Introduction
Parasites of the phylum Acanthocephala have an indirect life cycle in which the vertebrate definitive host becomes infected by ingesting larvae (cystacanths) contained within the haemocoel of an arthropod intermediate host (Taraschewski, Reference Taraschewski2005). Some species are pathogenic to terrestrial and marine birds (Richardson & Nickol, Reference Richardson and Nickol2008) and have frequently been associated with mortality in populations of aquatic birds (Sanford, Reference Sanford1978; Liat & Pike, Reference Liat and Pike1980; Camphuysen et al., Reference Camphuysen, Ens, Heg, Hulscher, van der Meer and Smit1996, Reference Camphuysen, Berrevoets, Cremers, Dekinga, Dekker, Ens, van der Have, Kats, Kuiken, Leopold, van der Meer and Piersma2002). Acanthocephalans are also known for their capacity to influence predator–prey interactions through behavioural, physiological or phenotypic manipulation of intermediate hosts such as crabs (Pulgar et al., Reference Pulgar, Aldana, Vergara and George-Nascimento1995; Haye & Ojeda, Reference Haye and Ojeda1998; Latham & Poulin, Reference Latham and Poulin2001, Reference Latham and Poulin2002), frequently leading to a higher risk of predation by definitive hosts (Latham & Poulin, Reference Latham and Poulin2001, Reference Latham and Poulin2002).
The Bahía Blanca Estuary, in Argentina, is inhabited by large populations of Cyrtograpsus angulatus and Neohelice granulata crabs (Spivak, Reference Spivak1997), which are intermediate hosts to cystacanths of the acanthocephalan Profilicollis chasmagnathi (Alda et al., Reference Alda, La Sala, Marcotegui and Martorelli2011). This estuary is the most important breeding site for Olrog's gull (Larus atlanticus) (OG), a vulnerable species (IUCN, 2011) endemic to the Atlantic coast of Argentina, Uruguay and southern Brazil (Yorio et al., Reference Yorio, Bertellotti and García Borboroglu2005). During their breeding season, OG parents prey primarily on N. granulata (Delhey et al., Reference Delhey, Carrete and Martínez2001) and C. angulatus (Petracci, pers. comm.), which are fed to the young from day 1 of hatching. Repeated exposure to parasites ingested from crabs has been shown to be associated with annual mortality of OG chicks in the largest known breeding colony for this species (La Sala & Martorelli, Reference La Sala and Martorelli2007).
Based on the feeding ecology of OG, the epidemiology of acanthocephalan infections in OG populations is likely dictated by the infection dynamics at the population level of the intermediate host. However, there is a gap in knowledge of the impact acanthocephalan infections have on populations of these crabs from the Bahía Blanca Estuary.
An observational, cross-sectional study was performed in populations of C. angulatus and N. granulata to identify risk factors for infection by P. chasmagnathi under the hypotheses that: (1) infections are associated with mortality of intermediate hosts; and (2) the risk of infection inflicted on OG varies depending on the crab prey species.
Materials and methods
Sampling procedures
Samples were collected in a feeding area of OG in the Bahía Blanca Estuary (38°44′S, 62°22′W) in south-western Buenos Aires province, Argentina, between July 2006 and July 2007. In the study site, C. angulatus inhabit mainly subtidal muddy sediments, and N. granulata are found both in the subtidal area and on mudflats of the intertidal zone. Both crab species studied here are bottom feeders in mud flats and salt marshes (Spivak, 1997). Cyrtograpsus angulatus was sampled from the subtidal zone using baited nets, whereas N. granulata was sampled systematically along transects inside a predefined area (50 × 200 m) on a large intertidal mudflat.
Sampling was stratified by season. Minimum sample size for each season was estimated using the Win Episcope V. 2.0 software (De Blas et al., Reference De Blas, Ortega, Frankena, Noordhuizen and Thrusfield2000) based on the following assumptions, for each species: (1) infinite population size; (2) an expected prevalence of 50%; (3) an accepted error of 10%; and (4) a confidence level of 95%. Calculations resulted in a minimum sample size of 96 crabs of each species for each season.
Examination of crabs
Crabs collected in the OG feeding area were transported live to the laboratory (Centro de Estudios Parasitológicos y de Vectores, CONICET-UNLP), humanely killed by stunning (cooling at 4°C) followed by destruction of nerve centres (Murray, Reference Murray2006), and dissected. The crabs were sexed and measured to the nearest 0.01 mm (carapace width at the level of first pair of lateral spines, and carapace length). The number of cystacanths of P. chasmagnathi per crab was recorded.
Statistical analysis
Prevalence of infection, 95% confidence interval (CI) and mean intensity (number of parasites per number of infected hosts) were computed and used as measures of frequency, confidence level and intensity of infection, respectively. Unpaired Student's t-tests were used to compare carapace length and width between sampling sites in each species. All analyses were considered statistically significant at P ≤ 0.05.
Host size (carapace length and width, in mm) was used as a proxy for host age (McLay, Reference McLay1988). To test the first hypothesis, that acanthocephalan infection is associated with mortality of intermediate hosts, methods proposed by Rousset et al. (Reference Rousset, Thomas, De Meeûs and Renaud1996) and Lester (Reference Lester1984) were implemented. For each species: (1) the relationship between infection prevalence/intensity and host size; and (2) the aggregation level of infection as a function of host size, were assessed as approximations to a causal relationship between infection and mortality. As suggested by the above-mentioned authors, a decrease of the mean parasite load, prevalence and/or aggregation level in relation to host size was interpreted as indirect evidence of parasite-induced mortality. Considering the hypothesis being tested here and the analytical approaches followed, the analyses initially included only those crabs sampled alive from the OG feeding area. Further analyses also included crabs collected from the OG breeding colony to assess the difference in prevalence, intensity and aggregation level between predated and non-predated crabs of each species.
All analyses were performed using the statistical package R (R Development Core Team, 2010). A two-stage model-fitting approach was employed to assess the role of crab size as a risk factor for infection status. First, generalized additive models (GAM) with non-parametric smoothers were fitted to explore the shape of the response (infected/not infected) to the continuous predictor variables (carapace length and width). Next, generalized linear models (GLM) with a binary, logistic response were used to reproduce the shapes identified in the GAMs by adding appropriate parametric terms in the model. For both GAMs and GLMs, binomial distributions with logit link functions were used.
GAMs were fitted using the cubic regression spline method implemented in the ‘mgcv’ R package (Wood, Reference Wood2006), and GLMs were fitted using the R ‘stats’ package. GLMs were constructed using a stepwise process, starting from a saturated model which included all independent variables and two-way interactions. Terms were successively eliminated to identify the most parsimonious and explicative model based on the Akaike information criterion (AIC) (Akaike, Reference Akaike1974). Terms (variables or their interactions) were eliminated if variable inclusion did not reduce the AIC by more than two units (Burnham & Anderson, Reference Burnham and Anderson2002).
A set of models was constructed for each crab species using ‘carapace length’ (mm) and ‘carapace width’ (mm) as main explanatory variables, and ‘sex’ (male/female), ‘sampling season’ (spring, summer, fall, winter) and ‘sampling site’ (feeding area/breeding colony) as covariates. Because carapace length and width were highly correlated (>70%), they were examined separately to avoid collinearity, and the variable yielding to the lowest AIC was included in the model. For continuous variables, the assumption of linearity was tested by plotting the midpoints of the quartiles of the distribution against the log-odds of the outcome for each category (Dohoo et al., Reference Dohoo, Martin and Stryhn2003). The association between infection status and independent variables was quantified by estimation of the odds ratio (OR). The OR for dichotomous independent variables (e.g. sampling site), which was computed as the exponential of the regression coefficient, indicates how much more likely it is for the outcome to be present in individuals of the reference group compared to those in the baseline group. For continuous independent variables, such as carapace length, the regression coefficient gives the change in the log-odds associated with an increase of one unit (mm) in the independent variable. Hence the exponential of its coefficient indicates the percentage increase in the odds for a one-unit increase in carapace length.
The other outcome of interest, ‘intensity of infection’, was zero-inflated (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009). Therefore, several types of GLMs were fitted to quantify the association between infection intensity and host size while accounting for covariates described above and handling over-dispersion (Zeileis et al., Reference Zeileis, Kleiber and Jackman2008). Based on the AIC criterion, zero-altered negative binomial models (ZANB), also known as hurdle models, provided a better fit to the data than Poisson, negative binomial, zero-inflated Poisson or zero-inflated negative binomial models. In ZANB models, a negative binomial GLM is used to fit the count process excluding zeros (zero-truncated count model), whereas a binomial GLM is used to fit the probability of measuring a positive count. The logistic component of the ZANB model is presented in the table but not interpreted because this was accomplished using the logistic GLMs described above. For the continuous variable in the negative binomial model, exp(β) indicated the percentage change in the dependent variable for a one-unit increase in carapace length.
Analyses were conducted using the ‘pscl’ R package (Zeileis et al., Reference Zeileis, Kleiber and Jackman2008). The significance of each final model was tested by comparing it against a null model without predictors using likelihood ratio tests with the ‘lmtest’ R package (Zeileis & Hothorn, Reference Zeileis and Hothorn2002).
To assess the association between parasite aggregation and host size, crabs of each species were assigned to one of four carapace length categories based on the quartiles of the distribution. The degree of parasite aggregation was calculated (regardless of the crabs' size) for each season by using the variance-to-mean ratio (Elliot, Reference Elliot1977). Spring level aggregation (when sampling was conducted both in the OG colony and their feeding area) was calculated for each sampling site to assess the degree of aggregation among predated crabs, because predation by OG contributes considerably to crab mortality during this season at this study site, and prey selection may be influenced by the intensity of infection in the crabs. Parasite aggregation was also estimated (regardless of sampling season) for each size-class in feeding area only crabs.
To test the second hypothesis, that the risk of infection imposed on OG varies by crab species, a subset of data for both species which included: (1) all crabs collected from nests in the colony; and (2) only those crabs from the feeding area with carapace width within the range of crabs collected from OG colony nests, was used. This was done to restrict the analysis to a dataset consisting only of hosts more likely to be predated by OG based on the species' prey size preference. A GLM with a binary, logistic response which included ‘infection status’ (infected/not infected) as the dependent variable, and ‘species’ (C. angulatus/N. granulata) as the primary independent variable was fitted. The model included the explanatory variables used to assess the first hypothesis and was assembled following the same procedures.
Results
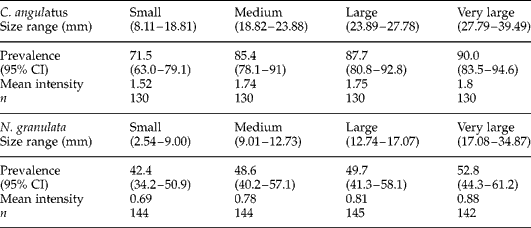
The differences in carapace size (length) between sampling sites for each crab species are presented in table 1. A total of 520 C. angulatus and 578 N. granulata were collected in all four seasons, which included 98 C. angulatus and 147 N. granulata collected dead (predated) from nests in a nearby colony of OG in the spring. Prevalence with 95% CIs, mean intensity of infection, and sample sizes stratified by size-class (carapace length) are provided for each species in table 2.
Table 1 Carapace length (mm) in C. angulatus and N. granulata sampled from feeding area and breeding site of Olrog's gull.

Table 2 Prevalence, 95% CIs and mean intensity of infection by P. chasmagnathi in crabs from the Bahía Blanca Estuary are shown with number (n) of crabs sampled categorized by size-class.
Hypothesis 1: infection is associated with mortality of intermediate hosts
The fitted GAMs showed positive linear associations between infection status (infected/not infected) and crab size (carapace length and width) for both species. Therefore, only linear variable terms were included in the final GLMs. The probability of infection in both species was better described by the length of the carapace than by its width, so only carapace length was included in the models. The crude relationship (i.e. unadjusted by other covariates) between carapace length and the probability of infection for both species is shown in fig. 1. The final models assessing the variables associated with infection in C. angulatus and N. granulata from both sites are detailed in tables 3 and 4, respectively.

Fig. 1 Fitted probability of infection by P. chasmagnathi as a function of carapace length in C. angulatus (a) and N. granulata (b). Clumped black circles at 0 and 1 on the y-axis represent the observed data and show the ranges of carapace length for infected (1) and non-infected (0) crabs.
Table 3 Logistic regression model describing the association between risk factors and infection by cystacanths of P. chasmagnathi in C. angulatus.

Baseline category: breeding colony.
† AIC value increment if the single term is dropped.
Table 4 Logistic regression model describing the association between risk factors and infection by cystacanths of P. chasmagnathi in N. granulata.
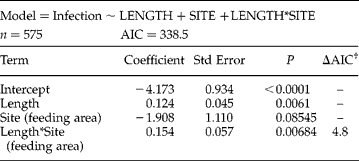
Baseline category: breeding colony.
† AIC value increment if the single term is dropped.
In C. angulatus, the odds of infection increased by 7% (OR: 1.07; 95% CI: 1.03–1.12) for each millimetre increase in the size, and were ~17 times greater (OR: 16.74; 95% CI: 2.16–129.36) in crabs sampled from the OG feeding area compared to those sampled from nests in the breeding colony. In N. granulata, there was an interaction that suggests that the relationship between size and infection status depended on the sampling site. In crabs collected from the breeding colony and the feeding area, the odds of infection increased by 13% (OR: 1.13; 95% CI: 1.01–1.27) and 32% (OR: 1.32; 95% CI: 1.18–1.48), respectively, for each millimetre increase in host size.
In C. angulatus, the intensity of infection was not associated with carapace length or width when the analysis was restricted to crabs from the feeding area, nor when it included crabs from both sites and the ‘sampling site’ was introduced as covariate. Conversely, in N. granulata, the mean intensity of infection increased by 11.7% for each additional millimetre of carapace length when the analysis included only crabs from the feeding area. When crabs from both sampling sites were included in the model, the intensity of infection rose by 16.5% for every unit increase in the size (table 5).
Table 5 Zero-altered negative binomial model describing the association between carapace length and infection intensity by cystacanths of P. chasmagnathi in N. granulata.

LRT (likelihood ratio test): χ2 = 102.8; P < 0.0001.
Variability in infection level (variance-to-mean ratio) by size-class in C. angulatus was concave-down shaped and increased from small-sized crabs reaching a local maximum in large-sized crabs, with a precipitous drop of 41.4% between large and very large crabs (fig. 2). In N. granulata, variability in infection level was similar between small and medium-sized crabs, but increased steadily between the latter and very large crabs, where aggregation reached its highest level (fig. 2).

Fig. 2 Changes in parasite aggregation (variance-to-mean ratio) with host size-class in C. angulatus (■) and N. granulata (▲) sampled from the feeding area of OG. Host size (carapace length) is expressed as four categories (S, small; M, medium; L, large; VL, very large).
Seasonal patterns of parasite aggregation are presented in table 6. Aggregation levels in N. granulata varied (range 1.76–2.45) throughout the year in the feeding area only and the level of aggregation was 173.3% higher among crabs collected in the spring from nests in the OG colony compared to those sampled in the feeding area. Aggregation levels were more variable (range 1.78–3.51) in C. angulatus but, contrary to what was observed in N. granulata, the level of aggregation was 251% lower among crabs collected in the OG colony compared to those sampled in the feeding area.
Table 6 Parasite aggregation (variance-to-mean ratio) by sampling season. The spring sample collection for both species was stratified by sampling site.

* Not applicable because samples were not collected.
Hypothesis 2: risk of infection varies with crab species
The odds of infection were over 2.7 times (OR: 2.74; 95% CI: 0.95–7.87) higher for N. granulata than for C. angulatus, but this difference was not statistically significant.
Discussion
This work represents the first assessment of epidemiological factors affecting acanthocephalan infection in populations of grapsid crabs in general and in C. angulatus and N. granulata in particular.
The results suggest that acanthocephalan infections are associated with mortality of intermediate hosts through predator–prey interactions. We showed that: (1) host size is the most significant risk factor for acanthocephalan infections in populations of C. angulatus and N. granulata; (2) the probability of infection does not differ between species; (3) parasite aggregation in the spring is higher in N. granulata crabs predated by OG compared with non-predated ones sampled in the feeding area; and (4) C. angulatus in the largest size-class have the lowest level of parasite aggregation.
Early works have revealed that a decrease of the mean parasite load/prevalence, or parasite aggregation level, as a function of host age, suggests the presence of parasite-induced host mortality (Lester, Reference Lester1984; Rousset et al., Reference Rousset, Thomas, De Meeûs and Renaud1996). The GAM and GLM models, in this study, showed positive linear associations between crab size and the odds of infection in both species. A positive linear association between crab size and intensity for N. granulata was evident in the ZINB model, whereas a non-significant association was observed between size and intensity for C. angulatus in this analysis. Interestingly, although these models failed to reveal a causal association between Profilicollis infections and mortality in the populations studied, the analyses of aggregation levels suggest the opposite effect. The observation of a decreasing level of aggregation in C. angulatus in the largest size-class indicates that a smaller fraction of the largest and most heavily infected crabs is removed from the population. In contrast to the observation of parasite aggregation in C. angulatus, the aggregation pattern in N. granulata, which increased across size-classes, attaining its highest level in very large crabs, does not support the presence of parasite-associated mortality.
As mentioned in the introduction, the phylum Acanthocephala is widely known for its ability to mediate predator–prey interactions through various mechanisms (Pulgar et al., Reference Pulgar, Aldana, Vergara and George-Nascimento1995; Haye & Ojeda, Reference Haye and Ojeda1998; Latham & Poulin, Reference Latham and Poulin2001, Reference Latham and Poulin2002). OG numbers in the study area peak during the species' breeding season in the austral spring and, as described above, gull parents feed chicks and themselves almost exclusively on C. angulatus and N. granulata during this period. This strong trophic specialization, combined with the capacity of acanthocephalans to manipulate their intermediate hosts, initially suggested that parasite-related mortality in C. angulatus was associated with predation by OG. However, predation by OG could be ruled out as the main source of mortality because C. angulatus predated by OG and collected in the colony: (1) were considerably smaller than those sampled from the feeding area; (2) had a negligible (0.01%) prevalence of infection; and (3) showed no parasite aggregation as a consequence of (2). Alternatively, other species known for preying on these crab species, such as sharks (Cortés, Reference Cortés1999) and fish which occur in the study site, might be responsible for the suspected C. angulatus mortality.
Despite the apparent lack of association initially observed between infection and mortality in N. granulata, there was a remarkably higher level of parasite aggregation (regardless of size-class) among crabs collected in nests from the colony during the spring. This may indicate that N. granulata with the highest infection intensity are being removed from the population via predation by OG. Given the host manipulation capacity of acanthocephalans, it is plausible that this biological trait somehow leads to an increased likelihood of predation in heavily infected N. granulata, which eventually would be reflected by the higher parasite aggregation observed among predated individuals.
The odds of infection were higher for N. granulata than for C. angulatus, although this difference was not significant. This lack of significance could be explained by the life history of crab species, such as habitat use and feeding ecology, and by characteristics of the parasite's life cycle. Adult females of P. chasmagnathi infecting vertebrate final hosts shed their eggs through faeces. These relatively small eggs are picked up inadvertently over time by C. angulatus and N. granulata through feeding, and eventually reach their cystacanth stage in these intermediate hosts. The bottom-feeding foraging habit of these crabs makes infection a random process which is expected to follow a density-independent infection pattern, as suggested by Latham & Poulin (Reference Latham and Poulin2002) for other species of shore crabs. Therefore, infection patterns in both species should have similar parasite infection transmission rates, explaining the similar rates of infection observed.
In conclusion, our results suggest that mortality of the largest and most heavily infected C. angulatus is associated with predators other than OG, whereas mortality among the most heavily infected N. granulata does seem to be associated with a higher risk of predation by OG. The latter findings underline the importance of incorporating, whenever possible, data from both live and dead hosts when studying the epidemiology of helminth infections in intermediate hosts within systems where their predators can be identified and predated hosts can be sampled.
Acknowledgements
The authors thank especially the Club Náutico Gral, Daniel Cerri for institutional support and Joaquín Cereguetti for fieldwork assistance. We thank the anonymous reviewers whose suggestions resulted in substantial improvements to this article. This study was partly funded by Agencia Nacional de Promoción Científica y Tecnológica (PICT 34412/05).










