INTRODUCTION
Lowland tropical evergreen forests in South-East Asia are among the most species-rich biomes in the world and support a high proportion of endemic biodiversity (Sodhi et al. Reference SODHI, KOH, BROOK and NG2004a, Reference SODHI, POSA, LEE, BICKFORD, KOH and BROOK2010). However, these forests are subject to the highest relative rates of deforestation and degradation anywhere in the humid tropics (Achard et al. Reference ACHARD, EVA, STIBIG, MAYAUX, GALLEGO, RICHARDS and MALINGREAU2002) owing largely to agriculture and commercial logging (Lambert & Collar Reference LAMBERT and COLLAR2002, Sodhi & Brook Reference SODHI and BROOK2006), and results in a patchwork landscape of forest fragments (Wright Reference WRIGHT2005). Effects of deforestation-driven fragmentation on tropical faunal communities, notably birds have been extensively documented (Laurance et al. Reference LAURANCE, LOVEJOY, VASCONCELOS, BRUMA, DIDHAM, STOUFFER, GASCON, BIERREGAARD, LAURANCE and SAMPAIO2002, Marsden et al. Reference MARSDEN, WHIFFIN and GALLETI2001, Renjifo Reference RENJIFO1999). A second kind of habitat fragmentation occurs when the construction of large hydroelectric dams causes low-lying areas upstream to be flooded, creating land-bridge islands from hills and ridges in the original terrain (Diamond Reference DIAMOND2001), as exemplified in South America and China (Cosson et al. Reference COSSON, RINGUET, CLAESSENS, MASSARY, DALECKY, VILLIERS, GRANJON and PONS1999, Fournier-Chambrillon et al. Reference FOURNIER-CHAMBRILLON, FOURNIER, GAILLARD, GENTY, HANSEN and VIE2000, Wu et al. Reference WU, HUANG, HAN, XIE and GAO2003). Unlike fragmentation arising from anthropogenic clearance of forest that results in a mosaic of non-forest matrix habitats (e.g. scrub, pasture, cultivation), flooding forms an open-water matrix that poses considerable barriers to the dispersal of many animals. Studies examining fragmentation effects arising from dam-induced flooding can thus provide a clearer picture of impacts on isolated faunal communities by minimizing confounding effects of species usage of matrix habitat in contemporary studies of fragmentation (Cosson et al. Reference COSSON, RINGUET, CLAESSENS, MASSARY, DALECKY, VILLIERS, GRANJON and PONS1999). Moreover, local community dynamics of land-bridge island faunas may be different from that of habitat fragments and cannot be easily understood using knowledge inferred from terrestrial fragments.
Nonetheless, faunal communities on land-bridge islands are analogous to those in forest fragments and are directly impacted by habitat loss and isolation. Birds are currently the best-examined taxonomic group in forest fragments, although existing studies mainly focus on the Neotropics (Arriaga-Weiss et al. Reference ARRIAGA-WEISS, CALME and KAMPICHLER2008, Ferraz et al. Reference FERRAZ, NICHOLS, HINES, STOUFFER, BIERREGAARD and LOVEJOY2007, Giraudo et al. Reference GIRAUDO, MATTEUCCI, ALONSO, HERRERA and ABRAMSON2008, Renjifo Reference RENJIFO1999, Stouffer et al. Reference STOUFFER, BIERREGAARD, STRONG and LOVEJOY2006). There is broad consensus that forest fragmentation leads to alteration of original bird communities through local extirpation, leading to lowered richness, abundance and diversity (Sodhi et al. Reference SODHI, KOH, BROOK and NG2004a) as well as changes in taxonomic composition (Renjifo Reference RENJIFO1999). Post-fragmentation bird communities are often unevenly structured, being dominated by few species. Moreover, the fact that bird species consume a variety of food items means that responses to habitat disturbances differ, depending on feeding guild (Gray et al. Reference GRAY, BALDAUF, MAYHEW and HILL2007). Insectivorous and frugivorous birds have been shown to be more strongly affected by forest fragmentation (Sodhi et al. Reference SODHI, LIOW and BAZZAZ2004b) than other major feeding guilds. For instance, both Sekercioğlu et al. (Reference SEKERCIOĞLU, EHRLICH, DAILY, AYGEN, GOEHRING and SANDI2002) and Stouffer et al. (Reference STOUFFER, BIERREGAARD, STRONG and LOVEJOY2006) reported significant reductions of insectivorous bird abundance and diversity in forest fragments in Costa Rica and Amazonian Brazil while Terborgh et al. (Reference TERBORGH, LOPEZ, TELLO, YU, BRUNI, Laurance and Bierregaard1997) documented greater losses among known insectivorous birds such as antbirds and woodcreepers on forested islands in Lake Guri, Venezuela.
One possible explanation for the decline of insectivores is the decline of arthropod prey abundance, though limited evidence is available (Stratford & Robinson Reference STRATFORD and ROBINSON2005) and some studies in fact show the contrary (Didham Reference DIDHAM, Watt, Stork and Hunter1997). Additionally, Sekercioğlu et al. (Reference SEKERCIOĞLU, EHRLICH, DAILY, AYGEN, GOEHRING and SANDI2002) has argued that ability to disperse through surrounding matrix habitat is a critical factor underlying the persistence of insectivorous birds in forest fragments in Costa Rica. Indeed, better dispersal ability together with the ability to exploit or tolerate matrix habitat (Antongiovanni & Metzger Reference ANTONGIOVANNI and METZGER2005) may help buffer bird subpopulations in fragments from extinction, though this is less relevant for land-bridge islands due to dispersal limitations posed by water. Furthermore, insectivorous birds that exhibit highly specialized behaviours, for example ant-following or mixed-flocking, may also be more vulnerable to forest fragmentation (Sekercioğlu Reference SEKERCIOĞLU2007, Sodhi et al. Reference SODHI, LIOW and BAZZAZ2004b, Willis Reference WILLIS1974). Dispersive constraints, ecological specialization and other stochastic events may then act synergistically to shape bird communities in forest fragments.
To date, there have been a number of studies investigating forest fragmentation effects on bird communities in South-East Asia (Castelleta et al. Reference CASTELLETA, THIOLLAY and SODHI2005, Pattanavibool & Dearden Reference PATTANAVIBOOL and DEARDEN2002). Studies addressing responses of birds in different functional groups are however still lacking and more so for studies on land-bridge islands where we found none in the literature. In our study, we examined the bird communities of six land-bridge islands and two adjacent mainland forest sites in Kenyir, a large man-made lake in Peninsular Malaysia, two decades following flooding and hypothesized that insectivorous birds would be the worst affected dietary guild. We examined bird species richness, abundance and diversity for three dietary guilds, insectivores, frugivores and omnivores on islands and on mainland sites, and analysed how these three different guilds responded to fragmentation caused by post-damming flooding.
METHODS
Site description
Our study was carried out in Lake Kenyir (5°00′N, 102°48′E), a large man-made lake in the north-eastern state of Terengganu in Peninsular Malaysia formed by the damming of the upper tributaries of the Terengganu River (Figure 1). The completion of the Kenyir Dam in 1986 flooded low-lying forest along tributaries of the Terengganu River, particularly the Kenyir and created a freshwater impoundment of 370 km2 at 145 m asl (Furtado et al. Reference FURTADO, SOEPADMO, SASEKUMAR, LIM, ONG, DAVISON and LIEW1977). Lake Kenyir is estimated to contain over 340 land bridge islands ranging in area from less than 1 ha to over 1000 ha (Furtado et al. Reference FURTADO, SOEPADMO, SASEKUMAR, LIM, ONG, DAVISON and LIEW1977). These islands are non-flooded remnants of former highlands, hill and ridge tops and are mostly forested. Most of the catchment area surrounding Lake Kenyir is densely forested. Although forest on the islands and surrounding mainland was logged 30–50 y ago, the vegetation in our study area share similar logging histories and consists mainly of a mixture of primary and tall secondary lowland dipterocarp forests.
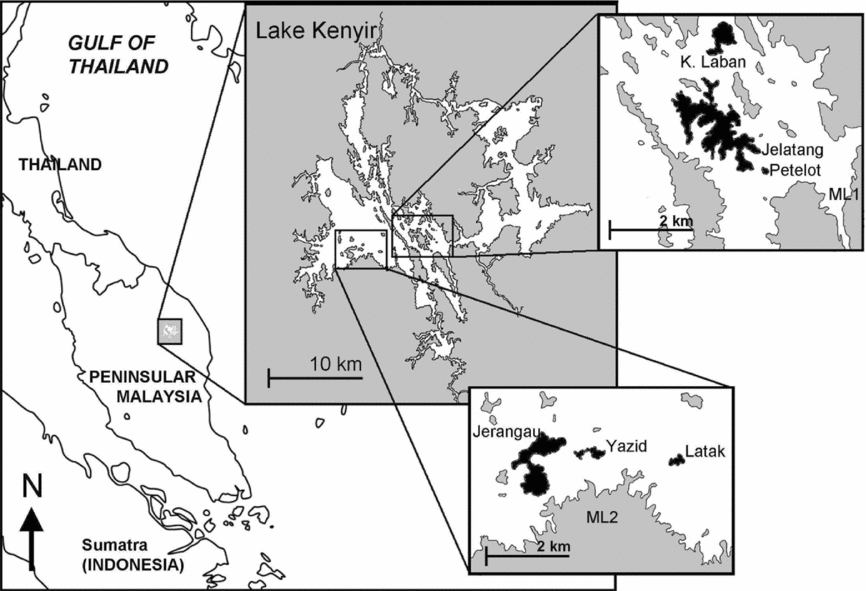
Figure 1. Location of Lake Kenyir in Peninsular Malaysia. Inset: Map of the Lake Kenyir area showing the six islands and two mainland sites sampled.
Eight study sites were chosen using topographic maps and on-site surveys prior to sampling. Of the eight sites, six are islands of different sizes grouped into three area classes while two are control sites on the mainland. Islands were grouped into ‘large islands’, with area of > 100 ha, ‘medium islands’ with area of 20–50 ha and ‘small islands’, with area of < 20 ha. All six islands sampled are topographically undulating as they were former hill tops or ridges and all are covered with logged tall forest. Our mainland control sites are topographically similar to the islands and estimated to have an area of 434 300 ha, given their contiguity with the large forested Taman Negara National Park.
We conducted bird surveys using point counts to determine the composition of bird communities at our eight study sites. Four cycles of bird sampling was conducted from 11 June 2007 to 18 February 2008. Bird sampling cycles were conducted over the months of June, August, October and February and are thus not biased by seasonal variations as it encompasses both dry and wet (October–January) seasons. The number of sampling points varied from seven to 38 depending on the size of the site. To minimize observer bias, all bird surveys were carried out by the same observer (DLY) using a pair of 10 × 42 binoculars. Sampling points were systematically selected to comprehensively cover our study sites.
All 179 sampling points identified were at least 50 m from the forest edge to minimize edge effects. Successive points chosen along entry paths were spaced at least 100 m apart while keeping to time constraints taken to travel between points although this may not be sufficient to minimize interdependence. Birds were sampled between 07h30 and 12h00 at all sites. At each point, all birds seen or heard within a fixed radius of 50 m within 10 min were recorded and their behaviour noted, following Peh et al. (Reference PEH, SODHI, JONG, SEKERCIOĞLU, YAP and LIM2006). Raptors, migratory, crepuscular and aerial species (e.g. swiftlets) were excluded from our study. Our point counts were conducted only during good weather (i.e. no rain and strong winds). Taxonomic sequence and nomenclature for birds follows Robson (Reference ROBSON2002).
Estimation of species richness
Species rarefaction curves based on smoothed species accumulation were plotted for all study sites to assess completeness and intensity of sampling efforts across all sites using EstimateS (Version 8.2, R. K. Colwell, http://purl.oclc.org/estimates). The use of species rarefaction and accumulation curves provides a standardized benchmark for comparing species richness between sites, as opposed to using simple species lists (Gotelli & Colwell Reference GOTELLI and COLWELL2001). As sampling effort is never able to detect all species present (Nichols et al. Reference NICHOLS, BOULINIER, HINES, POLLOCK and SAUER1998), relevant estimators can be used to extrapolate true species richness based on the observed species richness. We used the mean of four non-parametric estimators (Chao1, Chao2, Jack1, Jack2) to compute the estimated ‘true’ species richness, which can then be used to calculate the proportion of species detected to determine sampling completeness. These four estimators have been shown to perform best among many estimators, being the most accurate and least biased overall (Walther & Martin Reference WALTHER and MARTIN2001, Walther & Moore Reference WALTHER and MOORE2005). Lastly, we used Shannon's diversity index H (Magurran Reference MAGURRAN1988) as a measure of overall avian faunal diversity.
Classification of guilds and families
To test if patterns exist for persistence on land-bridge island bird communities with respect to taxonomic level (family) and feeding guild, we collated data on these attributes from various sources. Taxonomic information was compiled from Robson (Reference ROBSON2002) and Wells (Reference WELLS1999, Reference WELLS2007). Guild information mainly followed that of Wong (Reference WONG1986) and where not available, we assigned species into dietary guilds based on our field observations of foraging and dietary information in the literature (Kinnaird & O'Brien Reference KINNAIRD and O'BRIEN2007, Wells Reference WELLS1999, Reference WELLS2007). Supplementary information on guild membership (e.g. diet, foraging techniques) was obtained from correspondence with researchers. We identified three major dietary guilds, namely insectivores, which included birds with predominantly arthropod-based diets, frugivores, which included species that consume mostly fruits and figs and omnivores, which consist of species which have significant proportions of fruits, nectar and arthropods in their diet.
Statistical analyses
We compared bird community composition on our study sites by performing Sørensen (Bray–Curtis) cluster analysis on an absence-presence matrix of all species sampled. This was carried out using the PC-ORD 2.0 software (MjM Software, Gleneden Beach, Oregon, USA). Subsequently, we employed an information-theoretic approach to examine in a heuristic manner (Burnham & Anderson Reference BURNHAM and ANDERSON2002) the effects of two components of fragmentation – fragment area and isolation – on insectivorous, frugivorous and omnivorous birds on the islands under study at Lake Kenyir. For each of the three response variables, species richness, abundance and Shannon's diversity index H, a set of six a priori candidate general linear mixed-effects models were evaluated using ‘Site’ as a random effect. In the set of candidate models, the global model included three main effects, Area, Isolation and Guild, as well as two interaction terms, Area × Guild and Isolation × Guild. The main effects of Area and Guild entered all candidate models based on both prior knowledge and the early exploration of our data. Isolation was measured as the distance from the nearest mainland. Area and Isolation were log10-transformed to account for non-normality. Because Area and Isolation were collinear (Pearson's r = −0.93, P < 0.0001 on log10-transformed values), models with both of these two predictor variables were evaluated with caution. All models were checked for the homogeneity of their residuals. We compared and ranked models using Akaike's information criterion corrected for small sample size (AICc) following Burnham & Anderson (Reference BURNHAM and ANDERSON2002). AICc weights (wAICc) provided relative weight of any particular model which varied from 0 (no support) to 1 (complete support) relative to the entire model set (Burnham & Anderson Reference BURNHAM and ANDERSON2002). All statistical analyses were carried out using the R Package version 2.9.2 (R Development Core Team, Vienna).
RESULTS
Our sampling recorded 124 bird species representing 23 families at the eight study sites, with species occupancy ranging from 11 to 92 species (Appendix 1). Most of our species accumulation curves (sample-rarified and rescaled to number of individuals) appeared to be reaching asymptotes (Figure 2), indicating a high level of sampling completeness. Comparing observed number of species with the mean of four non-parametric estimators, 74.6%–87.8% of the forest birds present at the sites were detected (Table 1). As the observed species richness and the means of the estimators were highly collinear across all sites (Pearson's r = 0.997, P < 0.0001), we used the observed species richness for subsequent analyses. Our analysis of bird communities showed that mainland control sites were most similar to each other in species composition. The three smallest island sites had a highly similar bird community and are more similar to each other than any of the control sites or large islands (Figure 3). When classified into feeding guilds, richness of all three guilds declined with area, however both insectivorous and frugivorous birds showed comparably steeper declines compared with omnivorous birds (Figure 4).

Figure 2. Species accumulation curves for birds (rescaled to number of individuals), with estimated areas, at six islands and two mainland sites.
Table 1. Estimated bird richness using the four best non-parametric estimators (Chao1, Chao 2, Jack 1, Jack 2) and mean proportion of species detected for each of the eight sites sampled.


Figure 3. Dendrogram showing per cent similarity of bird communities sampled from six islands and two mainland sites, using a simple species absence-presence matrix. Per cent similarity is based on agglomerative cluster analysis using the Bray–Curtis index.

Figure 4. Bar chart showing number of bird species at each site sampled, classified by respective dietary guilds: omnivore, insectivore and frugivore. (Abbreviations used – ML, Mainland; JLT, Jelatang; JRG, Jerangau; LBN, Laban; YZD, Yazid; LTK, Latak; PTL, Petelot).
Species richness
The model with Area, Guild and their interaction as predictors ranked the highest (wAICc = 0.833) and is over five times more likely to be the best approximating model than the second-ranked model (~Area + Guild + Area × Guild + Isolation). The top-ranked model suggests that species richness of all bird dietary guilds decreased with decreasing island area and that this effect varied among the guilds (Figure 5a). Notably, the effects of isolation appear relatively weak overall, as suggested by our candidate models (Table 2). Insectivorous birds showed the steepest decline in species richness with decreasing island area (Figure 5a, slope coefficient = 7.62 ± 0.72) compared to omnivores or frugivores. While insectivorous bird richness was highest on the control sites, it declined steadily with respect to island area such that on the smallest site, omnivore richness exceeds that of insectivores (Figure 4).

Figure 5. Observed species richness (a), abundance (b) and Shannon's diversity index H (c) plotted against log10-transformed area for all sites. Regression lines (solid lines) for the bird dietary guild Insectivore (solid squares), Frugivore (solid circles) and Omnivore (solid triangles) with ± SE of estimated slopes (broken lines) as confident intervals were drawn according to the general linear mixed effect model ~ Area + Guild + Area: Guild. Area: Guild denotes the interaction between Area and Guild. For the response variable abundance, the most plausible model does not include the interaction term and the confidence intervals of the three regression lines overlap.
Table 2. General linear mixed-effects models and summary statistics for species richness, abundance, and Shannon's diversity index H. Predictor variables included the main effects of Area, Isolation and Guild and two interaction terms, Area: Guild and Isolation: Guild. All models used Site as a random effect. K = number of model parameters, LL = minimum negative log-likelihood, AICc = Akaike's information criterion corrected for small sample size, DAICc = difference between AICc of the top-ranked and current model; wAICc = wAICc weight.

Abundance
The top-ranked model for Abundance included only the main effects of Area and Guild. However the wAICc ratio for this model versus the second-ranked model (~Area + Guild + Area × Guild) is only 2.14 (0.529/0.247, Table 2) suggesting that the effect of Area-Guild interaction should not be overlooked. To illustrate this point we based the plot on the second-ranked model instead of the top-ranked one (Figure 5b) and as shown by the regression lines and their confidence intervals, the insectivorous birds again appeared to have the steepest slope albeit with greater uncertainty compared to species richness (Figure 5a, b). The other candidate models are unlikely to be good approximating models of our data judging by their AICc and wAICc values (Table 2).
Shannon's diversity index
Again the top-ranked model included Area, Guild and their interaction as predictor variables and it is 3.59 (0.690/0.192, Table 2) times more likely than the second-ranked model (~Area + Guild). Interestingly Shannon's H declined most rapidly in frugivorous birds with decreasing area (Figure 5c), suggesting greater unevenness. Again, Shannon's H for omnivorous birds is higher than that of insectivores on the smallest site.
Overall analyses excluding mainland sites
To ensure that the large area effects exerted by mainland sites do not confound the relationships that we have demonstrated, we repeated our mixed-effects models using the best predictors from our previous models for Species Richness, Abundance and Shannon's diversity index H (Table 2), but excluding data from both mainland sites. The same relationships persist and the resulting model rankings are not affected (Appendix 2). For instance, the model with Area, Guild and their interaction as predictors again ranked the highest and hence the best supported (wAICc = 0.527) for species richness. Likewise, the models considering the effects of Area and Guild for abundance (wAICc = 0.601) and Shannon's diversity index (wAICc = 0.733) remain the best supported ones. Clearly, this shows that while the effects of the large area of mainland sites do influence our models, the relative strengths of the models are unchanged.
DISCUSSION
Forest fragmentation effects on tropical bird communities have been well investigated, especially in the Neotropics and demonstrate a number of predictable consequences. More than ample evidence exists to show that diversity and richness in forest fragments erode with time as bird species become extinct, altering the composition of the residual bird community (Karr Reference KARR1982, Terborgh et al. Reference TERBORGH, LOPEZ, TELLO, YU, BRUNI, Laurance and Bierregaard1997). Using an information-theoretic approach, we showed that a model taking into consideration effects of area, feeding guild and the interaction between these two factors best explained the species richness of the residual bird communities in forest islands. Our study suggests that the decrease in habitat area had a stronger impact on species richness of insectivorous birds than that of frugivorous or omnivorous (generalists) birds and also provides supporting evidence that small forest fragments have simpler bird communities with less functional groups at a feeding guild level (Table 3).
Table 3. Observed species richness of the six insectivorous foraging guilds on six islands and two mainland control sites sampled. Foraging guild classification follows Wong (Reference WONG1986).

An increased sensitivity of insectivorous birds to fragmentation effects has been implicated in a number of studies in South America (Sekercioğlu et al. Reference SEKERCIOĞLU, EHRLICH, DAILY, AYGEN, GOEHRING and SANDI2002, Stouffer & Bierregaard Reference STOUFFER and BIERREGAARD1995). Studies that examined understorey insectivorous birds like antbirds and flycatchers have demonstrated that insectivore richness declined rapidly after fragmentation (Stouffer & Bierregaard Reference STOUFFER and BIERREGAARD1995, Stratford & Stouffer Reference STRATFORD and STOUFFER1999) and extinction of insectivores may continue even long after isolation (Karr Reference KARR1982) with little compensation by re-colonizers (Robinson Reference ROBINSON1999). Although our results do not show temporal changes in a bird community, it highlights the sensitivity of insectivorous birds to fragmentation, and that resident insectivorous birds may be the first to go extinct after fragmentation, compared with frugivorous or omnivorous species. To understand this better, it is important to examine the constituent bird fauna in Malaysian lowland forests and how these fare in our island fragments.
The majority of Malaysian insectivorous birds are oscine passerines and include some species-rich families like babblers (Sylviidae), crows (Corvidae) and flycatchers (Muscicapidae) while non-passerine insectivores include woodpeckers (Picidae) and trogons (Trogonidae). Representatives from these families featured prominently on the largest islands and the mainland control sites, but are generally reduced or entirely absent on the three smallest islands, which notably exhibited very similar bird communities. For example, not a single species of flycatcher or woodpecker and only one trogon (Harpactes oreskios) persisted on the three smallest islands. Among the babblers, most of which are small understorey or terrestrial insectivores, a steep reduction in species richness can be observed across all sites and corroborates with studies examining similar guilds in South America (Lees & Peres Reference LEES and PERES2008). For example, the terrestrial black-capped babbler (Pellorneum capistratum) occurs on the control sites, but is absent from all the islands (Appendix 1). While the two largest islands harboured as many as six to eight babbler species, only two species occurred on the two smallest islands by comparison, of which one, the striped tit-babbler (Macronous gularis) also occurred at all sites and is known to be able to tolerate heavy habitat disturbance (Lim Reference LIM2009, Wells Reference WELLS2007). Even when compared with the mainland control sites, the largest islands are still relatively depauperate in babbler richness considering that over 12 species occur on the mainland. Lastly, when we considered omnivorous families, all the islands still supported bulbuls, flowerpeckers and sunbirds although family-level richness was markedly lowered. However, in the absence or paucity of insectivores and frugivores, omnivorous species like bulbuls (e.g. Pycnonotus simplex) became more conspicuously dominant and abundant, suggesting ecological release.
It is possible that various changes in ecological conditions in the island fragments drove a greater loss of insectivorous species as indicated by both reduced richness and abundance, thus the poor persistence as suggested by our findings. In fact, of a dozen hypotheses proposed to explain the loss of insectivorous species, many addressed ecological alterations like microclimate changes, food resources reduction and increased nest predation. In Lago Guri for example, the loss of some bird species were attributed to increased nest predation incurred from trapped populations of coatis and capuchin monkeys on islands (Terborgh et al. Reference TERBORGH, LOPEZ, TELLO, YU, BRUNI, Laurance and Bierregaard1997) while Willis (Reference WILLIS1974) directly documented high mortalities attributable to loss of nest, nestlings and fledglings of antbirds in Barro Colorado. Artificial nest predation experiments conducted in forest fragments have also documented higher nest predation rates (Gibbs Reference GIBBS1991, Wong et al. Reference WONG, SODHI and TURNER1998). On Kenyir's islands, direct evidence of increased nest predation is lacking, but observed troops of omnivorous long-tailed macaque (Macaca fascicularis), lar gibbon (Hylobates lar) and Callosciurus sp. squirrels, all of which are opportunistic nest raiders, imply that nest predation may affect resident birds to some extent.
Decreased prey abundance associated with forest fragmentation is a convenient hypothesis to explain insectivorous bird decline and has found some support by temperate studies like Burke & Nol (Reference BURKE and NOL1998). However, this is now no longer widely accepted as newer tropical studies like Sekercioğlu et al. (Reference SEKERCIOĞLU, EHRLICH, DAILY, AYGEN, GOEHRING and SANDI2002) have found no link between arthropod abundance and fragmentation. In fact, Didham (Reference DIDHAM, Watt, Stork and Hunter1997) has pointed out that different arthropod groups are affected differently by fragmentation and that arthropod prey, on the contrary, could increase in overall abundance after fragmentation. Lastly, although microclimatic variables were not measured in our study, conditions in fragments may also have been altered after fragmentation and this could have impacted some of the understorey insectivores, which are known to be highly adapted to understorey environmental conditions where variation in temperature, humidity and light are very small (Stratford & Robinson Reference STRATFORD and ROBINSON2005). Changes in microclimatic conditions could potentially impose great physiological stress on these highly sensitive species and thus limit their occurrence, as is suggested by Sekercioğlu et al. (Reference SEKERCIOĞLU, LOARIE, OVIENDO BRENES, EHRLICH and DAILY2007), where subject species were shown to track even small differences in humidity and temperature.
One key difference between our study and those examining forest fragments is that we addressed true islands surrounded by a water matrix. Non-forest matrix surrounding forest fragments often contain some form of vegetative cover (e.g. scrub, plantation) and may still be exploited by some of the more adaptable bird species (Mitra & Sheldon Reference MITRA and SHELDON1993). The same does not apply for open water which has no ecological value for forest birds. Many forest birds, being associated with close-canopy habitats and which includes a number of insectivores are poor dispersers and cannot cross open bodies of water (Moore et al. Reference MOORE, ROBINSON, LOVETTE and ROBINSON2008, Stratford & Robinson Reference STRATFORD and ROBINSON2005), roads (Laurance et al. Reference LAURANCE, STOUFFER and LAURANCE2004) and also avoids forest gaps and edges (Lees & Peres Reference LEES and PERES2009), limiting their mobility greatly. This dispersal limitation imposes a major hurdle for recolonization once subpopulations of insectivorous birds on smaller islands are extirpated. Empirical evidence in Robinson (Reference ROBINSON1999) showed that minimal recolonization occurred to compensate for numerous insectivorous bird extinctions on Barro Colorado. Considering that local extinctions of resident bird populations on tropical islands are often permanent, it is possible that metapopulation dynamics play only a limited role in shaping tropical land-bridge island bird communities although more field evidence is needed.
While many frugivores like barbets, pigeons and hornbills were clearly absent on the small islands, our study in fact showed that frugivores as a guild are relatively less affected compared with insectivores. We suggest that higher vagility and consequently increased dispersal amongst frugivores may explain this difference in persistence (Lees & Peres Reference LEES and PERES2008, Sodhi et al. Reference SODHI, LIOW and BAZZAZ2004b). Being largely canopy birds, frugivores would be able to better tolerate microclimatic changes in fragments compared with many insectivores since canopy conditions are clearly more variable (Stratford & Robinson Reference STRATFORD and ROBINSON2005). Furthermore, considerable evidence exists to show that many frugivorous birds disperse widely to find food. Hornbills, which are among the best-studied Malaysian frugivores, show large temporal density fluctuations, and certain species entirely disappear from study areas, clearly indicating widespread wandering in search of fruits (Kinnaird & O'Brien Reference KINNAIRD and O'BRIEN2007).
Other studies have shown that apart from hornbills, smaller frugivores like pigeons and barbets also range widely for fruit (Lambert Reference LAMBERT1989a, Reference LAMBERT1989b; Wells Reference WELLS1999). For instance, a radio-tagged yellow-crowned barbet (Megalaima henricii) was detected 700 m away from a fruit-source (Lambert Reference LAMBERT1989b). Given this, it is thus possible that frugivores in our study island hop widely and that these islands actually constitute only temporary sink habitats for individuals dispersing from mainland forests and not representative of a residual resident population. This may also explain why our observed Shannon's diversity index declined with area, indicating an increasingly uneven frugivore community. We acknowledge that in the absence of long-term studies, it is impossible to distinguish whether frugivorous birds detected on the islands were truly members of a remnant population or occasional dispersants from elsewhere and thus while a frugivorous bird may be locally extinct, it can continue to occur on our sites as dispersants from the mainland.
In summary, our study echo the findings of many South American studies of birds in habitat fragments by showing that area and feeding guild (insectivore, omnivore, frugivore) best explained the bird community composition on land-bridge islands. It also shows that insectivores are the most severely impacted guild, as seen in greater reductions of abundance and overall richness when compared with frugivores and omnivores, with potentially detrimental consequences for predator–prey interactions given the diversity and richness of insectivorous birds in Malaysian lowland forests. Future studies would need to examine the Malaysian lowland insectivorous bird community in greater resolution to determine if behavioural specialization (e.g. foraging, clutch sizes) predisposes specialized guilds to local extinction, although the lack of detailed life history and ecological information for many tropical Asian insectivorous birds and the continuing high rates of deforestation in South-East Asia may render this a challenging task.
Future studies should also attempt to test basic predictions of island biogeography and metapopulation theories by measuring survival/extinction rates over a prolonged study regime and compare dispersal abilities of remnant bird populations in different guilds by using radio-tracking, mark–recapture techniques and mist nets (Sekercioğlu et al. Reference SEKERCIOĞLU, LOARIE, OVIENDO BRENES, EHRLICH and DAILY2007, Sieving & Karr Reference SIEVING, KARR, Laurance and Bierregaard1997), which at present is evidently lacking for South-East Asia. These studies should also monitor changes in environmental variables to determine if factors associated with local extinctions in tropical South-East Asia are consistent with those in the Neotropics. Although it may be pointed that a study such as ours is more applicable to land-bridge island ecosystems and highlights ecological decay directly driven by large hydroelectric dams, it nevertheless emphasizes that effective preservation of intact insectivore assemblages demand preservation of large contiguous forest tracts. Furthermore, it demonstrates the severity of damming-induced fragmentation on forest bird communities, a feature which will increasingly become a prevalent feature of the South-East Asian landscape (Goodland Reference GOODLAND, Dorcey, Steiner, Acreman and Orlando1997, Tobias et al. Reference TOBIAS, DAVIDSON and ROBICHAUD1998) as the forested upper courses of many large rivers (e.g. Perak, Rajang, Nam Theun) become dammed for hydroelectricity.
ACKNOWLEDGEMENTS
We would like to thank the Economic Planning Unit (Malaysia) for providing the permits (UPE: 40/200/19/1539) to conduct this study. We would also like to thank Geoffrey Davison, Clive Mann and Richard Corlett for providing some of the ecological data used in our study. T.M.L. thanks W. Jetz for his support. Lastly, we are grateful to two anonymous reviewers whose constructive comments improved our manuscript greatly. This study is supported by the National University of Singapore Grant No. R-154-000-331-122. L.P.K. is supported by an ETH Fellowship and the Swiss National Science Foundation.
Appendix 1. List of bird species recorded on six island and two mainland sites sampled.

Appendix 2. General linear mixed-effects models and summary statistics for species richness, abundance, and Shannon's diversity index H (excluding mainland sites). The candidate models consist of island area, dietary guild of birds and their interaction term (area: guild). A null model is included in each model set as control. K = number of model parameters; LL = minimum negative log-likelihood; AICc = Akaike's information criterion corrected for small sample size; DAICc = difference between AICc of the top-ranked and current model; wAICc = AICc weight.













