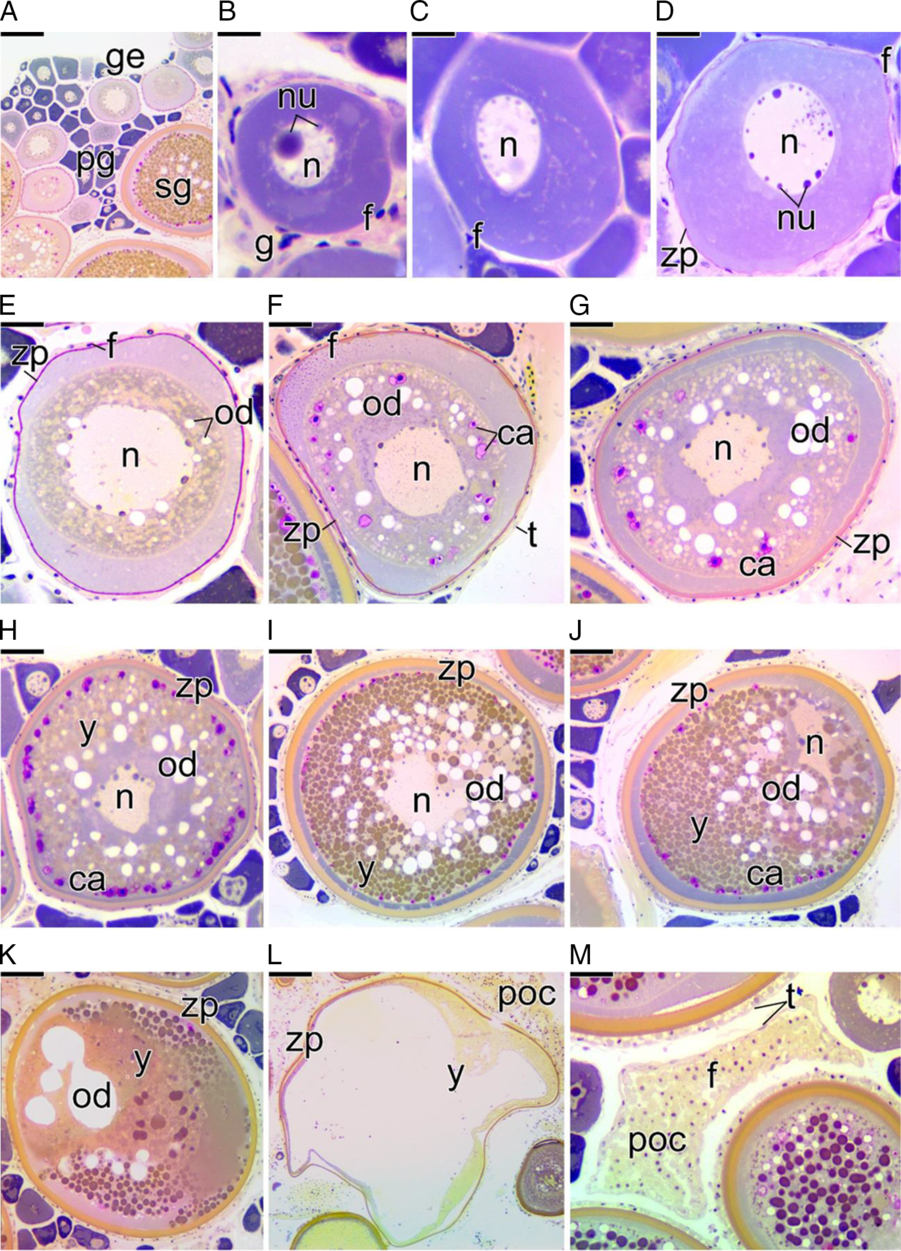
Figure 2. Cross-section of ovaries of Chaetodon striatus showing oocyte development. (A) Germinal epithelium (ge) delimiting the ovigerous lamellae. Note the presence of the oocytes at different stages: primary (pg) and a few secondary (sg) growth oocytes. (B) Detail of primary growth oocyte with the nucleolus (nu) quite developed in the nucleus (n). This oocyte, with cytoplasmic basophilia, is surrounded by follicle cells (f). (C, D) Primary growth oocyte development showing little nucleoli. In (D), the zona pellucida (zp) begins to be synthesized. (E) Primary growth oocyte showing the first oils droplets (od). The zona pellucida becomes evident. (F) Primary growth oocyte presenting first cortical alveoli (ca). The thecal cells (t) become evident. (G, H) Secondary growth oocyte in early (G) and intermediate (H) vitellogenesis. Note the deposition of yolk globules (y) in the cytoplasm. (I) Full-grown oocyte. Note the yolk globules and lipid droplets in large quantities. The nucleus remains in the centre of the oocyte. The zona pellucida becomes more developed. (J, K) Full-grown oocyte enters into maturation. The nucleus becomes eccentric (J) and the yolk globules and lipid droplets begin to fuse (K). (L) The oocyte is capable of undergoing ovulation. The yolk is fluid and the oocyte hydrated. (M) Postovulatory follicle complex (poc), formed by follicle (f) and thecal cells (t). Staining: MY. Bars: 100 µm (A), 10 µm (B), 20 µm (C, D), 40 µm (E–H), 70 µm (I–L), 30 µm (I).
With the permanence of the oocyte in diplotene, the oocyte begins to present a single developed nucleolus (Fig. 2B). The cytoplasm of these diplotene oocytes increases in volume and gradually becomes more basophilic, characterizing the primary growth period of the oocyte (Fig. 2C). Gradually, the cytoplasm of the primary growth oocyte becomes larger and less basophilic due to the increased amount of organelles that are distributed throughout the cytoplasm (Fig. 2D). The zona pellucida becomes visible at this stage. At the end of primary growth, an accumulation of oil droplets can be observed in the cytoplasm of the oocyte, near the nucleus (Fig. 2E). In the next step, small vesicles begin to form in the cytoplasm near the oocyte membrane, constituting the cortical alveoli, which are PAS positive (Fig. 2F). After the formation of cortical alveoli, deposition of yolk globules in the cytoplasm is observed, marking the entrance of the oocyte into secondary growth (Fig. 2G). Now, the oil droplets become more numerous, as do the cortical alveoli (Fig. 2G).
Throughout secondary growth, the oocyte increases in size again, also increasing the number of yolk globules, oil droplets, and cortical alveoli in its cytoplasm (Fig. 2H). At the end of secondary growth, the oocyte is fully developed, showing its maximum size (Fig. 2I). This oocyte is known as a full-grown oocyte. Its nucleus is central and its cytoplasm is completely filled with yolk globules. Oil droplets remain individualized and scattered throughout the cytoplasm among the yolk globules (Fig. 2I).
After the end of secondary growth, the full-grown oocyte enters into maturation (Fig. 2J–L). Maturation of the oocyte is characterized by migration of the nucleus towards the micropyle (Fig. 2J). The micropyle is viewed as an interruption in the zona pellucida. During maturation, the oil droplets and yolk globules gradually fuse (Fig. 2K) and the yolk become fluid, resulting in yolk hydration (Fig. 2L). Now, this oocyte may be subject to ovulation. Ovulation is the stage during which the oocyte emerges from the follicle and is released into the ovarian lumen. After ovulation, the postovulatory follicle complex, composed of follicle cells that surrounded the oocyte, the basement membrane and the theca, remain connected to the germinal epithelium (Fig. 2M).
Ovarian development
Ovaries of C. striatus undergo noticeable changes in colour, size, volume and blood supply throughout the life of the animal, being smaller and translucent when immature, and more developed and yellowish during periods of vitellogenesis. In this regard, five female gonadal patterns were described, characterizing the ovarian development stages found throughout the study period: (1) Developing stage; (2) Spawning-capable stage; (3) Actively spawning stage; (4) Regressing stage; and (5) Regenerating stage.
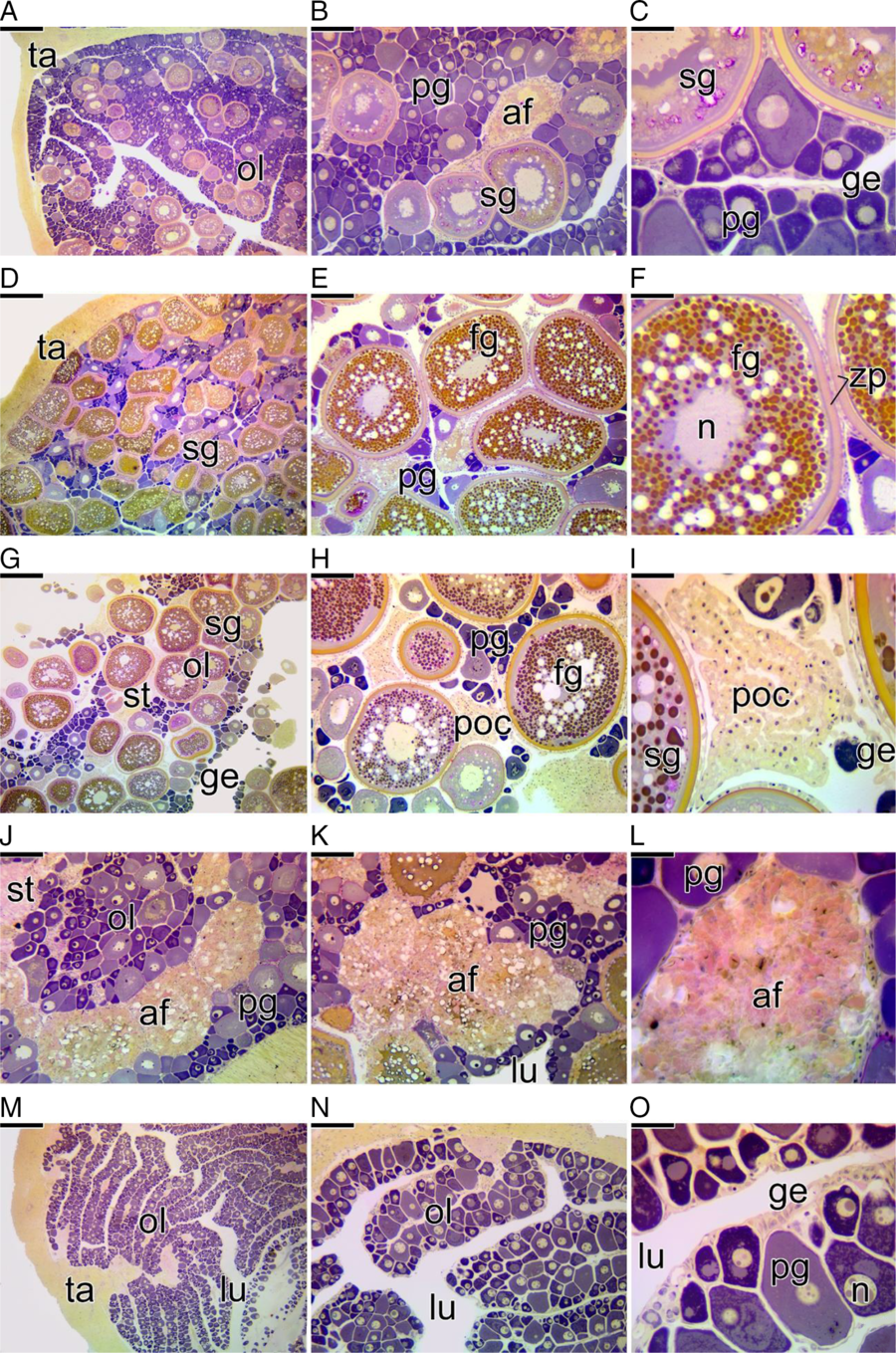
1. Developing stage: In this period of development, the ovary begins to present primary growth oocytes with oil droplets and cortical alveoli. The first oocytes in secondary growth in early and intermediate vitellogenesis can be observed (Fig. 3A–C). Some atretic follicles are observed (Fig. 3B). The germinal epithelium presents a few nests of oogonia and prophase oocytes (Fig. 3C).

Figure 3. Cross-section of the ovaries of Chaetodon striatus showing the female gonadal patterns found that characterize the stages of ovarian development. (A–C) Ovarian developing stage. The ovary is formed by a large number of primary growth oocytes (pg) and few secondary growth oocyte (sg). It is possible to observe the presence of atretic follicles (af). (B, C) Details of (A), showing the germinal epithelium (ge) that delimits ovigerous lamellae (ol). (D–F) Spawning-capable stage. During this stage, the ovary prepares for a possible spawning. The number of secondary growth oocytes (sg) increases considerably and the number of full-grown oocytes (fg) exceeds the number of primary growth oocytes (pg). (E, F) At this stage, full-grown oocytes have a central nucleus (n). (G–I) Actively spawning stage. The ovary has a large number of secondary growth and full-grown oocytes. (H, I) Oocytes in maturation were spawned, leaving a large amount of postovulatory follicles complex (poc) in the ovary. (I) Postovulatory complexes remain connected to the germinal epithelium (ge). (J–L) Ovarian regressing stage. After the spawning period, the remaining full-grown oocytes degenerate, giving rise to atretic follicles (af) in different phases of degeneration. There are no secondary growth oocytes. Primary growth oocytes (pg) increase in number again. (M–O) Ovarian regenerating stage. The ovigerous lamellae (ol), formed exclusively by primary growth oocytes, projects towards the ovarian lumen (lu), being bordered by the germinal epithelium (ge). Note the thick tunica albuginea (ta) surrounding the ovary. Ovarian stroma (st), zona pellucida (zp) Staining: MY. Bars: 200 µm (A, D, G, J, M), 100 µm (B, E, H, K, N), 50 µm (C, F, I, L, O).
2. Spawning-capable stage: At this stage, there is a large amount of secondary growth oocytes, most of these in final vitellogenesis as full-grown oocyte (Fig. 3D–F), that is they have already fully incorporated the necessary yolk, but still have a central nucleus (Fig. 3E, F). Some oocytes may be observed in early and intermediate vitellogenesis as well as in primary growth (Fig. 3D–F). Germ nests become quite scarce (Fig. 3F).
3. Actively spawning stage: This period is very similar to the previous stage. Most of the oocytes are found as full-grown oocyte or in maturation (Fig. 3G, H). Many postovulatory follicle complex are present (Fig. 3H, I).
4. Regressing stage: Primary growth oocytes are observed in larger numbers when compared with the antecedent stage (Fig. 3J, K). The remaining secondary growth oocytes, that have failed ovulation, degenerate and enter into atresia, increasing the number of atretic follicles (Fig. 3J–L). The period for maturation of the oocyte was not observed.
5. Regenerating stage: Regenerating ovaries have a large number of germ nests, containing oogonia or prophase oocytes in the germinal epithelium (Fig. 3M–O). In this period of ovarian development, only primary growth oocytes are observed throughout the gonad (Fig. 3M–O). However, there are no oocytes in the final stage of primary growth (with oil droplets or cortical alveoli). There are also no secondary growth oocytes, but some atretic follicles associated with large melanomacrophage centres have been observed. The tunica albuginea is quite thick compared with the ovarian capsules of immature ovaries.
Testicular structure and the male germinal epithelium
Testes of C. striatus are even and oval organs, partially fused by connective tissue from the tunica albuginea that surrounds them externally, forming a capsule. The testes are located dorsally in the abdominal cavity, opening into the urogenital papillae. They are attached to the dorsal wall of this cavity by a connective tissue called the mesorchium.
Male gonads of C. striatus are formed by two compartments: interstitial and germinal compartments (Fig. 4A–C), separated from each other by a basement membrane (Fig. 4D–F). The germinal compartment is formed by digitiform structures called testicular lobules that form a blind-end in the peripheral region of the gonad, characterizing lobular type organization of testis (Fig. 4A, B). Cysts of spermatogonia, as well as cysts of other germ cells types, are found along the entire length of the testicular lobules, characterizing the testis of C. striatus as the unrestricted lobular type (Fig. 4B, C). This pattern of testicular organization was easily detected by RM (silver impregnation) that labels the basement membrane of the germinal epithelium of the C. striatus (Fig. 4D–F).

Figure 4. Sagittal section of the testes of Chaetodon striatus showing the type of testicular organization. (A) Overview of the testis. Note the arrangement of the germinal and interstitial compartments (in), forming the testicular lobules (lo). (B, C) Details of (A) showing the testicular lobules, delimited by connective tissue that forms the interstitial compartment (in). (C) Detail of (A), showing the male germinal epithelium (ge), consisting of germ cysts in different stages of differentiation. Note the distribution of spermatogonia (g) throughout the germinal epithelium, characterizing a lobular unrestricted testis. (D) Overview of the testis, labelled with silver impregnation, showing the arrangement of the basement membrane (arrows) along the gonadal tissue. (E, F) Details of D showing the testicular lobules, formed by the germinal epithelium that is supported on the basement membrane. Spermatocyte (c), spermatid (t), spermatozoa (z). Staining: MY (A–C), reticulin method (D–F). Bars: 200 µm (A, D), 100 µm (B, E), 20 µm (C, F).
The germinal compartment in C. striatus is formed by somatic cells (Sertoli cells) and germ cells in different stages of development, which associate forming germ cysts (Fig. 5). Spermatogenesis begins within the cysts (Fig. 5A–C). The process occurs synchronously inside each cyst, that is within each cyst, the germ cells are in the same developmental phase (Fig. 5D–G). Primary spermatogonia proliferate within the cyst, forming secondary spermatogonia (Fig. 5D). These enter meiosis giving rise to primary and secondary spermatocytes (Fig. 5E, F). When meiosis finishes, the cysts begin to present the spermatids (Fig. 5G). After that, spermiogenesis is completed and spermatozoa are released into the testicular lumen (Fig. 5H).
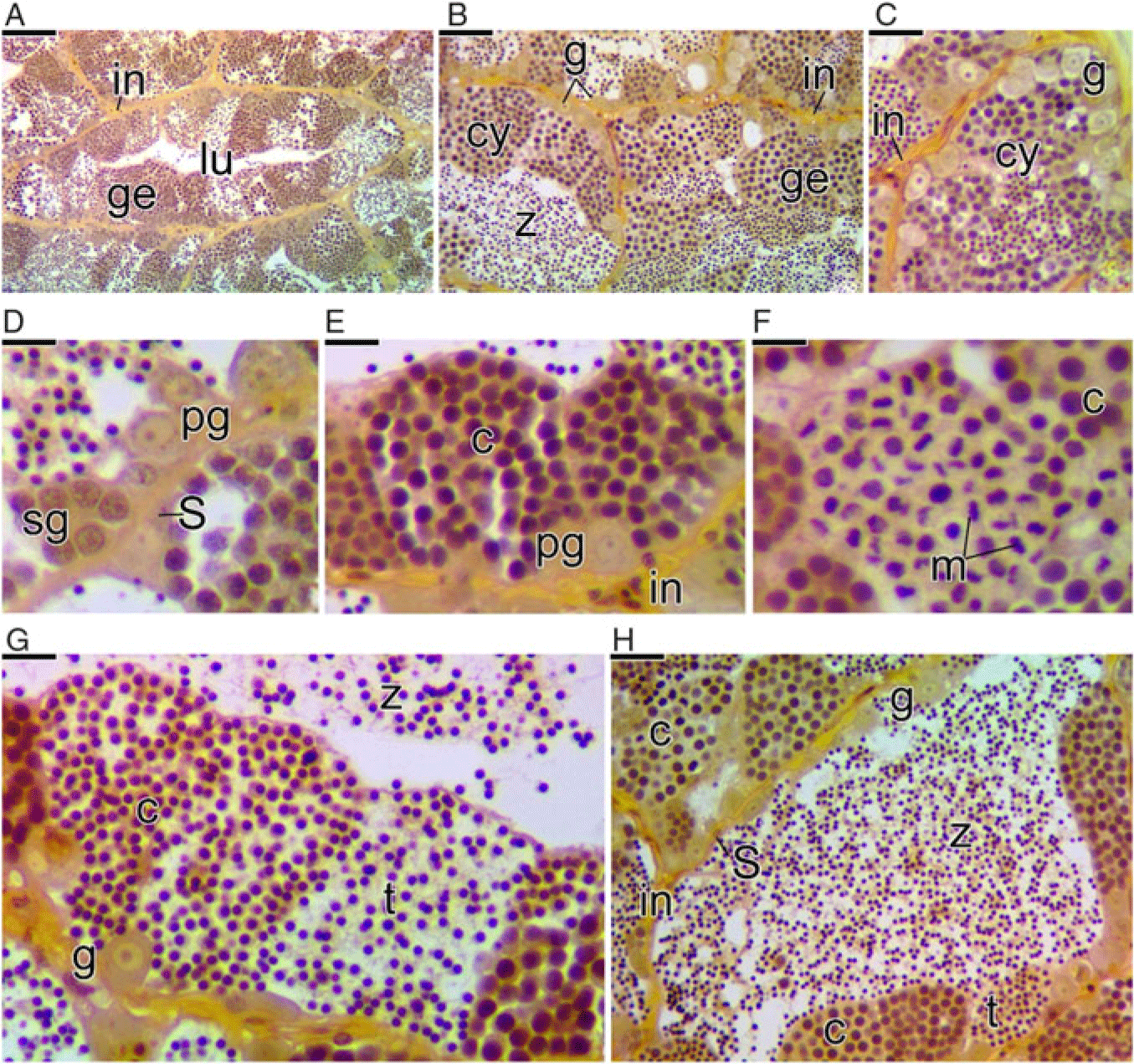
Figure 5. Cross-section of the testes of Chaetodon striatus showing the steps of the spermatogenesis. (A) Testicular lobule formed by germline cysts. Note the germinal epithelium (ge) continuous and interstitial compartment (in). (B, C) Details of (A) showing the germinal epithelium constituted by germline cysts (cy) and other germ cells types. (D) Cysts of primary (pg) and secondary (sg) spermatogonia delimited by Sertoli cells (S). (E) Cysts of primary spermatocytes (c). (F) Cysts of secondary spermatocytes, recognized by the meiotic division figures (m). (G) Cysts of spermatocytes (c) and spermatids (t). (H) Spermatozoa (z) in the lumen of the lobules. Testicular lumen (lu), spermatogonia (g). Staining: MY. Bars: 100 µm (A), 50 µm (B), 20 µm (C), 10 µm (D–G), 20 µm (C, F), 40 µm (H).
Testicular development
The testes of C. striatus undergo noticeable changes in colour, size and volume throughout the life of the animal, appearing to be smaller and translucent when immature, and more developed and whitish in the breeding periods. In this respect, five male gonadal patterns were described (Fig. 6), characterizing the stages of testicular development found throughout the study period: (1) Developing stage; (2) Reproducing-capable stage; (3) Actively reproducing stage; (4) Regressing stage; and (5) Regenerating stage.
1. Developing stage: In this stage, the testes of C. striatus initiate spermatogenesis (Fig. 6A–C). Cysts with cells at different stages of the spermatogenesis (spermatogonia, spermatocytes and spermatids) can be found to constitute the germinal epithelium (Fig. 6C). The lumen of the testicular lobules is still mostly empty, with a greatly reduced amount of spermatozoa (Fig. 6B, C). The germinal epithelium is continuous throughout the testis (Fig. 6B, C). The interstitial compartment is thin (Fig. 6C), formed by fibroblasts, collagen fibres and blood vessels. The animals found at this stage of testicular development are not capable of breeding.
2. Reproducing-capable stage: In this step, germ cysts can be visualized throughout the testis at different stages of spermatogenesis (spermatogonia, spermatocytes and spermatids) (Fig. 6D–F). The lumen of the testicular lobules is fully filled with spermatozoa, mainly in the central region of the testis (Fig. 6D–F). The germinal epithelium becomes progressively discontinuous (Fig. 6D–F), especially in the regions near the testicular duct, due to release of sperm into the testicular lumen. The animals found at this stage of testicular development are about to enter their reproductive period.
3. Actively reproducing stage: Now, the cysts of spermatogonia, spermatocytes and spermatids are few (Fig. 6G–I). The lumen of the testicular lobules is full of spermatozoa. With rupture and opening of cysts for sperm release, the germinal epithelium tends to become totally discontinuous (Fig. 6G–I). The animals found at this stage of testicular development are capable of reproducing.
4. Regressing stage: This stage is characterized by the presence of cysts of spermatogonia and spermatocytes throughout the testis, as well as residual sperm in the lumen of the testicular lobules (Fig. 6J–L). In the interstitial compartment, macrophages associated with the connective tissue remodelling are observed. The animals found at this step of testicular development are not capable of breeding, although there are still spermatozoa in the testicular duct and lumen, but in insufficient numbers.
5. Regenerating stage: The germinal epithelium is only formed by cysts of spermatogonia, becoming continuous again (Fig. 6M–O). There are no other types of germ cysts in the testis. The lumen of the testicular lobules is small and has residual spermatozoa (Fig. 6O). The interstitial compartment is more developed than in the previous stage (Fig. 6N–O).

Figure 6. Sagittal section of the testes of Chaetodon striatus showing the male gonadal patterns found that characterize the stages of testicular development. (A–C) Testicular developing stage. The testicular lobules (lo) are formed by a continuous germinal epithelium (ge) constituted by cysts (cy) of spermatogonia (g), spermatocytes (c) and spermatids (t). The testicular lumen (lu) is quite large, with a small amount of spermatozoa (z). Note the interstitial compartment (in) between two adjacent lobules. (D–F) Reproducing-capable stage. The testes become more developed and the testicular lobules are filled with large amounts of spermatozoa. (F) Detail of (D) showing the germinal epithelium, which varies from continuous to discontinuous. (G–I) Actively reproducing stage. At this stage, the testis produces large amounts of sperm and the animal is able to reproduce. (I) Detail of (G) showing the lumen fully filled with spermatozoa and discontinuous epithelium, formed by spermatogonia and Sertoli cells (S) only. (J–L) Testicular regressing stage. After the reproductive period, the testis is reduced in size and the gonadal tissue appears to be disorganized. (K) In the testicular duct and lobule, residual sperm are observed. The interstitial compartment is formed by a loose connective tissue. (L) In the germinal compartment, it is possible to observe some cysts of spermatogonia and spermatocytes, as well as residual sperm in the lumen. (M–O) Testicular regenerating stage. After the reproductive period and regression of the gonadal tissue, the testis shows a reduction in the testicular lumen. (N–O) The interstitium becomes more developed, enabling the detection of Leydig (L) cells. (O) The germinal epithelium, again continuous, is formed only by cysts of spermatogonia. In the testicular lumen, the presence of residual sperm is still noted. This stage is marked by intense proliferation of spermatogonia to restart the germinal epithelium. Staining: MY. Bars: 200 µm (A, D, G, J, M), 100 µm (B, E, H, K, N), 40 µm (C, F, I, L, O).
Discussion
Ovarian structure and the female germinal epithelium
The ovaries of Chaetodon striatus analyzed here have the same patterns of morphological organization and anatomical position found in most Teleostei. This structure determines the condition of the cyst ovary, in which the ovarian cavity is continuous with the gonoduct, extending along the abdominal cavity to the urogenital papillae (Nagahama, Reference Nagahama, Hoar, Randall and Donaldson1983; Selman and Wallace, Reference Selman and Wallace1986; Grier et al., Reference Grier, Uribe and Parenti2007). Externally, the ovaries of C. striatus present a considerable connective tissue capsule that allows external fusion of the gonads in the middle region, causing the ovaries to remain in a parallel position along the abdominal cavity of the animal. This connective tissue constitutes an adventitial-like layer, being common to both ovaries, without presenting a mesothelium between them. In addition, the germinal epithelium and the connective tissue emits septa into the ovarian tissue, forming the ovigerous lamellae, as in other species (Grier et al., Reference Grier, Uribe-Aranzábal, Patino and Jamieson2009; Lubzens et al., Reference Lubzens, Young, Bobe and Cerdà2010). The ovaries of C. striatus show compartmentalization of the gonadal tissue, a common pattern among Teleostei, in which the germinal compartment is made up of germinal epithelium and separated from the stromal compartment by a basement membrane (Grier, Reference Grier2002; Grier et al., Reference Grier, Uribe and Parenti2007; Quagio-Grassiotto et al., Reference Quagio-Grassiotto, Grier, Mazzoni, Nóbrega and Amorim2011; Mazzoni and Quagio-Grassiotto, Reference Mazzoni, Quagio-Grassiotto and Carreira2017). Quite common among all Teleostei, formation of ovarian follicles and oocyte development will occur from this germinal epithelium throughout the reproductive life of the animal (Grier et al., Reference Grier, Uribe-Aranzábal, Patino and Jamieson2009). Therefore, at the beginning of the primary growth, the C. striatus oocyte is already completely surrounded by their follicle cells, which synthesize a basement membrane around the ovarian follicle.
The ovarian follicle is composed of the germ cell, i.e. the oocyte and the follicle cells, the somatic cells derived from the epithelium (Grier et al., Reference Grier, Uribe and Parenti2007; Lubzens et al., Reference Lubzens, Young, Bobe and Cerdà2010). Around the follicle, thecal cells from the ovarian stroma (Grier, Reference Grier2000; Grier et al., Reference Grier, Uribe and Parenti2007; Mazzoni et al., Reference Mazzoni, Grier and Quagio-Grassiotto2010) encompass it. Therefore, the theca together with the ovarian follicle will constitute the follicle complex (Grier et al., Reference Grier, Uribe and Parenti2007).
Testicular structure and the male germinal epithelium
The basic organization of the testis, with spermatogenic and androgenic function, is common to all vertebrates, including Teleostei (Grier and Uribe-Aranzábal, Reference Grier, Uribe-Aranzábal and Jamieson2009; Schulz et al., Reference Schulz, França, Lareyre, Legac, ChiarinI-Garcia, Nóbrega and Miura2010). The testes are divided into two compartments: the interstitial compartment and the germinal compartment, which are separated by a basement membrane, regardless of type of testicular pattern, lobular or tubular (Grier and Uribe-Aranzábal, Reference Grier, Uribe-Aranzábal and Jamieson2009; Schulz et al., Reference Schulz, França, Lareyre, Legac, ChiarinI-Garcia, Nóbrega and Miura2010). In C. striatus, it was possible to classify the testicular organization pattern of the species as the lobular unrestricted type, clearly observed using RM. In the lobular unrestricted type testes, spermatogonia are found along the entire length of the lobules (Grier, Reference Grier1981). These data were already expected, as this type of testicular organization among fish is characteristic of derived taxa such as the Perciformes (Parenti and Grier, Reference Parenti and Grier2004; Mazzoni et al., Reference Mazzoni, Grier and Quagio-Grassiotto2014), the order belonging to the Chaetodontidae.
The male germinal epithelium of C. striatus undergoes morphophysiological changes throughout its life history that allows characterization of similar stages found in other Teleostei, during their reproductive cycle. The presence or absence of germline cysts along the germinal epithelium characterizes it as continuous or discontinuous (Grier and Taylor, Reference Grier and Taylor1998). In addition, the presence of a large quantity of spermatozoa in the lumen of the testicular lobules of C. striatus, as well as the number of germline cysts forming the germinal epithelium and changes in the interstitial compartment, allowed identification of potentially reproductive specimens during the study period.
Gonadal development and reproductive stages
In the present study, we proposed a terminology based on the dynamics of the female and male germinal epithelia to recognize stages similar to those proposed by Brown-Peterson and collaborators (Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-barbieri2011) to identify phases over the reproductive cycle of other Teleostei, namely Developing, Spawning-capable, Regressing and Regenerating. However, we prefer not to define stages as ‘reproductive phases’ within a reproductive cycle because we do not associate this type of gonadal development with seasons. Therefore, we could identify alterations that occur in the germinal epithelium without determining the reproductive period of C. striatus.
Characterization of the reproductive stages in the female C. striatus takes into account the presence and predominance in the gonad of oogonial proliferation and entrance into meiosis, morphological changes in the cytoplasm of the oocytes from the primary to secondary growth, and full-grown to mature oocytes and the spawning event. As regards males, characterization of the reproductive stages takes into account the presence and predominance in the gonad of spermatogonial proliferation and entrance into meiosis, differentiation of the gametic cells from spermatids to spermatozoa, and the release and presence of sperm in the lumen of the testicular lobules and duct.
The morphological data shown here confirm how informative is cellular dynamics of the germinal epithelium for understanding cyclic gonadal events during the adult reproductive lifecycle of fish in general. The presence of characteristic structures of gonadal remodelling, such as granulocyte-associated melanomacrophagic centres, reinforces the idea that these animals undergo constant alterations in germinal epithelium (Mazzoni et al., Reference Mazzoni, Lo Nostro, Antoneli and Quagio-Grassiotto2018). Another aspect that shows that the animals studied are sexually mature and have already completed reproductive cycles is the presence of a thick tunica albuginea. This capsule formed by connective tissue becomes thicker with each completed reproductive cycle (Brown-Peterson et al., Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-barbieri2011). The connective tissue that forms the interstitial compartment also becomes more developed after gamete extrusion (Brown-Peterson et al., Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-barbieri2011). This occurs due to granulocytes invasion for tissue remodelling, allowing renewal of the germinal compartment for the next reproductive period.
This knowledge on gametogenesis and reproductive stages of marine species, as in butterflyfish analyzed here, can become a fundamental tool for development of new strategies for captive breeding of several species. Therefore, the data presented here can contribute to knowledge on the life history of C. striatus and butterflyfish in general, as couple formation and reproductive behaviours are moderately conserved among Chaetodon (Nowicki et al., Reference Nowicki, O’Connell, Cowman, Walker, Coker and Pratchett2018).
In addition, information provided in this study supports the hypothesis that the mechanisms involved in changes in germinal epithelium are conserved during the evolutionary process of the Teleostei fish, showing only minor modifications and adaptations resulting from the specific reproductive biology of each species or group.
Acknowledgements
The authors would like to thank the Brazilian Agencies CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior); Instituto de Pesca – Base de Ubatuba; Biologists Ana Paula dos Santos and Francisco Costa; Veterinary Veronica Takatsuka and Vitor Spandri; and Zootechnist Otávio Mesquita de Souza.
Author contributions
Isabelle Leite Bayona Perez performed sample collection and preparation, performed the experiments, analyzed data and discussed the results. Talita Sarah Mazzoni helped to supervise the project, contributed to the experiments, analyzed data and wrote final version of the manuscript. Irani Quagio-Grassiotto conceived and designed the experiments, and reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical Standards
Not applicable.