Introduction
Climatic conditions in the area of release of biological control agents can influence whether establishment succeeds or fails and can determine whether the agent species will proliferate sufficiently to have an impact on the target weed (Debach & Rosen, Reference Debach and Rosen1991; Dent, Reference Dent1991; Day & Neser, Reference Day, Neser and Spencer2000). In order to predict the range of conditions that will suit an agent species, information is needed on the development, survival and reproduction of the insects under different climatic conditions. Of these, temperature probably has the greatest influence on the geographic distribution and abundance of insects (Howe, Reference Howe1967; Campbell et al., Reference Campbell, Franzer, Gilbert, Gutierrez and Mackauer1974; Kramer et al., Reference Kramer, Stinner and Hain1991; Lamb, Reference Lamb1992; Gilbert & Raworth, Reference Gilbert and Raworth1996; Woodson & Jackson, Reference Woodson and Jackson1996; Liu & Meng, Reference Liu and Meng1999; Gaston, Reference Gaston2003; Kalyebi et al., Reference Kalyebi, Overholt, Schulthess, Mueke and Sithanantham2006).
The root-feeding beetle, Longitarsus bethaeSavini & Escalona (Reference Savini and Escalona2005) (Coleoptera: Chrysomelidae), was collected from Mexico in 2000 and introduced into South Africa as a potential biocontrol agent for the alien invasive weed Lantana camara L. (Verbenaceae) (Simelane, Reference Simelane2005). A taxonomic revision indicated that L. bethae was a new species (Savini & Escalona, Reference Savini and Escalona2005), and that its distribution outside the collection site in Mexico is yet to be established. The life history of L. bethae was described by Simelane (Reference Simelane2005). The adult L. bethae perforate the epidermis and feed on the mesophyll tissue, producing an irregular smattering of pits. The eggs are laid singly or in small clusters of up to four eggs on the surface of the soil. Larvae burrow into rootlets and feed internally, producing elongate tunnels. Larvae pupate within 5 cm of the soil surface. Longitarsus bethae had been reared on lantana plants (variety 009 Light Pink) for over nine generations prior to the present study. Based on the host-specificity test results (Simelane, Reference Simelane2005), permission to release L. bethae into South Africa was granted by one (Department of Agriculture) of the two regulatory authorities in 2005.
The flea beetle predominantly overwinters in the egg stage (D.O. Simelane, personal observation). About 75% of eggs taken from plants that had been exposed to ovipositing females during autumn (April–May) failed to hatch until late September when temperatures were increasing during spring (September–October). Some early and middle stage larvae were also found in the roots during winter (June–July), suggesting that larval stages could overwinter as well. In 2005, average daily temperature at Rietondale Research Centre (S25°43′36.7″; E23°14′03.3″), Pretoria, ranged from 10 to 21, 6 to 19 and 10 to 27°C during autumn, winter and spring seasons, respectively. For L. bethae to succeed, egg hatch should coincide with the onset of summer rains because soil moisture has been shown to influence both egg development and larval survival (Simelane, Reference Simelane2006). The development of a degree-day (DD) model for predicting the time of egg hatch in the field could be useful in determining the likelihood of larvae appearing when soil conditions are suitable. The threshold temperature for egg hatch was therefore determined, and a DD model was developed in the laboratory to predict the time of egg hatch.
Photoperiod is another abiotic factor that affects the biology and behaviour of insects, sometimes resulting in the development of seasonal morphs (McPherson, Reference McPherson1974; Denlinger et al., Reference Denlinger, Giebultowicz and Saunders2001; Goehring & Oberhauser, Reference Goehring and Oberhauser2002; Danks, Reference Danks2003). Photoperiod is probably the main factor regulating reproductive diapause (McPherson, Reference McPherson1975; Denlinger, Reference Denlinger2002; Chocorosqui & Panizzi, Reference Chocorosqui and Panizzi2003). A decline in reproductive output of L. bethae was observed during four successive autumn seasons in a naturally-lit quarantine glasshouse in Pretoria, suggesting induction of reproductive diapause by shortening day length (Simelane, Reference Simelane2006). Therefore, a study was undertaken to determine whether photoperiod affected oviposition, percentage egg hatch and duration of egg development of L. bethae.
Relative humidity (RH) is a third climatic factor that could also influence survival of L. bethae eggs because the eggs are deposited on or very near the soil surface (Simelane, Reference Simelane2006) where they are subject to greater fluctuations in humidity than at greater depths. Desiccation could be particularly problematic if favourable DD stimulates embryonic development prior to the onset of seasonal rains (Hinton, Reference Hinton1981). As a result, the influence of humidity on oviposition and egg hatch were investigated in the laboratory.
Materials and methods
Rearing and extraction of L. bethae eggs
Large quantities of eggs were required for experiments on the effects of temperature and relative humidity on egg hatch. To concentrate oviposition, a group of approximately 400 newly emerged, unsexed L. bethae adults was enclosed in a gauze cage (0.55×0.55×0.95 m) with a single potted plant grown in a large pot (10 l) for eight days. Females started laying after a pre-oviposition period of about five days. To facilitate egg recovery, eggs were laid on sandy soil placed at a depth of 3 cm from the soil surface of each potted plant (Simelane, Reference Simelane2006). Eggs were later extracted from the sand using a sieve-flotation procedure (Foster et al., Reference Foster, Ruesink and Luckmann1979). In this procedure, soil was gently washed through a series of three-stacked sieves of downwardly decreasing mesh size (1.00 mm, 0.4 mm and 0.2 mm). Eggs together with the soil matter of the same size were collected on the last sieve. The mixture of eggs, mineral and organic material collected in this sieve was thoroughly rinsed out into a flask and later passed through a filter funnel and the filtrate was checked for eggs under a dissecting microscope. Eggs were removed from the filtrate using a fine brush.
Degree-day (DD) requirements and temperature effects on egg hatch and survival
Developmental rates and percentage survival of L. bethae eggs were determined in five separate temperature-controlled growth chambers set at constant temperatures of 12, 17, 22, 27 and 32°C, respectively. Temperatures in each growth chamber were checked regularly with a digital thermometer. Newly laid eggs (<24 h old) were placed in batches of 50 on moist filter paper in Petri dishes. Five batches, giving five replicates at each temperature, were enclosed in each of the growth chambers and were checked on a daily basis to record incubation periods and the proportion of eggs hatched. The threshold temperature for egg hatch was estimated by the x-intercept method derived from linear regressions of incubation periods against temperature (Arnold, Reference Arnold1959). In this method it was assumed that the rate of egg development and the range of temperature tested were linear, and that the x-intercept of the regression line indicates the minimum temperature at which egg development occurred. The DD above the temperature threshold required to complete egg development was estimated by the reciprocal of the slope of the fitted regression line (i.e. DD=1/x). A negative exponential curve was used to describe the relationship between the temperature and the duration of egg development. To obtain a graph for the exponential curve, a non-linear regression of the form t=a−bx was used, where t=duration in days, and x=temperature (°C). The parameters a and b were estimated by regression. The percentage survival curve of eggs kept under constant temperatures was graphically presented as means and standard errors.
Effect of photoperiod on oviposition and subsequent egg hatch of L. bethae
A comparative study of the influence of day length on oviposition and subsequent egg hatch was conducted under two photoperiodic regimes (16:8 h L:D and 8:16 h L:D) in the laboratory. Ten pairs of newly emerged L. bethae beetles were enclosed with a potted lantana plant (009 LP variety) in a clear plastic cage (30×30×40 cm) covered with a plastic gauze, and kept in a growth chamber for 10 days under a constant temperature (28±1°C), RH of 60%, and at either long (16:8 h) or short (8:16 h) photoperiods. Sand was placed at 3-cm depth on the surface of each pot to facilitate the recovery of eggs. The experiment was replicated 10 times, with each cage (plant) representing a replicate. Eggs were collected from each plant, and subsamples in batches of 100 eggs were placed on a Petri dish with a moist filter paper and kept at a room temperature (25±1°C), and in complete darkness inside a ventilated black box till they hatched. Comparisons of egg counts, pre-eclosion period and percentage egg hatch between the two photoperiodic regimes were made. Students' t-test was used to determine significances between the two photoperiodic regimes (Statistica, 2004). In order to stabilize the variance, the data were initially transformed to square roots before being subjected to parametric Students' t-test.
Effect of relative humidity on oviposition
Ten pairs of newly emerged adults were housed in isolation cages (30×30×40 cm), and were placed in glasshouses with either low (20–45%) or high (70–95%) RH for a period of 14 days. In both humidity regimes, the temperature ranged between 22±2°C at night and 28±2°C during the day, and the photoperiod was maintained at 14:10 h (L:D) throughout the study. In each isolation cage, eggs were laid on the sand to a depth of 3 cm of the surface to facilitate egg recovery. After a 14-day oviposition period, eggs recovered from the sand at both humidity regimes were counted and compared. Students' t-test was used to determine comparisons of oviposition and egg hatch of L. bethae between the two humidity regimes. In order to stabilize the variance, the data were initially transformed to square roots.
Effect of relative humidity on egg hatch
Relative humidity levels were maintained at constant levels within glass screw-capped jars (10×10×20 cm) using saturated salt solutions as described by Winston & Bates (Reference Winston and Bates1960). The jars were kept in separate controlled growth chambers set at a constant temperature (25°C). Six humidity levels were achieved: 21±1% with sodium hydroxide (NaOH); 45±1% with pure honey; 64±1% with 25 ml honey+10 g sugar; 74±1% with sodium chloride (NaCl); 86±1% with potassium chloride (KCl); and 95% with potassium dichromate (K2Cr3O7). Vial caps, measuring 2 cm in diameter, were used to place a batch of 50 newly laid L. bethae eggs (24 h old). The eggs were placed on a meshed platform raised 5 cm above the salt solution and left for 3, 6, 9 and 12 days before being transferred onto moist filter paper in a Petri dish at room temperature (25°C). The incubation of eggs at 25°C was done in order to determine their viability after exposure to the various humidity levels for different lengths of time. The experiment was repeated three times. Data were subjected to analysis of variance (ANOVA), and Fisher's protected least significant difference (LSD) was applied for separation of means (Statistica, 2004). The square-root transformation of counts did not change the significance of the analysis; thus the results and means from untransformed data are reported.
Results
Degree-day requirements and temperature effects on egg hatch and survival
The effect of temperature on the rate of development of L. bethae eggs is presented in fig. 1. At 12°C, eggs failed to hatch. Development rate increased rapidly with increasing temperatures between 12 and 27°C, for which the regression equation was found to be y=0.0056x−0.063 (r2=0.98; P<0.05). Calculated as the reciprocal of the slope of this regression line, the DD required for egg hatch was found to be 178.6. The regression line shows that the temperature threshold (To) of 11.3°C was required for egg hatch. The relationship between duration of egg development and temperature was best described by a negative exponential equation (t=125.7−0.086x), where t=duration in days, and x=temperature, which shows that developmental times decreased with increasing temperatures.

Fig. 1. Duration of development (pre-eclosion period, ▲) and development rate (●) of eggs of Longitarsus bethae under constant temperatures.
Mean survival of L. bethae eggs varied from 27 to 56% at 32 and 17°C, respectively, and was optimum between 17 and 25°C (fig. 2). Although the pre-eclosion period was longer at 17°C, egg survival at 17 and 22°C did not differ significantly, averaging 56 and 55%, respectively (fig. 2). A significant decline in egg survival was observed when temperature was increased from 27 to 32°C (F=53.61; P<0.001; n=50), decreasing from 45% at 27°C to 27% at 32°C. One hundred percent mortality was observed when eggs were exposed to 37°C, indicating that the upper lethal temperature limit was between 32 and 37°C.

Fig. 2. Percentage survival (mean±SE) of Longitarsus bethae eggs under five constant temperatures.
Effect of photoperiod on oviposition and subsequent egg hatch of L. bethae
There was no significant difference in oviposition between females exposed to short and long photoperiod regimes during the 10-day period (table 1). Exposure of newly emerged adults to either short or long day length during oviposition had no effect on subsequent egg-hatch and incubation periods (table 1). Production of viable eggs also showed that mating activity was not hampered by the reduction of photoperiod.
Table 1. Oviposition by five females Longitarsus bethae during a 10-day period and subsequent egg hatch under two photoperiod regimes.

Data are mean±SE.
Effect of relative humidity on oviposition
Oviposition was almost the same at both low and high humidity regimes (t=−0.255; P=0.80). During the 14-day period, a group of 10 females laid 745.1±38.4 (±SE) and 759.9±43.1 eggs under low and high humidity conditions, respectively.
Effect of relative humidity on egg hatch
Longitarsus bethae eggs were sensitive to aridity, and no egg hatch occurred after eggs were exposed to humidity levels below 63% for more than 3 days at 25°C (table 2). Only 1.5% eggs hatched when they were exposed to 65±1% RH for up to 3 days. About 47.5% of eggs hatched when exposed to 74±1% RH for 3 days but viability declined when the eggs were kept at this humidity level for more than 3 days, with only 2% hatching after 12 days of exposure to 74±1% RH. Optimum egg survival was observed when eggs were kept between 86±1 and 95% RH for up to 12 days. Although a slight decline in egg hatch was observed on the 12th day at 85±1% RH, the viability of eggs kept at 95% RH did not decline during the 12-day pre-eclosion period. The data indicated no significant influence of humidity on the duration of the pre-eclosion periods (table 3).
Table 2. Percentage survival of Longitarsus bethae eggs kept at six constant humidity levels and at a constant temperature (25°C) for 3 to 12 days.
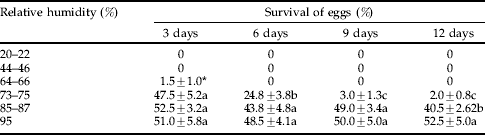
Means within the same humidity level followed by the same letter are not significantly different, P=0.05, using LSD test.
* Value was not included in statistical analysis.
Table 3. Pre-eclosion period of Longitarsus bethae eggs kept at six constant humidity levels and at a constant temperature (25°C) for 3 to 12 days.

–Indicates complete mortality before egg hatch.
Discussion
The establishment of biological control agents depends upon a number of factors, including climatic conditions in the area of release conducive to the survival and growth of insects (Debach & Rosen, Reference Debach and Rosen1991; Dent, Reference Dent1991). In the current study, both humidity and temperature had an effect on egg development. Although climatic factors interact under natural conditions, the present study suggests that both temperature and humidity could play some role in the population dynamics of L. bethae. In the event that eggs are the overwintering stage, as observed by the author during preliminary studies, the DD model determined in the present study will enable prediction of egg hatch in the field. The data analysis showed that the temperature threshold for eggs to hatch was 11.3°C, and yet no egg hatch was observed at 12°C. Perhaps the most likely explanation for this discrepancy may be that continuous exposure to low temperatures allows some embryonic development but not the physiological adjustments necessary for eclosion of neonates from the egg (Force & Messenger, Reference Force and Messenger1964). The complete arrest of egg hatch during constant exposure to 12°C and a resumption of normal development on restoration to 25°C may be of some use in preserving and distributing the agent during a mass-release programme. However, there are limitations on how long this type of manipulation can be exploited because mortality of L. bethae eggs increased after constant exposure to 12°C for more than two months (D.O. Simelane, personal observation).
There was no indication that photoperiod alone could induce reproductive diapause. The diapause phase observed during autumn in a naturally-lit glasshouse could have been a result of several factors acting in concert such as photoperiod and temperature signals and the host plant (Zaslavski et al., Reference Zaslavski, Zinovjeva, Umarova and Reznik1999; Denlinger, Reference Denlinger2002; Goehring & Oberhauser, Reference Goehring and Oberhauser2002; Perdikis et al., Reference Perdikis, Lykouressis and Economou2004). It is also likely that the onset of diapause in L. bethae requires signals received during earlier life stage(s) (Denlinger, Reference Denlinger2002). There is also evidence that larvae of some insect species enter diapause in response to high temperature and declining day length rather than to low temperatures, thus avoiding producing a next generation that might fail because of subsequent low temperatures (Saunders, Reference Saunders1971). It is also likely that low soil temperature may either delay the development or trigger diapause of immature stages of L. bethae during winter months.
Whilst the study showed that L. bethae could lay within a wide range of humidity levels, eggs need high humidity or damp surface layers of soil for successful development. For this reason, during periods of low rainfall under normal summer temperatures, egg survival will probably be adversely affected, especially in exposed habitats where the surface of the soil becomes extremely dry. It seems probable that levels of humidity necessary for egg survival will prevail only during the wet summer season. As demonstrated in previous investigations (Simelane, Reference Simelane2006), most of the unfed first-instar larvae and pupae could be eliminated by moisture stress which prevails in dry soil conditions. Because differing levels of humidity did not influence the period of egg development, the timing of egg hatch would be dictated by temperature, regardless of the levels of humidity. Such a phenomenon also occurred in six African egg parasitoids (Hemiptera: Trichogrammatidae) (Kalyebi et al., Reference Kalyebi, Overholt, Schulthess, Mueke and Sithanantham2006). However, temperature and humidity regimes in the summer rainfall regions of South Africa where L. camara is a problem are anticipated to be well within the ranges that are tolerated by L. bethae.
In order to fully understand the population dynamics of L. bethae, future investigations should endeavour to give a full description of the phenology of this agent, which will take into account the effects of temperature on development of all life stages, including pre-oviposition and oviposition periods (Skinner et al., Reference Skinner, Ragsdale, Hansen, Chandler and Moon2004; Schaub et al., Reference Schaub, Graf and Butturini2005). To formulate a field emergence model for L. bethae, a field-based DD model similar to that developed by Skinner et al. (Reference Skinner, Ragsdale, Hansen, Chandler and Moon2004) whereby plants with soil cores containing an overwintering stage were held at constant temperatures, should be developed after the insect has been released into the environment. However, the knowledge obtained in the present study will be very useful for mass rearing as well as field release programmes for L. bethae.
Acknowledgements
The author is indebted to Prof. J.H. Hoffmann of the University of Cape Town, Dr A.J. Urban and Mr Robert S. Nofemela, both of the Plant Protection Research Institute (PPRI), for valuable advice and comments on earlier drafts of this manuscript. Special thanks to Mr Matlala Phenye and Molefe Thobakgale for their technical support during the study. The project was financially supported by the Working for Water programme of the Department of Water Affairs and Forestry and PPRI of the Agricultural Research Council.


