INTRODUCTION
The species composition of communities in particular localities is shaped by both ecological and evolutionary factors (Vuilleumier and Simberloff, Reference Vuilleumier and Simberloff1980; Ricklefs, Reference Ricklefs1987; Wiens, Reference Wiens1989). From an ecological perspective, it is determined by the availability of necessary resources for all species and whether the latter can acquire these resources (e.g., Soberon, Reference Soberon2007), while from an evolutionary perspective, a community is composed of species that either originated locally or migrated from elsewhere (e.g., Wiens Reference Wiens1989). However, for organisms that utilize other organisms as their resources, the availability of resources or habitats themselves has a substantial evolutionary component. In particular, this is true for parasites than cannot exist without their hosts and for which community composition may be shaped by combined ecological, evolutionary and biogeographical forces underlying host assemblages (see review by Hoberg and Brooks, Reference Hoberg, Brooks, Morand and Krasnov2010).
Parasite communities represent important and convenient models for investigations of spatial rules of community assembly and the growing field of spatial networks (Gravel et al. Reference Gravel, Massol, Canard, Mouillot and Mouquet2011). This is because (a) parasites form a large proportion of the diversity of life, probably outnumbering free-living organisms (Windsor, Reference Windsor1998; Poulin and Morand, Reference Poulin and Morand2000); (b) parasites of the same taxon share a trophic level; and (c) the resource niche of a parasite is easily defined (the set of host species used). The spatial distribution of parasite communities is fragmented among host individuals, among host species within a location, and among locations (Esch et al. Reference Esch, Shostak, Marcogliese, Goater, Esch, Bush and Aho1990; Poulin, Reference Poulin2007). The structure and assembly rules of parasite communities at host individual and species levels have been extensively studied during the last 2 decades (see Poulin, Reference Poulin2007 for review). In contrast, the most encompassing hierarchical level, that of communities of parasites consisting of all parasites on all hosts in a given location, or compound communities (sensu Esch et al. Reference Esch, Shostak, Marcogliese, Goater, Esch, Bush and Aho1990), has received less attention. One of the reasons for this is that parasite species in compound communities are less likely to interact with each other than those in communities at lower hierarchical levels (Combes, Reference Combes2001; Poulin, Reference Poulin2007). As a result, the mechanisms shaping compound communities of parasites are still unclear.
Positive relationships between species diversity and habitat diversity are well documented (e.g., Rosenzweig, Reference Rosenzweig1992). Given that (a) hosts are of the utmost importance for parasites and represent not only their resources, but also their habitats, and (b) a parasite is able to exploit successfully only a (more or less) limited spectrum of host species, it is not surprising that the species richness of parasite communities also correlates positively with that of host communities (e.g., Watters, Reference Watters1992; Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Degen2004a), although there are some exceptions (Zhang et al. Reference Zhang, Gong, Feng, Duna, Wu, Weng and Lu2002; Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Poulin2007a; see below). For example, in small mammals and their flea parasites, species richness and composition of the parasite community harboured by a particular host species have been shown to depend on the species richness and composition of the surrounding host community (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Degen2004b, Reference Krasnov, Shenbrot, Mouillot, Khokhlova and Poulin2005), so that different host populations from the same species can harbour very different flea assemblages if they are part of highly dissimilar host communities (Krasnov et al. Reference Krasnov, Shenbrot, Mouillot, Khokhlova and Poulin2005). As a result, when the entire flea assemblages and entire small mammalian host assemblages of different locations are compared, dissimilarity in flea species composition is strongly affected by dissimilarity in host species composition, although the roles of environmental dissimilarity and geographical distance are also substantial (Krasnov et al. Reference Krasnov, Mouillot, Shenbrot, Khokhlova and Poulin2010a). However, this has only been tested for the Palaearctic (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Poulin2007a). It is important to note though that the relationships between host community structure and parasite community structure may differ among biogeographical realms due to differences in levels of host specialization by parasites, history of host-parasite associations, and/or landscape effects on parasite diversification.
As noted above, the species composition of a local community has both ecological and evolutionary (historical) components. With respect to parasites, the ecological component involves the occurrence of host species that a parasite can successfully exploit, while the evolutionary component consists of both the host species on which a parasite originated and other hosts to which it switched to from its original host (e.g., Paterson and Gray, Reference Paterson, Gray, Clayton and Moore1997). This component can be inferred from a comparison between host and parasite phylogenies (Brooks, Reference Brooks1988; Paterson and Banks, Reference Paterson and Banks2001). For the sake of determining how parasite communities were formed, the effects of these two components should be disentangled (Poulin and Krasnov, Reference Poulin, Krasnov, Morand and Krasnov2010), but the lack of necessary tools has precluded this in earlier studies. The new metric of phylogenetic community dissimilarity (PCD) proposed by Ives and Helmus (Reference Ives and Helmus2010) now allows distinction between the roles of history and ecology in shaping species composition of parasites or any other community of organisms. This metric can be partitioned into 2 components, namely (a) a purely compositional (hereafter referred to as compositional) non-phylogenetic component reflecting shared species between 2 communities, and (b) a phylogenetic component that reflects phylogenetic relationships among non-shared species. One of the advantages of this metric is that a species not shared between 2 communities increases their similarity if the community from which it is absent nevertheless contains species that are phylogenetically related to it. This is especially important for studies of community composition of parasites because different but phylogenetically-close host species often share their parasites (Poulin, Reference Poulin2010).
To distinguish between ecological versus evolutionary effects of host assemblages on the species composition of parasite assemblages, we examined relationships between compositional and phylogenetic components of community dissimilarity of fleas, and compositional and phylogenetic components of community dissimilarity of their small mammalian hosts, respectively, across distinct regions. This was carried out at 2 scales, namely (a) within each of 4 biogeographical realms (Afrotropics, Palaearctic, Neotropics, Nearctic), and (b) within the Old World (Afrotropics, Palaearctic) and the New World (Neotropics, Nearctic). Our hypothesis was that compositional and/or phylogenetic dissimilarity of host assemblages determines compositional and/or phylogenetic dissimilarity of flea assemblages; our study design allows it to be tested separately in different realms, as well as at different scales. The approach implemented here differs from those of our earlier studies. In contrast to Krasnov et al. (Reference Krasnov, Mouillot, Shenbrot, Khokhlova and Poulin2010a), here we consider not only compositional but also phylogenetic dissimilarity of parasite assemblages between host communities. In contrast to Krasnov et al. (Reference Krasnov, Mouillot, Shenbrot, Khokhlova, Vinarski, Korallo-Vinarskaya and Poulin2010b), we now consider both compositional and phylogenetic dissimilarity of parasite assemblages between host communities rather than between host species. For the first time, we investigate patterns of dissimilarity between parasite assemblages not only in the Palaearctic but also in 3 other biogeographic realms.
Indeed, several studies have demonstrated that the relationships between the structure of exploiter communities and that of exploited communities can vary across the globe. For example, the diversity of nectarivorous birds and bats has been shown to be affected by the diversity of their food plants in the New World but not in the Old World (Fleming, Reference Fleming2005), while the opposite was true for the species richness of fleas and their small mammalian hosts (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Poulin2007a). Here, we further hypothesized that the relationships between compositional and/or phylogenetic dissimilarities of host and flea assemblages would vary among biogeographical realms. In addition, biogeographical realms, by definition, are areas with more or less homogenous fauna or flora. This homogeneity can be both compositional and phylogenetic. The latter implies that different communities across a realm are composed of species belonging to the same phylogenetic lineages, while different phylogenetic lineages characterize different realms. Consequently, we predicted that the effect of phylogenetic dissimilarity of host assemblages on that of parasite assemblages is more likely to be realized or, at least, is more pronounced, at the larger scale.
The relationships between compositional and/or phylogenetic similarity of assemblages of parasites and those of their hosts might be confounded by the effect of geographical distances among assemblages. In fact, one of the most ubiquitous geographical patterns is distance decay of similarity (Nekola and White, Reference Nekola and White1999), whereby similarity in plant or animal community composition between any 2 localities decreases with increasing geographical distance between them. Even if distance decay of similarity applies to plants and animals, free-living and parasitic, and terrestrial and aquatic groups (Poulin, Reference Poulin2003; Oliva and González, Reference Oliva and González2005; Soininen et al. Reference Soininen, McDonald and Hillebrand2007; La Sorte et al. Reference La Sorte, McKinney, Pyšek, Klotz, Rapson, Celesti-Grapow and Thompson2008), it mainly ignores trophic relationships while they may shape those patterns as highlighted by the emerging field of trophic geography (Gravel et al. Reference Gravel, Massol, Canard, Mouillot and Mouquet2011). Thus, to control for the possible confounding effect of geographical distance, we included it in our models.
MATERIALS AND METHODS
Composition of flea and host assemblages and geographical distances
Data were obtained from 63 published studies (7 from Afrotropics, 28 from Palaearctic, 8 from Neotropics and 20 from Nearctic) that reported flea species found on each small mammal species (Didelphimorphia, Paucituberculata, Macroscelidea, Erinaceomorpha, Soricomorpha, Lagomorpha and Rodentia) in a particular region (see Supplementary material, Online version only; see Krasnov et al. (Reference Krasnov, Mouillot, Shenbrot, Khokhlova, Vinarski, Korallo-Vinarskaya and Poulin2010b) for map of the Palaearctic regions). In total, the database comprised data on 433 flea and 392 mammal species. Cosmopolitan flea species parasitic on commensal rodents across the world (Xenopsylla cheopis, Leptopsylla segnis, Nosopsyllus fasciatus) and cosmopolitan commensal rodents (Rattus norvegicus, Mus musculus) were excluded from the analysis. From these data and for each realm, we compiled matrices of flea and host species occurrence in a region. The geographical distances between pairs of regions were calculated as the linear distance between the centres of each region (described in the respective sources), obtained from a map using the ArcGIS Desktop 9.2 software. These distances were log+1-transformed prior to analyses.
Phylogenetic community dissimilarity (PCD)
A variety of metrics has been proposed to compare communities based on the phylogenetic relationships of the species they comprise (e.g., Rao, Reference Rao1982; Warwick and Clarke, Reference Warwick and Clarke1995; Pavoine et al. Reference Pavoine, Dufour and Chessel2004; Chave et al. Reference Chave, Chust, Thebaud, Storch, Marquet and Brown2007; Hardy and Senterre, Reference Hardy and Senterre2007; Bryant et al. Reference Bryant, Lamanna, Morlon, Kerkhoff, Enquist and Green2008). From this variety, 2 groups of metrics can be distinguished (Ives and Helmus, Reference Ives and Helmus2010). One group of metrics is based on Rao's quadratic entropy (1986) for phylogenetic distance. For example, ΠST of Hardy and Senterre (Reference Hardy and Senterre2007) (see Chave et al. Reference Chave, Chust, Thebaud, Storch, Marquet and Brown2007 for similar metric) involves calculating pairwise phylogenetic distances between species taken from the same communities and those between species from all communities, and measuring the degree to which the former exceed the latter. Metrics in the other group measure species turnover while taking into account phylogenetic information. For example, UniFrac of Lozupone and Knight (Reference Lozupone and Knight2005) represents the sum of the branch lengths that 2 communities share on a phylogenetic tree. Similarly, the Phylosor index (Bryant et al. Reference Bryant, Lamanna, Morlon, Kerkhoff, Enquist and Green2008) uses the phylogenetic diversity of Faith (1992), which is the sum of branch lengths in a phylogenetic tree joining the basal node to the tips of all the species in a community. Then Phylosor measures the phylogenetic diversity of species shared between communities divided by the average phylogenetic diversity of species belonging to each community (e.g., Morlon et al. Reference Morlon, Schwilk, Bryant, Marquet, Rebelo, Tauss, Bohannan and Green2011).
Recently, Ives and Helmus (Reference Ives and Helmus2010) proposed another method to compare communities using phylogenetic information. Furthermore, they demonstrated that their index of phylogenetic community dissimilarity (PCD) has advantages in comparison to earlier metrics. First, in contrast to UniFrac, for example, it is independent of the species richness of communities. Second and most important, after removal of phylogenetic information, this index collapses to a modified Sørensen's index (see below). As a result, PCD can be partitioned into non-phylogenetic (compositional; PCDc) and phylogenetic (PCDp) components dependent on non-shared species (see above). Calculation of PCD is based on calculation of the phylogenetic species variability metric (PCV; Helmus et al. 2007) measuring the phylogenetic diversity of species in a community (see Ives and Helmus, Reference Ives and Helmus2010 for details and equations). The compositional component (PCDc) is measured as 1 minus the Sørensen's index modified by multiplying the denominator by the total number of species in order to remove its dependence on community size. Obviously, if all species are phylogenetically independent, then PCD=PCDc, while the phylogeny-dependent component of PCD (PCDp) is calculated as the quotient of PCD and PCDc. This partitioning allows one to assess the importance of phylogeny in community structure. In addition, the simulation study of Ives and Helmus (Reference Ives and Helmus2010) demonstrated that statistical properties of PCD prevail over those of both UniFrac (Lozupone and Knight, Reference Lozupone and Knight2005) and ΠST (Hardy and Senterre, Reference Hardy and Senterre2007).
Data analyses
We calculated matrices of PCDc and PCDp separately for host and parasite assemblages across all possible pairs of regions within a realm and within a hemisphere (i.e. Old and New Worlds) using the package ‘picante’ (Kembel et al. Reference Kembel, Cowan, Helmus, Cornwell, Morlon, Ackerly, Blomberg and Webb2010) implemented in the R 2.13.0 software environment (R Development Core Team, 2011). Hereafter, compositional and phylogenetic dissimilarity are treated in separate analyses. Phylogenetic trees were constructed based on trees of Bininda-Emonds et al. (Reference Bininda-Emonds, Cardillo, Jones, MacPhee, Beck, Grenyer, Price, Vos, Gittleman and Purvis2007) for hosts and Whiting et al. (Reference Whiting, Whiting, Hastriter and Dittmar2008) for fleas. The latter was modified as detailed in Krasnov et al. (Reference Krasnov, Poulin and Mouillot2011).
Then, we analysed the effect of compositional or phylogenetic dissimilarity in host regional assemblages, and geographical distance between assemblages, on the compositional or phylogenetic dissimilarity of flea regional assemblages within a realm, respectively, using multiple regressions on distance matrices (MRM; see Manly, Reference Manly1986; Legendre and Legendre, Reference Legendre and Legendre1998; Lichstein, Reference Lichstein2007 for details). MRM is an extension of partial Mantel analysis used to investigate relationships between a multivariate response distance matrix (in our case, pairwise dissimilarity in flea assemblages) and any number of explanatory distance matrices (in our case, pairwise dissimilarity in host assemblages and pairwise geographical distances) (Lichstein, Reference Lichstein2007). The significance of the model and regression coefficients was tested by permuting a response matrix while the explanatory matrices are held constant. The rows and corresponding columns in the response matrices are permuted simultaneously and the coefficient of determination of the model and regression coefficients are calculated for each permutation to generate a null distribution (Legendre and Legendre, Reference Legendre and Legendre1998; Lichstein, Reference Lichstein2007). All probabilities were based on 10 000 permutations. Analyses were performed using the package ‘ecodist’ (Goslee and Urban, Reference Goslee and Urban2007) implemented in the R 2.13.0 software environment (R Development Core Team, 2011).
The effect of compositional or phylogenetic dissimilarity in host regional assemblages on the compositional or phylogenetic dissimilarity of flea regional assemblages across realms within a hemisphere was also analysed using MRM. However, we did not include geographical distance in these analyses because of (a) oceanic and/or terrestrial gaps between realms and (b) the weak (if any) effect of geographical distance in within-realm analyses (see Results section).
Since our explanatory matrices were not independent (because distance decay of similarity for host assemblages was likely), we implemented additional partial multiple regressions on distance matrices to estimate the ‘pure’ effect of each explanatory matrix for within-realm analyses. To extract a ‘pure’ effect for each independent variable, we calculated coefficients of determination (R2) for the entire model with 2 independent matrices and for the model without one of the matrices (matrix of interest). The ‘pure’ effect of this matrix of interest was then obtained following the decomposition of Legendre and Legendre (Reference Legendre and Legendre1998) for multiple fractions of explanations (see also Borcard et al. Reference Borcard, Legendre and Drapeau1992; Lichstein, Reference Lichstein2007). We report R 2 values for the entire models as well as the associated P-values, whereas we express the fraction of variation explained by each ‘pure’ effect as a percentage. Detailed explanations and discussion of this method can be found elsewhere (Borcard et al. Reference Borcard, Legendre and Drapeau1992; Legendre and Legendre, Reference Legendre and Legendre1998; Legendre et al. Reference Legendre, Borcard and Peres-Neto2005; Tuomisto and Roukolainnen, Reference Tuomisto and Roukolainen2006; Krasnov et al. Reference Krasnov, Mouillot, Shenbrot, Khokhlova, Vinarski, Korallo-Vinarskaya and Poulin2010b).
RESULTS
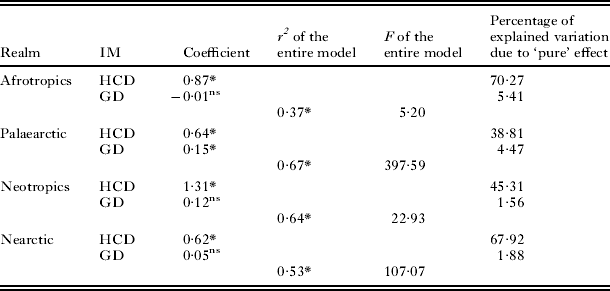
The multiple regressions of matrices of compositional dissimilarities of flea assemblages against matrices of compositional dissimilarities of host assemblages within realms demonstrated that dissimilarity in the former significantly increased with an increase in dissimilarity of the latter in all studied realms (Table 1). The lowest coefficient of determination was found for the Afrotropic realm, whereas coefficients of determination were rather similar in the regressions for the remaining 3 realms. An illustrative example for the Palaearctic realm is presented in Fig. 1. A significant effect of geographical distance was found only for the Palaearctic, but its ‘pure’ effect on flea dissimilarity was much lower than that of host dissimilarity. When the relationships between compositional dissimilarities of flea assemblages and compositional dissimilarities of host assemblages were analysed at the scale of hemisphere, they were found to be significantly positive in both the Old and the New World (coefficients 0·82 and 0·81, R2=0·80 and R2=0·63, F=2429·37 and F=640·75, respectively; P<0·0001 for both).

Fig. 1. Relationship between pairwise compositional dissimilarity of flea assemblages (PCDc fleas) and pairwise compositional dissimilarity of host assemblages (PCDc hosts) among distinct regions within the Palaearctic realm.
Table 1. Results of multiple regressions on distance matrices using a permutation method with the matrix of pairwise compositional dissimilarity of flea assemblages between regions as the dependent matrix, and matrices of pairwise compositional dissimilarity of host assemblages (HCD) and geographical distance (GD) between regions as independent matrices (IM)

* P<0·05, ns, non-significant (P>0·05).
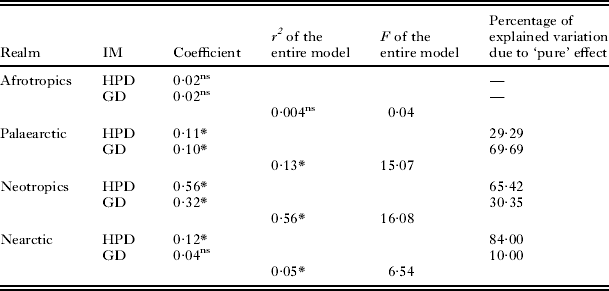
No relationship between phylogenetic dissimilarities of flea versus host assemblages across regions was found in the Afrotropics (Table 2). In contrast, phylogenetic dissimilarity of flea assemblages was affected by phylogenetic dissimilarity of host assemblages in the remaining realms (Table 2). In addition, when the effect of host phylogenetic dissimilarity on flea phylogenetic dissimilarity in the Palaearctic was analysed without accounting for geographical distance, it was found to be non-significant (R2=0·07, F=5·60, P=0·13). Although the effect of phylogenetic dissimilarity of host assemblages on that of flea assemblages in the Nearctic was significant, it was relatively weak (note R2 values in Table 2) (Fig. 2). The effect of geographical distance on flea phylogenetic dissimilarity was significant in the Palaearctic and Neotropics only with a stronger effect for the latter. The ‘pure’ effect of geographical distance was higher than that of host phylogenetic dissimilarity in the Neotropics, while the ‘pure’ effects of geographical distance and host phylogenetic dissimilarity were similar in the Palaearctic. Analyses at the higher scale (hemisphere) resulted in significant positive relationships between phylogenetic dissimilarities of flea and host assemblages in both the Old and the New World (coefficients 0·69 and 0·67, R2=0·31 and R2=0·47, F=262·67 and F=340·42, respectively; P<0·0001 for both) (see illustrative example with the New World in Fig. 2).

Fig. 2. Relationship between pairwise phylogenetic dissimilarity of flea assemblages (PCDp fleas) and pairwise phylogenetic dissimilarity of host assemblages (PCDp hosts) among distinct regions of the New World (across the Neotropic and the Nearctic realms).
Table 2. Results of multiple regressions on distance matrices using a permutation method with the matrix of pairwise phylogenetic dissimilarity of flea assemblages between regions as the dependent matrix, and matrices of pairwise phylogenetic dissimilarity of host assemblages (HPD) and geographical distance (GD) between regions as independent matrices (IM)
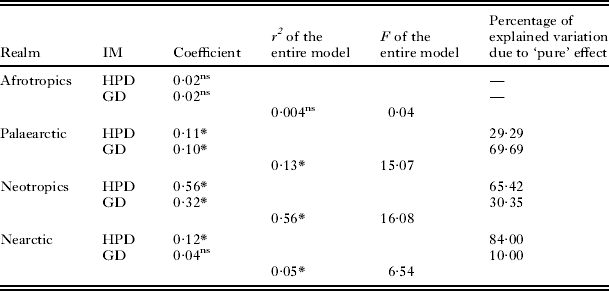
* P<0·05, ns, non-significant (P>0·05).
DISCUSSION
This study produced 3 main results. First, compositional dissimilarity in host assemblages strongly affected compositional dissimilarity in flea assemblages across regions independently of whether this relationship was considered within a realm or within a hemisphere. Second, the effect of phylogenetic dissimilarity of host assemblages on that of flea assemblages varied geographically and was scale dependent. At lower (within a realm) scale, the effect of phylogenetic dissimilarity of host assemblages on that of flea assemblages was found mainly in the realms of the New World. This effect was absent in the Afrotropics, while in the Palaearctic (a) it was revealed only if geographical distances were taken into account, and (b) its ‘pure’ effect was smaller than that of geographical distance (the opposite was true for the Neotropics). At the larger (within a hemisphere) scale, the effect of host phylogenetic dissimilarity on that of fleas was found in both hemispheres. In addition, the relationship between flea and host phylogenetic dissimilarities was weaker than that based on compositional dissimilarities (compare R2 values in Tables 1 and 2). In other words, the association between species compositions of fleas and hosts across regions was geography- and scale-invariant, while the association between the phylogenetic structures of flea and host assemblages (a) was most prominent in the New World and (b) was manifested at larger rather than smaller scale. Third, the effect of geographical distance on compositional and phylogenetic dissimilarity of flea assemblages was found in some, but not all realms.
It is commonly accepted that flea origins and diversification are associated with mammalian hosts (Traub, Reference Traub, Traub and Starke1980, Reference Traub and Kim1985; Medvedev, Reference Medvedev2005; Whiting et al. Reference Whiting, Whiting, Hastriter and Dittmar2008). The strong relationship between compositional dissimilarities of flea and host assemblages, with the concomitant weak (or lack of) relationship between their phylogenetic dissimilarities, suggests that the great proportion of fleas inhabiting a region did not co-speciate with co-occurring hosts. Instead, fleas occurring in a region either arrived to this region from elsewhere by switching from the host on which they originated, or originated in this region and switched to their current host after their original host became extinct. In both scenarios, the new host is only distantly related to the original host.
Host switching (i.e. colonization of a host from a different taxon) is a common historical event in the co-evolution of parasites and their hosts (Beveridge and Chilton, Reference Beveridge and Chilton2001; Roy, Reference Roy2001; Brooks et al. Reference Brooks, León-Règagnon, McLennan and Zelmer2006; Nuismer and Thompson, Reference Nuismer and Thompson2006). In particular, flea-mammal co-evolution has presumably involved numerous host switches (Krasnov and Shenbrot, Reference Krasnov and Shenbrot2002; Lu and Wu, Reference Lu and Wu2005). For example, jerboas (Dipodoidea) originated in central Asia (Shenbrot et al. Reference Shenbrot, Sokolov, Heptner and Kovalskaya2008), while pulicid fleas undoubtedly have an African origin (Traub, Reference Traub and Kim1985). Consequently, parasitism of pulicid fleas on jerboas can only be explained by switching from other hosts such as Gerbillinae (Krasnov and Shenbrot, Reference Krasnov and Shenbrot2002) that originated in Africa and dispersed to Eurasia no later than in the Miocene (Wessels, Reference Wessels1998). Obviously, switching is expected to be easier if the new host is closely related to the original host because closely-related hosts are likely to be more similar in their ecological, physiological and/or immunological traits than distantly-related host species (Brooks and McLennan Reference Brooks and McLennan1991; Harvey and Pagel, Reference Harvey and Pagel1991). However, switching of fleas from an original to a distantly-related host also seems to be rather common. In fact, the results of the study of flea phylogeny and host associations by Whiting et al. (Reference Whiting, Whiting, Hastriter and Dittmar2008) allowed to infer at least 4 inter-class host shifts (from Mammalia to Aves) and at least 1 inter-order host shift (from Rodentia to Chiroptera). Switching to distantly-related hosts can sometimes be facilitated by the lack of specific defence mechanisms (either immunological or behavioural or both) in these hosts. As a result, parasites exploiting a new host, only distantly related to the original one, may achieve even higher feeding and reproductive success as compared to those on the original host (Krasnov et al. Reference Krasnov, Korine, Burdelova, Khokhlova and Pinshow2007b).
A parasite can exploit any host with which it spatially co-occurs and from which it is able to extract the necessary resources and convert them into offspring (Combes, Reference Combes2001). Obviously, the spatial co-occurrence of a parasite and a host within a region does not depend on host phylogeny. Our results suggest that the ability of a parasite to exploit a host successfully also does not necessarily depend on it. Consequently, a large proportion of associations in a regional assemblage may result from ecological fitting (Janzen, Reference Janzen1985; Brooks et al. Reference Brooks, León-Règagnon, McLennan and Zelmer2006). The prerequisites of ecological fitting are that (a) many hosts share some resource necessary for a parasite independently of their phylogeny, and (b) the parasite tracks this resource rather than a host per se (Janzen, Reference Janzen1985; Brooks et al. Reference Brooks, León-Règagnon, McLennan and Zelmer2006). These prerequisites apply well to fleas (Krasnov et al. Reference Krasnov, Mouillot, Shenbrot, Khokhlova, Vinarski, Korallo-Vinarskaya and Poulin2010b).
Nevertheless, the phylogenetic dissimilarity of host assemblages had a certain effect on that of flea assemblages in the New World realms, although this effect was weak in the Nearctic. Earlier, we reported a difference between the Old World and the New World in 2 ecological patterns relating to flea-mammal associations. The first was that flea species richness appeared strongly positively correlated with host species richness across regions in the Old but not in the New World (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Poulin2007a). Secondly, there was a significant phylogenetic signal in the size of a flea's host spectrum for the Old but not the New World taxa (Krasnov et al. Reference Krasnov, Mouillot, Shenbrot, Khokhlova and Poulin2010a). We explained these differences by contrasting the history of flea-mammal associations in both hemispheres. This history may also be a reason behind the difference between the Old World and the New World in the existence of a relationship between flea versus host phylogenetic dissimilarity. Several facts hint that the history of flea-host associations has been shorter in the New World than in the Old World. First, hosts that support the majority of extant flea species belong to several lineages of rodents (Arvicolinae, Murinae, Gerbillinae, Cricetinae) and insectivores (Soricidae) that originated in the Old World (see Traub, Reference Traub, Traub and Starke1980 and references therein). Second, the total number of flea species is higher in the Old World than in the New World (although species richness is similar in the Neotropic and the Afrotropic realms, the flea fauna in the Palaearctic is three times richer than that of the Nearctic; Medvedev Reference Medvedev2005). Third, flea-host interactions in the Old World (the Palaearctic) have been shown to be relatively specialized compared to those in the New World (the Nearctic), so that each flea species interacts with relatively fewer host species in the former (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Poulin2007a). In other words, fleas in the New World may simply have had less evolutionary time to switch between distantly-related hosts than fleas in the Old World.
However, the phylogenetic dissimilarity of host assemblages significantly affected the phylogenetic dissimilarity of flea assemblages when considered at the scale of an entire hemisphere. The reason behind this is likely the relative phylogenetic homogeneity of both flea and host faunas within a realm and the sharp contrasts in phylogenetic structure of fleas and hosts between realms (in our case, in the Afrotropics versus the Palaearctic, and the Neotropics versus the Nearctic). For example, flea assemblages of the Afrotropic realm are characterized by the presence of families such as Xiphiopsyllidae and Chimaeropsyllidae and subfamilies such as Dinopsyllinae, that do not inhabit the Palaearctic, while the Palaearctic subfamily Amphipsyllinae has not been found in Sub-Saharan Africa. Similarly, tribes characteristic of the Neotropics such as Agastopsyllini and Ctenopariini do not occur in the Nearctic, while the Nearctic subfamily Anomiopsyllinae is absent from the Neotropics (Traub, Reference Traub, Traub and Starke1980; Medvedev, Reference Medvedev2005). Regarding hosts, rodent families such as Spalacidae, Myospalacidae, Dipodidae and Sminthidae and subfamilies such as Cricetinae and Calomyscinae inhabit the Palaearctic, but not the Afrotropics, while the opposite is true for the order Macroscelidea, rodent families Anomaluridae, Petromuridae, Bathyergidae, and Pedetidae, and rodent subfamilies Dendromurinae, Lophiomyinae and Mystromyinae. In the New World, the small mammal fauna of the Neotropics differs from that of the Nearctic by the presence of several families of hystricognath rodents as well as the orders Paucituberculata (Caenolestidae) and Microbiotheria (Microbiotheridae), and by the absence of rodent families Aplodontidae, Heteromyidae and Geomyidae (Wilson and Reeder, Reference Wilson and Reeder2005).
One of the surprising results of this study is that in many cases we failed to find distance decay of similarity. According to Soininen et al. (Reference Soininen, McDonald and Hillebrand2007), there are 3 main groups of mechanisms that act separately or together to produce the distance decay of similarity, namely (a) a decrease in environmental (abiotic and/or biotic) similarity with increasing distance; (b) the probability that a hard geographical barrier blocks dispersal increases with increasing distance; and (c) the limited mobility of many species. Given that a host represents a parasite's environment, our results suggest that the first group of mechanisms acts on flea assemblages, so that when environmental (=host) similarity effect is controlled for, geographical distance does not have a ‘pure’ effect of its own. Similarly, Krasnov et al. (Reference Krasnov, Shenbrot, Mouillot, Khokhlova and Poulin2005) showed that similarity in flea communities across different populations of the same rodent host was explained better by differences in rodent faunal composition between localities than by geographical distance. In addition, it is also possible that the phenomenon of distance decay of similarity is not as universal as previously thought (see also Vinarski et al. Reference Vinarski, Korallo, Krasnov, Shenbrot and Poulin2007).
Concluding, it should be noted that the relatively weak (if any) relationships between phylogenetic components of dissimilarity of flea versus host assemblages within realms might be associated with the fact that the majority of flea species are, in general, not highly host specific. The effect of host phylogeny on the structure of assemblages of more host-specific parasites (such as, for example, lice or monogenean flatworms) could be more pronounced, while this effect for parasites with complex life cycles could be more complicated. Consequently, the relationships between compositional and phylogenetic dissimilarity of parasite assemblages and those of host assemblages require further investigation using other parasite-host associations.
ACKNOWLEDGEMENTS
We thank two anonymous referees for their helpful comments. This is publication no. 752 of the Mitrani Department of Desert Ecology.
FINANCIAL SUPPORT
This study was partly supported by Israel Science Foundation (Grant no. 27/08 to B.R.K. and I.S.K.).